Abstract
Objective
To evaluate the association between the severity of endometriosis and the preoperative neutrophil-to-lymphocyte ratio (NLR) and serum level of cancer antigen 125 (CA 125).
Methods
Data were obtained from the medical records of 419 patients who underwent laparoscopic conservative surgery for ovarian endometrioma between April 2005 and March 2013. Each patient's preoperative complete blood count was recorded and the endometriosis score was assessed.
Results
The endometriosis score was not associated with either the NLR or the serum level of CA 125. The endometriosis score was negatively related to preoperative hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration. The only positive association was between NLR and the patients' age. NLR and preoperative serum anti-Müllerian hormone level were found to be negatively related.
Conclusion
The severity of endometriosis was not associated with the serum level of CA 125 or the NLR. The presence of a negative correlation between the severity of endometriosis and red blood cell dynamics needs further investigation.
Keywords: Blood cell count, CA-125 antigen, Endometriosis, Hemoglobins, Lymphocytes, Neutrophils
Introduction
Endometriosis is defined as the presence of endometrial tissue in ectopic locations including the ovaries, pelvic peritoneum, and rectovaginal space. The estimated prevalence of endometriosis is 5%-15% in the general population and up to 20%-48% in infertile women [1,2]. Despite a large number of studies, the etiology and pathophysiology of the disease have not been fully elucidated [3]. Laparoscopic surgery and histological examination of the tissue are needed to confirm the presence of the disease. There are no reliable non-invasive methods to diagnose endometriosis, especially early-stage endometriosis [4]. Nonetheless, cancer antigen 125 (CA 125) has been studied extensively as a non-invasive serological marker for endometriosis. Several studies have reported elevated CA 125 levels in the serum, menstrual blood, and peritoneal fluid of women with endometriosis [5,6,7]. However, a meta-analysis of 23 studies showed that using CA 125 for detecting early-stage endometriosis has limited utility [8].
Mounting evidence suggests that endometriosis is a chronic inflammatory disease, and its pathogenesis is associated with increased neutrophil counts and decreased lymphocyte counts [9,10]. Peritoneal macrophages from patients with endometriosis express a higher level of cyclooxygenase-2 and release a higher amount of prostaglandins, suggesting that inflammation plays an important role in the pathogenesis of endometriosis [11]. Moreover, several studies have compared the levels of inflammatory markers such as C-reactive protein (CRP), interleukin 6, interleukin 8, and tumor necrosis factor alpha in patients with endometriosis, healthy women, and women with ovarian cysts [12,13,14,15]. As a result, these cytokines and chemokines were implicated in cascade reactions and impaired immune system response. This evidence suggests that endometriosis may be considered a disease involving both local and systemic inflammatory disease.
It is well known that the neutrophil-to-lymphocyte ratio (NLR) increases in response to systemic inflammatory reactions [16]. Some evidence suggests that the NLR may be an index of the systemic inflammatory response and a prognostic factor for numerous diseases [17,18,19]. A recent study demonstrated that the mean NLR in patients with minimal to mild endometriosis was significantly higher than in healthy women, indicating that the NLR might be a more sensitive marker than serum CA 125 [20]. The mean NLR and the combined value of the NLR and serum CA 125 level in women with advanced stage endometriosis were also found to be significantly higher than in patients with benign tumors or in healthy individuals [21].
In light of the above findings, the NLR may increase proportionally with the advancing stages of endometriosis, because stage-dependent changes are expected if a certain biomarker is involved in the pathophysiology of the disease. Therefore, in the present study, we investigated the association between the severity of endometriosis with the NLR and with the serum levels of CA 125.
Methods
We reviewed 419 consecutive patients who underwent elective surgery at the Seoul National University Bundang Hospital between April 2005 and March 2013. This study was approved by the Institutional Review Board of the Seoul National University Bundang Hospital (IRB no. B-1312-232-107).
Preoperative medical history questionnaires and pelvic examinations were conducted. All patients underwent routine preoperative laboratory studies, including a complete blood count test performed within one month before the surgery. Patients who underwent surgery primarily for ovarian endometrioma, which was later confirmed pathologically, and who underwent a laparoscopic surgical procedure were eligible for inclusion in our study. Pathological confirmation or clinical suspicion of pregnancy, leiomyoma, adenomyosis, pelvic inflammatory disease, tuberculosis, abnormal liver function, hematonosis, endocrine disease, or immune system diseases were the exclusion criteria.
