Key Points
Genetic elimination of the coagulation transglutaminase fXIII limits arthritis incidence and severity in mice.
FXIII supports arthritis pathogenesis through distinct mechanisms linked to joint inflammation and osteoclastogenesis.
Abstract
Rheumatoid arthritis is a chronic inflammatory disease characterized by synovial hyperplasia, inflammatory cell infiltration, irreversible cartilage and bone destruction, and exuberant coagulation system activity within joint tissue. Here, we demonstrate that the coagulation transglutaminase, factor XIII (fXIII), drives arthritis pathogenesis by promoting local inflammatory and tissue degradative and remodeling events. All pathological features of collagen-induced arthritis (CIA) were significantly reduced in fXIII-deficient mice. However, the most striking difference in outcome was the preservation of cartilage and bone in fXIIIA−/− mice concurrent with reduced osteoclast numbers and activity. The local expression of osteoclast effectors receptor activator of nuclear factor-κB ligand (RANKL) and tartrate resistant acid phosphatase were significantly diminished in CIA-challenged and even unchallenged fXIIIA−/− mice relative to wild-type animals, but were similar in wild-type and fibrinogen-deficient mice. Impaired osteoclast formation in fXIIIA−/− mice was not due to an inherent deficiency of monocyte precursors, but it was linked to reduced RANKL-driven osteoclast formation. Furthermore, treatment of mice with the pan-transglutaminase inhibitor cystamine resulted in significantly diminished CIA pathology and local markers of osteoclastogenesis. Thus, eliminating fXIIIA limits inflammatory arthritis and protects from cartilage and bone destruction in part through mechanisms linked to reduced RANKL-mediated osteoclastogenesis. In summary, therapeutic strategies targeting fXIII activity may prove beneficial in limiting arthropathies and other degenerative bone diseases.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by synovial hyperplasia, inflammation, and tissue destruction. The progression of clinically uncontrolled arthritis can lead to devastating inflammation-driven cartilage and bone destruction, resulting in irreversible joint damage. Therapeutic strategies at the level of inflammatory pathways (eg, antitumor necrosis factor (TNF) agents or B-cell depletion) have shown considerable efficacy in reducing synovitis and limiting progressive joint damage, but are not uniformly successful in treating RA.1,2 Indeed, nonresponsiveness to treatment and an inability to reverse cartilage and bone loss highlight the need for identifying novel targets to improve therapeutic efficacy and patient outcomes.
Coagulation system activity is a prominent feature of RA, and specific links between coagulation and inflammatory systems play a fundamental role in disease progression. The synovial fluid of RA patients contains reduced levels of coagulation factors in conjunction with corresponding increases in levels of thrombin-antithrombin complexes and fibrin degradation products, indicative of ongoing coagulation system activity.3-5 Functional studies in mice have shown that pharmacologic inhibition of thrombin activity or the genetic elimination of the clotting factor fibrinogen, significantly diminishes arthritis severity.6,7 Directly promoting local inflammation is one mechanism by which fibrin(ogen) supports arthritis pathogenesis as disruption of fibrin(ogen)-αMβ2 leukocyte integrin engagement significantly diminished arthritis in mice due to reduced local proinflammatory cytokine (eg, TNFα, IL-1β, and IL-6) expression.8 Although coagulation factors are increasingly recognized as powerful modifiers of inflammatory joint disease, the contribution, interplay, and mechanism(s) by which specific factors of diverse function (eg, serine proteases, G-protein coupled receptors) modify disease outcome remains ill-defined.
FXIII is a thrombin-activated transglutaminase that catalyzes the formation of covalent ε-N-(γ-glutamyl)-lysine crosslinks between residues of target proteins.9 FXIII is present in circulation as a heterotetrameric zymogen composed of 2 catalytic fXIIIA and 2 noncatalytic fXIIIB subunits. The preeminent function of activated fXIII is the stabilization of fibrin clot structures through crosslinking of fibrin polymers to increase stiffness, reduce stretch, and limit plasmin-mediated proteolysis.10,11 The vital contribution of fXIII to hemostasis is highlighted by the fact that patients with fXIII deficiency and fXIIIA-deficient mice display bleeding diatheses and significantly impaired wound healing.12,13 However, putative roles for fXIIIA in settings outside of coagulation and wound healing, including the mechanisms linked to inflammatory disease pathologies, are largely unexplored.
In the present study, we explored the contribution of fXIIIA to arthritis pathogenesis in the experimental setting of collagen-induced arthritis (CIA). For the first time, our studies revealed that fXIIIA is a potent determinant of inflammatory arthritis and eliminating fXIIIA can ameliorate arthritis severity. In addition, using a combination of in vivo and in vitro approaches, we report that fXIIIA appears to support arthritis pathogenesis by multiple distinct mechanisms, including pathways linked to local inflammation, which are likely fibrinogen-dependent, and pathways directing local osteoclast differentiation and function, which appear to be fibrinogen-independent.
Methods
Mice
Mice lacking the fXIII catalytic subunit A (fXIIIA−/−) and fibrinogen-deficient mice have been described previously.14,15 For CIA experiments, fXIIIA−/− and fXIIIA+/+ littermates were backcrossed to the DBA/1J genetic background for 8 generations. Males and females were enrolled in experiments as separate cohorts and analyzed. All mice were housed in the animal care facility of Cincinnati Children’s Hospital Research Foundation. The Cincinnati Children’s Hospital Animal Care and Use Committee approved the study protocols.