According to the above selection criteria, 189 women with stage III endometriosis and 230 women with stage IV endometriosis were included in the analysis. The endometriosis score was evaluated according to the revised American Society for Reproductive Medicine guidelines [22]. Preoperative serum values of CRP, anti-Müllerian hormone (AMH), carcinoembryonic antigen (CEA), CA 19-9, and CA 125 were also recorded if the tests were performed within one month of surgery. Dysmenorrhea and/or infertility before surgery were also recorded. The menstrual cycle date was not considered. The NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count.
Student's t-tests were used to compare differences in the means of the continuous variables between the groups of patients with stage III and IV endometriosis. The relationship between the endometriosis score or the NLR and each of the parameters was evaluated using linear and quadratic regression analyses. All statistical analyses were performed using PASW ver. 18.0 (SPSS, Chicago, IL, USA). A p-value <0.05 was considered statistically significant.
Results
As shown in Table 1, bilateral disease and bilateral ovarian cystectomies were more prevalent in women with stage IV endometriosis than in women with stage III endometriosis. However, the size of the largest endometrioma, percentage of women with dysmenorrhea, and infertility rates were similar between the two groups. Although over half of the data for the total population was missing, the preoperative serum values of CRP, AMH, CEA, CA 19-9, and CA 125 were similar between the two groups.
Table 1.
Comparison of clinical and serologic parameters between women with endometriosis stage III and stage IV
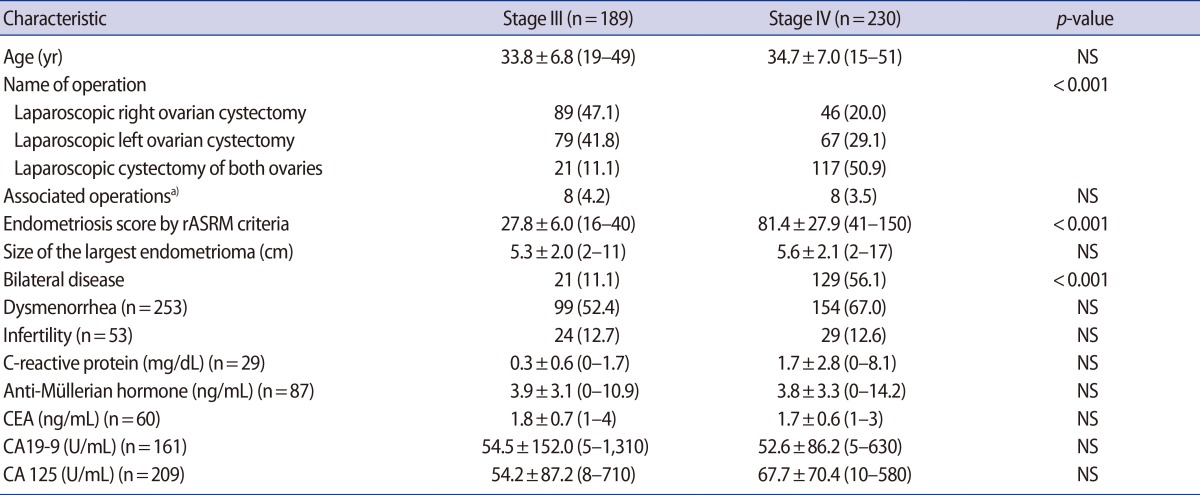
Values are presented as mean±SD (range) or number (%).
NS, not significant; rASRM, revised American Society for Reproductive Medicine; CEA, carcinoembryonic antigen; CA, cancer antigen.
a)Endometrial polypectomy.
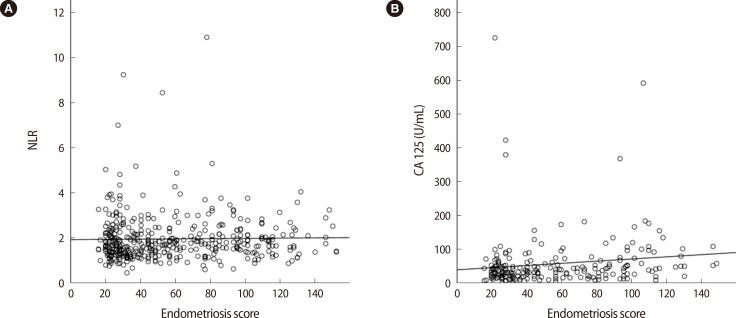
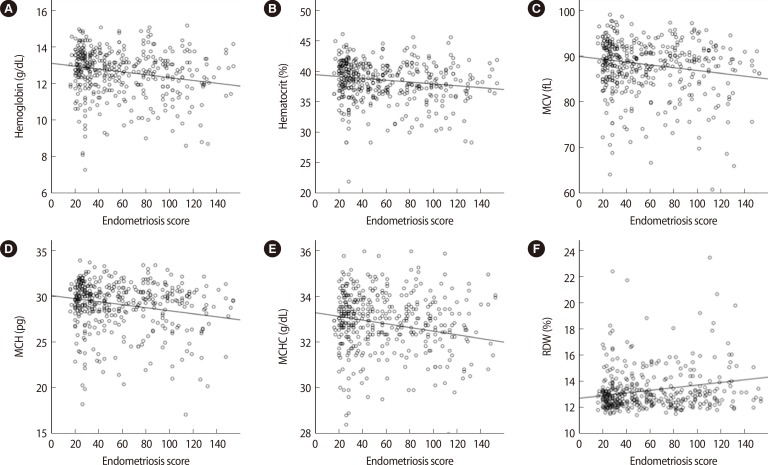
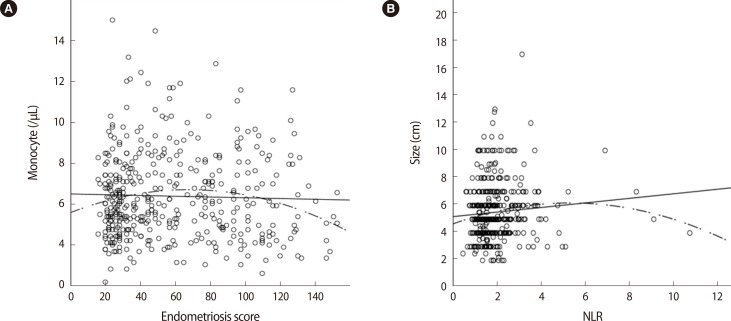
Using the endometriosis score from the 419 women, linear relationships were assessed (Table 2). The endometriosis score had no association with either the NLR or the serum level of CA 125 (Figure 1). The endometriosis score had a significant negative relationship with the preoperative values of hemoglobin, hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC), but a positive relationship with red cell distribution width (RDW). These findings were unexpected (Figure 2). No relationship between the endometriosis score and monocyte was observed in the linear analysis; however, a quadratic analysis revealed a significant negative correlation (r =-0.13, p=0.021) (Figure 3A).
Table 2.
Correlations between endometriosis score and preoperative serologic and hematologic markers
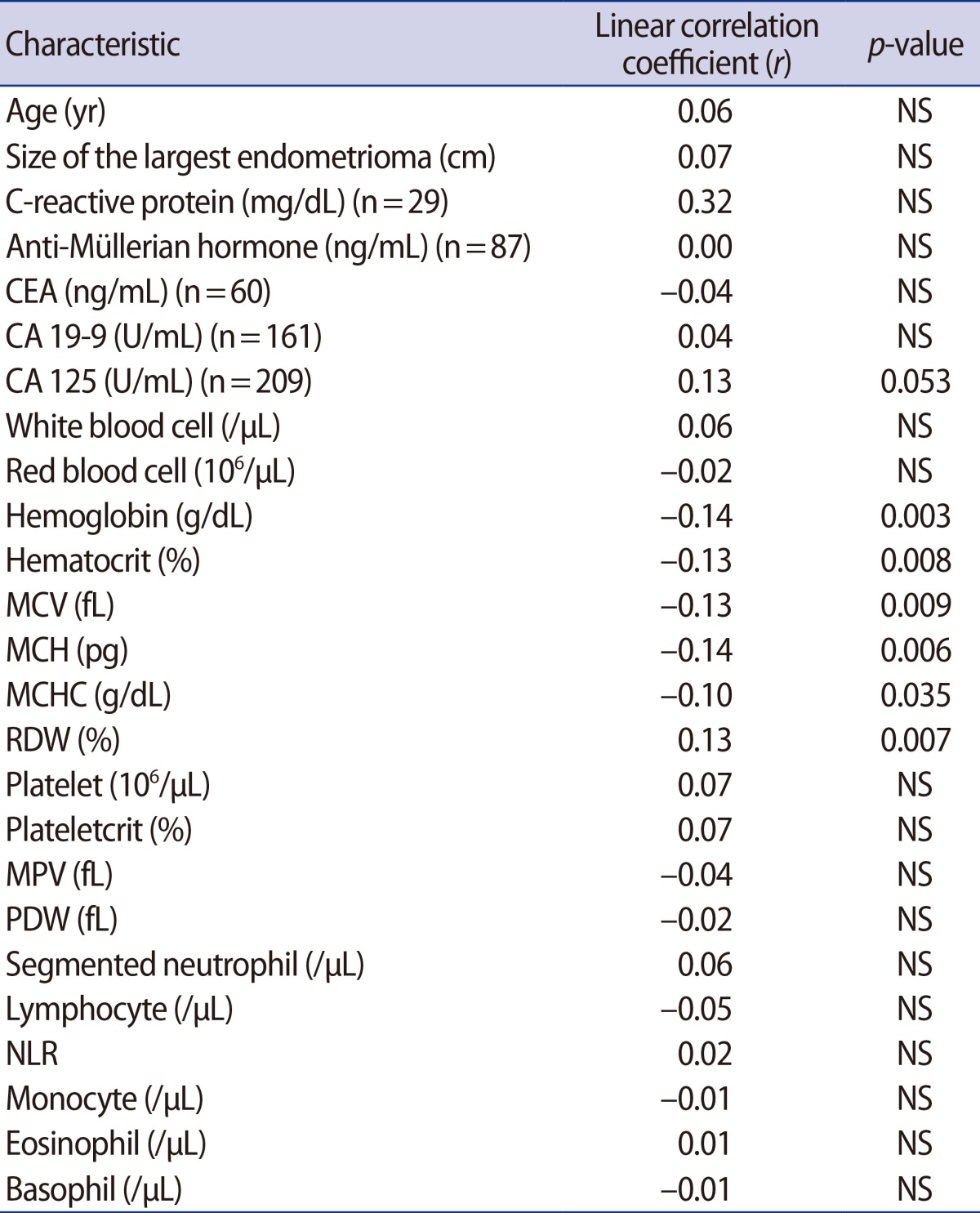
NS, not significant; CEA, carcinoembryonic antigen; CA, cancer antigen; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red cell distribution width; MPV, mean platelet volume; PDW, platelet distribution width; NLR, neutrophil-to-lymphocyte ratio.
Figure 1.
Relationships between the endometriosis score and the preoperative values of the neutrophil-to-lymphocyte ratio (NLR) and cancer antigen (CA) 125. (A) NLR, no correlation (r=0.02, p=0.711) and (B) CA 125, no correlation (r=0.13, p=0.053).
Figure 2.
Preoperative values of red blood cell dynamics showing significant linear correlations with the endometriosis score: (A) hemoglobin (r=-0.14, p=0.003), (B) hematocrit (r=-0.13, p=0.008), (C) mean corpuscular volume (MCV) (r=-0.13, p=0.009), (D) mean corpuscular hemoglobin (MCH) (r=-0.14, p=0.006), (E) mean corpuscular hemoglobin concentration (MCHC) (r=-0.10, p=0.035), and (F) red cell distribution width (RDW) (r=0.13, p=0.007).
Figure 3.
Preoperative values showing significant quadratic correlations: (A) relationship between the endometriosis score and monocyte (r=-0.13. p=0.021), (B) relationship between the neutrophil-to-lymphocyte ratio (NLR) and the size of the largest endometrioma (r=-0.13. p=0.036).
Using the NLR from the 419 women, linear relationships were assessed (Table 3). The NLR was directly related with the patient's age, but was negatively associated with the preoperative values of AMH. No relationship between the NLR and the size of the largest endometrioma was observed in the linear analysis; however, a quadratic analysis revealed a significant positive correlation (r=0.13, p=0.036) (Figure 3B).
Table 3.
Correlations between the neutrophil-to-lymphocyte ratio and preoperative serologic markers
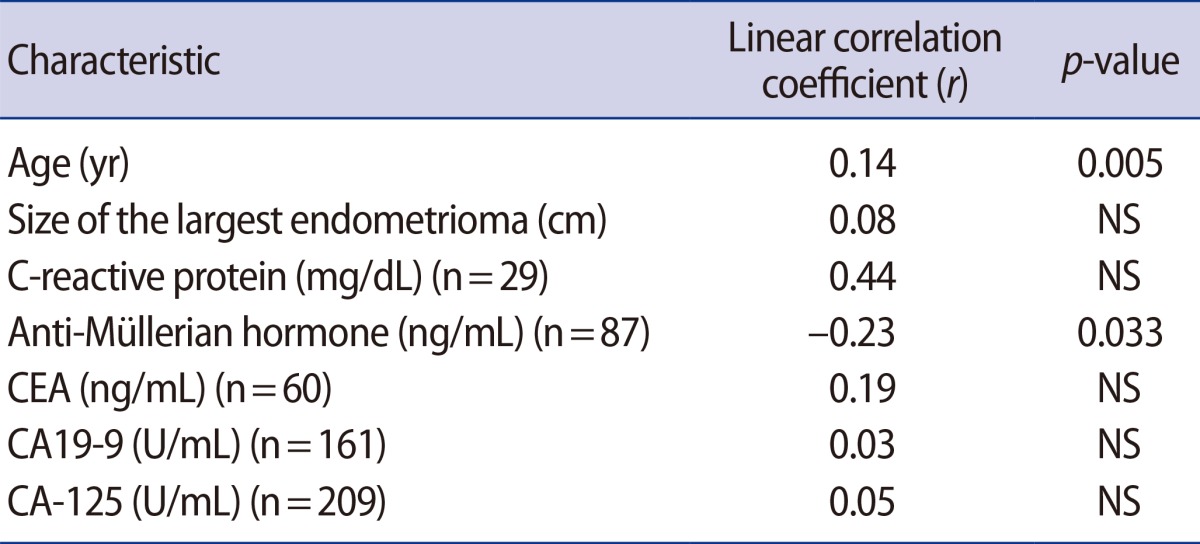
NS, not significant; CEA, carcinoembryonic antigen; CA, cancer antigen.
Discussion
Endometriosis is known to be related to local and systemic inflammatory processes. The peritoneal microenvironment in women with endometriosis includes an abundance of inflammatory cytokines, chemokines, and prostaglandins. Inflammation is also present in the ectopic endometrium of women with endometriosis [11]. However, only a few studies suggest that the NLR may be a potential biomarker of endometriosis. Two recent studies demonstrated a higher NLR in women with early-stage and advanced-stage endometriosis when compared with those without endometriosis [20,21]. Yang et al. [21] reported that the NLR alone as well as the combined value including serum CA 125 levels were significantly higher in women with moderate to severe endometriosis than in those with benign tumors or healthy controls. In these studies, the control groups may not have been appropriate because the control groups were selected without laparoscopic inspection.
In our study, the absolute count of segmented neutrophils or lymphocytes and NLR were not related to the severity of endometriosis. Thus, the severity of endometriosis appears to be unrelated to the degree of inflammation in advanced-stage endometriosis. Contrastingly, the NLR was found to be positively correlated with age and negatively correlated with AMH, in accordance with previous studies [23,24,25]; therefore, the NLR may be a marker of the aging process.
In the present study, we demonstrated that there was no association between the NLR and the endometriosis score in women diagnosed with endometrioma after laparoscopic and histological confirmation. Since our study population only included women with moderate to severe endometriosis, it can be concluded that the NLR might not be a good marker for moderate to severe endometriosis. However, we cannot rule out the possibility that the NLR is related to the severity of early-stage endometriosis.
CA 125 is a glycoprotein that has been extensively studied in connection with endometriosis. In our study, there was no association between the endometriosis score and CA 125 level. In contrast, a number of studies have shown that CA 125 levels are related to the stages of endometriosis [4,26,27,28]. Furthermore, a comprehensive meta-analysis and one recent study have suggested that CA 125 may be a more useful diagnostic marker for stage III and IV endometriosis than for early-stage endometriosis [8,29]. Although the serum CA 125 level appears to fluctuate according to the menstrual cycle [30], menstrual cycle dates were not available in the present study. Thus, further studies are needed to evaluate data from a larger cohort that includes the menstrual cycle in the analysis.
Significant associations between the endometriosis score and several indices related to red blood cell (RBC) dynamics such as hemoglobin, hematocrit, MCV, MCH, MCHC, and RDW are an intriguing finding in the present study. We cannot search out a literature contextualizing these findings.
However, a recent study has observed that mean platelet volume (MPV) was associated with endometriosis and adenomyosis; thus, MPV may be a significant clinical marker associated with the chronic inflammatory processes involved in both diseases [31]. However, we did not find such an association.
Increased hemoglobin and hematocrit levels are usually related to hemoconcentration. The MCV is a measured value of the mean RBC volume. The MCH is the mean mass of hemoglobin per RBC in a sample of blood. The MCHC is the measure of the concentration of hemoglobin in a given volume of packed RBCs. When calculating the MCV, MCH, and MCHC, both the hematocrit and hemoglobin levels are used; therefore, higher hemoglobin and hematocrit levels could explain higher MCV, MCH, and MCHC values. In contrast, higher RDW values indicate a greater variation in the size of the RBCs.
We suggest that more severe endometriosis is associated with lower hemoconcentration, along with the dysregulation of RBC or iron metabolism. Some evidence has shown that iron metabolism is potentially involved in the pathogenesis of endometriosis [32,33,34]. Iron overload in the pelvic cavity can affect numerous mechanisms involved in the development of endometriosis [32]. An excess concentration of iron has been found in various components of the peritoneal cavity (peritoneal fluids, endometriotic lesions, peritoneum, and macrophages) in women with endometriosis [33]. Furthermore, iron can generate free radical species that can induce cellular damage and alter gene expression via the regulation of transcription factors related to the pathophysiology of endometriosis, such as the nuclear factor kappa light-chain enhancer of activated B cells [34]. The results of the present study suggest that women with endometriosis have altered RBC or iron metabolism. However, further studies are needed to clarify the precise role of iron metabolism in the pathogenesis of endometriosis and subsequent changes in hematologic parameters.
The primary limitation of our study is its retrospective design. Furthermore, our study included only women who underwent surgery for ovarian endometrioma and were diagnosed with moderate to severe endometriosis. Thus, we cannot exclude that the NLR may be a marker for early-stage endometriosis.
In conclusion, our findings demonstrated that the NLR is not a good marker for evaluating the severity of endometriosis in patients with moderate to severe endometriosis. The NLR was only associated with the age of the patients. Moreover, we found that the serum level of CA 125 is nonetheless a good marker of endometriosis severity. Significant correlations between the endometriosis score and several indices relating to RBC dynamics were unexpected findings; therefore, further studies are needed to evaluate these findings.
Footnotes
This work was supported by grant no. A120043 from the Korea Health Care Technology R&D Project, Ministry of Health and Welfare, Republic of Korea.
No potential conflict of interest relevant to this article was reported.
References
- 1.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 2.Guo SW, Wang Y. The prevalence of endometriosis in women with chronic pelvic pain. Gynecol Obstet Invest. 2006;62:121–130. doi: 10.1159/000093019. [DOI] [PubMed] [Google Scholar]
- 3.Halis G, Mechsner S, Ebert AD. The diagnosis and treatment of deep infiltrating endometriosis. Dtsch Arztebl Int. 2010;107:446–455. doi: 10.3238/arztebl.2010.0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amaral VF, Ferriani RA, Sa MF, Nogueira AA, Rosa e Silva JC, Rosa e Silva AC, et al. Positive correlation between serum and peritoneal fluid CA-125 levels in women with pelvic endometriosis. Sao Paulo Med J. 2006;124:223–227. doi: 10.1590/S1516-31802006000400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbieri RL, Niloff JM, Bast RC, Jr, Scaetzl E, Kistner RW, Knapp RC. Elevated serum concentrations of CA-125 in patients with advanced endometriosis. Fertil Steril. 1986;45:630–634. doi: 10.1016/s0015-0282(16)49333-7. [DOI] [PubMed] [Google Scholar]
- 6.Koninckx PR, Riittinen L, Seppala M, Cornillie FJ. CA-125 and placental protein 14 concentrations in plasma and peritoneal fluid of women with deeply infiltrating pelvic endometriosis. Fertil Steril. 1992;57:523–530. [PubMed] [Google Scholar]
- 7.O' Shaughnessy A, Check JH, Nowroozi K, Lurie D. CA 125 levels measured in different phases of the menstrual cycle in screening for endometriosis. Obstet Gynecol. 1993;81:99–103. [PubMed] [Google Scholar]
- 8.Mol BW, Bayram N, Lijmer JG, Wiegerinck MA, Bongers MY, van der Veen F, et al. The performance of CA-125 measurement in the detection of endometriosis: a meta-analysis. Fertil Steril. 1998;70:1101–1108. doi: 10.1016/s0015-0282(98)00355-0. [DOI] [PubMed] [Google Scholar]
- 9.Osuga Y, Koga K, Hirota Y, Hirata T, Yoshino O, Taketani Y. Lymphocytes in endometriosis. Am J Reprod Immunol. 2011;65:1–10. doi: 10.1111/j.1600-0897.2010.00887.x. [DOI] [PubMed] [Google Scholar]
- 10.Berbic M, Fraser IS. Regulatory T cells and other leukocytes in the pathogenesis of endometriosis. J Reprod Immunol. 2011;88:149–155. doi: 10.1016/j.jri.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511–519. doi: 10.1016/j.fertnstert.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bedaiwy MA, Falcone T, Sharma RK, Goldberg JM, Attaran M, Nelson DR, et al. Prediction of endometriosis with serum and peritoneal fluid markers: a prospective controlled trial. Hum Reprod. 2002;17:426–431. doi: 10.1093/humrep/17.2.426. [DOI] [PubMed] [Google Scholar]
- 13.Darai E, Detchev R, Hugol D, Quang NT. Serum and cyst fluid levels of interleukin (IL)-6, IL-8 and tumour necrosis factor-alpha in women with endometriomas and benign and malignant cystic ovarian tumours. Hum Reprod. 2003;18:1681–1685. doi: 10.1093/humrep/deg321. [DOI] [PubMed] [Google Scholar]
- 14.Ulukus M, Ulukus EC, Seval Y, Zheng W, Arici A. Expression of interleukin-8 receptors in endometriosis. Hum Reprod. 2005;20:794–801. doi: 10.1093/humrep/deh675. [DOI] [PubMed] [Google Scholar]
- 15.Jee BC, Suh CS, Kim SH, Moon SY. Serum soluble CD163 and interleukin-6 levels in women with ovarian endometriomas. Gynecol Obstet Invest. 2008;66:47–52. doi: 10.1159/000119091. [DOI] [PubMed] [Google Scholar]
- 16.Jilma B, Blann A, Pernerstorfer T, Stohlawetz P, Eichler HG, Vondrovec B, et al. Regulation of adhesion molecules during human endotoxemia. No acute effects of aspirin. Am J Respir Crit Care Med. 1999;159:857–863. doi: 10.1164/ajrccm.159.3.9805087. [DOI] [PubMed] [Google Scholar]
- 17.Zahorec R. Ratio of neutrophil to lymphocyte counts: rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102:5–14. [PubMed] [Google Scholar]
- 18.Bishara S, Griffin M, Cargill A, Bali A, Gore ME, Kaye SB, et al. Pretreatment white blood cell subtypes as prognostic indicators in ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 2008;138:71–75. doi: 10.1016/j.ejogrb.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophillymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181–184. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 20.Cho S, Cho H, Nam A, Kim HY, Choi YS, Park KH, et al. Neutrophil-to-lymphocyte ratio as an adjunct to CA-125 for the diagnosis of endometriosis. Fertil Steril. 2008;90:2073–2079. doi: 10.1016/j.fertnstert.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 21.Yang H, Lang JH, Zhu L, Wang S, Sha GH, Zhang Y. Diagnostic value of the neutrophil-to-lymphocyte ratio and the combination of serum CA-125 for stages III and IV endometriosis. Chin Med J (Engl) 2013;126:2011–2014. [PubMed] [Google Scholar]
- 22.Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817–821. doi: 10.1016/s0015-0282(97)81391-x. [DOI] [PubMed] [Google Scholar]
- 23.Ilhan N, Daglioglu MC, Ilhan O, Coskun M, Tuzcu EA, Kahraman H, et al. Assessment of neutrophil/lymphocyte ratio in patients with age-related macular degeneration. Ocul Immunol Inflamm. 2014:1–4. doi: 10.3109/09273948.2014.921715. [DOI] [PubMed] [Google Scholar]
- 24.Bazzi WM, Dejbakhsh SZ, Bernstein M, Russo P. Neutrophil-lymphocyte ratio in small renal masses. ISRN Urol. 2014;2014:759253. doi: 10.1155/2014/759253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee DY, Hong SW, Chang YG, Lee WY, Lee B. Clinical significance of preoperative inflammatory parameters in gastric cancer patients. J Gastric Cancer. 2013;13:111–116. doi: 10.5230/jgc.2013.13.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen FP, Soong YK, Lee N, Lo SK. The use of serum CA-125 as a marker for endometriosis in patients with dysmenorrhea for monitoring therapy and for recurrence of endometriosis. Acta Obstet Gynecol Scand. 1998;77:665–670. doi: 10.1034/j.1600-0412.1998.770615.x. [DOI] [PubMed] [Google Scholar]
- 27.Martinez S, Garrido N, Coperias JL, Pardo F, Desco J, Garcia-Velasco JA, et al. Serum interleukin-6 levels are elevated in women with minimal-mild endometriosis. Hum Reprod. 2007;22:836–842. doi: 10.1093/humrep/del419. [DOI] [PubMed] [Google Scholar]
- 28.Rosa E Silva AC, Rosa E Silva JC, Ferriani RA. Serum CA-125 in the diagnosis of endometriosis. Int J Gynaecol Obstet. 2007;96:206–207. doi: 10.1016/j.ijgo.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 29.Maiorana A, Cicerone C, Niceta M, Alio L. Evaluation of serum CA 125 levels in patients with pelvic pain related to endometriosis. Int J Biol Markers. 2007;22:200–202. doi: 10.1177/172460080702200306. [DOI] [PubMed] [Google Scholar]
- 30.Kafali H, Artuc H, Demir N. Use of CA125 fluctuation during the menstrual cycle as a tool in the clinical diagnosis of endometriosis; a preliminary report. Eur J Obstet Gynecol Reprod Biol. 2004;116:85–88. doi: 10.1016/j.ejogrb.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 31.Bodur S, Gun I, Alpaslan Babayigit M. The significance of mean platelet volume on diagnosis and management of adenomyosis. Med Glas (Zenica) 2013;10:59–62. [PubMed] [Google Scholar]
- 32.Defrere S, Gonzalez-Ramos R, Lousse JC, Colette S, Donnez O, Donnez J, et al. Insights into iron and nuclear factor-kappa B (NF-kappaB) involvement in chronic inflammatory processes in peritoneal endometriosis. Histol Histopathol. 2011;26:1083–1092. doi: 10.14670/HH-26.1083. [DOI] [PubMed] [Google Scholar]
- 33.Defrere S, Lousse JC, Gonzalez-Ramos R, Colette S, Donnez J, Van Langendonckt A. Potential involvement of iron in the pathogenesis of peritoneal endometriosis. Mol Hum Reprod. 2008;14:377–385. doi: 10.1093/molehr/gan033. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Ramos R, Defrere S, Devoto L. Nuclear factor-kappaB: a main regulator of inflammation and cell survival in endometriosis pathophysiology. Fertil Steril. 2012;98:520–528. doi: 10.1016/j.fertnstert.2012.06.021. [DOI] [PubMed] [Google Scholar]