Arthritis induction and macroscopic scoring
CIA induction and evaluation in mice were performed as previously described.8 Briefly, cohorts of fXIIIA+/+ and fXIIIA−/− mice (6-10 weeks old) were given intradermal injections of 100 μg bovine type II collagen (CII, Elastin Products Co., Inc.) in complete Freund’s adjuvant on days 0 and 21. Details of arthritis scoring are provided in the supplemental Methods on the Blood Web site. For cystamine experiments, male DBA/1 mice were administered 900 mg/L cystamine dihydrochloride (Sigma-Aldrich) in their drinking water starting 14 days prior to the first immunization until the end of the experiment. Mice receiving plain drinking water were used as controls.
Histology
Qualitative and quantitative histologic evaluation of joint tissue was performed as previously described8 or as detailed in the supplemental Methods.
RNA isolation and quantitative real-time PCR
Frozen hind paws were homogenized in TRIzol (Invitrogen) and RNA was extracted according to the manufacturer’s protocol. Complementary DNA was synthesized from 2 μg total RNA using a High Capacity RNA-to-cDNA kit (Applied Biosystems). Real-time polymerase chain reaction was performed in a StepOne Plus instrument (Applied Biosystems) using TaqMan probes for IL-6: Mm00446190_m1, IL-1β: Mm01336189_m1, TNFα: Mm99999068_m1, IL-10: Mm00439614_m1, TGF-β: Mm00441724_m1, receptor activator of nuclear factor-κB ligand (RANKL): Mm01313943_m1, tartrate resistant acid phosphatase (TRAP): Mm00475698_m1, osteoprotegerin (OPG): Mm01205928_m1, nuclear factor of activated T cells c1 (NFATc1): Mm00479441_m1. β2M: Mm00437762_m1 was used as an endogenous control gene for all TaqMan-based real-time polymerase chain reaction (PCR) analyses.
Analysis of cellular and humoral response to CII immunization
Details of T-cell and B-cell adaptive immune responses after collagen immunization are available in the supplemental Methods.
In vitro osteoclastogenesis
Splenocytes from fXIIIA+/+ or fXIIIA−/− mice were plated at a density of 1 × 106 cells/well in a 24-well plate and cultured for 7 days in the presence of RANKL (100 ng/mL) and monocyte colony stimulating factor (M-CSF) (30 ng/mL) in α-minimum essential medium (containing 10% fetal bovine serum and antibiotics). Cells were harvested for gene expression analyses or stained for TRAP with a leukocyte acid phosphatase kit (Sigma-Aldrich), according to the manufacturer’s instructions to identify osteoclasts. TRAP+ cells containing >3 nuclei were counted as osteoclasts.
Statistical analysis
Kaplan-Meier log-rank analysis was used to compare arthritis incidence. Arthritis index and severity were evaluated using the Mann-Whitney U test. Histopathologic scores, messenger RNA (mRNA) quantification, safranin-O staining and quantification of osteoclasts were compared using 2-tailed Student t test or 2-way analysis of variance followed by the Student-Newman-Keuls posthoc test. P < .05 was considered statistically significant.
Results
Genetically-imposed fXIIIA deficiency results in reduced CIA incidence and severity
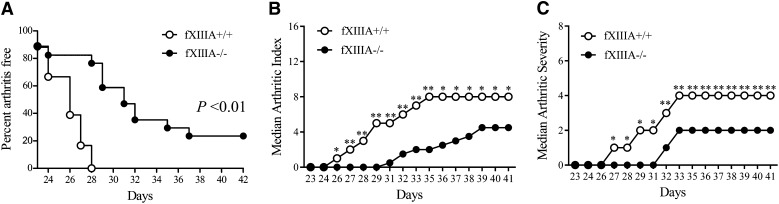
Analysis of CIA-challenged mice deficient in the catalytic A subunit of fXIII (ie, fXIIIA−/−) and wild-type littermate controls (fXIIIA+/+) revealed that fXIIIA deficiency resulted in significantly decreased CIA incidence relative to wild-type mice (Figure 1A). Approximately 20% of male fXIIIA−/− mice showed no evidence of macroscopic arthritis in the paws over the entire observation period, whereas 100% of wild-type mice in each study developed multiple arthritic joints. Furthermore, the number of paw joints affected (ie, arthritis index) was significantly reduced in fXIIIA−/− mice compared with wild-type mice beginning at day 26 after primary collagen immunization, and this pattern persisted to the end of the macroscopic evaluation period (Figure 1B). The degree of swelling in each paw (ie, arthritis severity) was also significantly reduced in fXIIIA−/− mice relative to wild-type mice (Figure 1C). A similar significant diminution in macroscopic disease was observed in CIA-challenged female fXIIIA−/− mice compared with female fXIIIA+/+ animals (supplemental Figure 1). These observations suggest that the elimination of fXIIIA limits the development and progression of inflammatory arthritis.
Figure 1.
Genetic elimination of coagulation fXIIIA results in diminished CIA incidence and severity. (A) The percentage of male mice with macroscopic arthritis in the paws is shown for the 3-week evaluation period after the second CII immunization. Note that approximately 20% of fXIIIA−/− mice remained arthritis-free based on visible inspection through the CIA study period. Kaplan-Meier log-rank analysis. (B) The median arthritis index revealed a significant diminution in number of arthritic joints in the absence of fXIIIA. (C) The median arthritis severity for the same cohort of mice revealed a significant reduction in paw swelling for CIA challenged fXIIIA−/− mice (n = 18 in fXIIIA+/+ group and n = 17 in fXIIIA−/− group). Mann-Whitney U test. *P < .05; **P < .01 (B-C).
FXIIIA deficiency is protective against CIA-driven histological pathologies including cartilage degradation and bone erosion
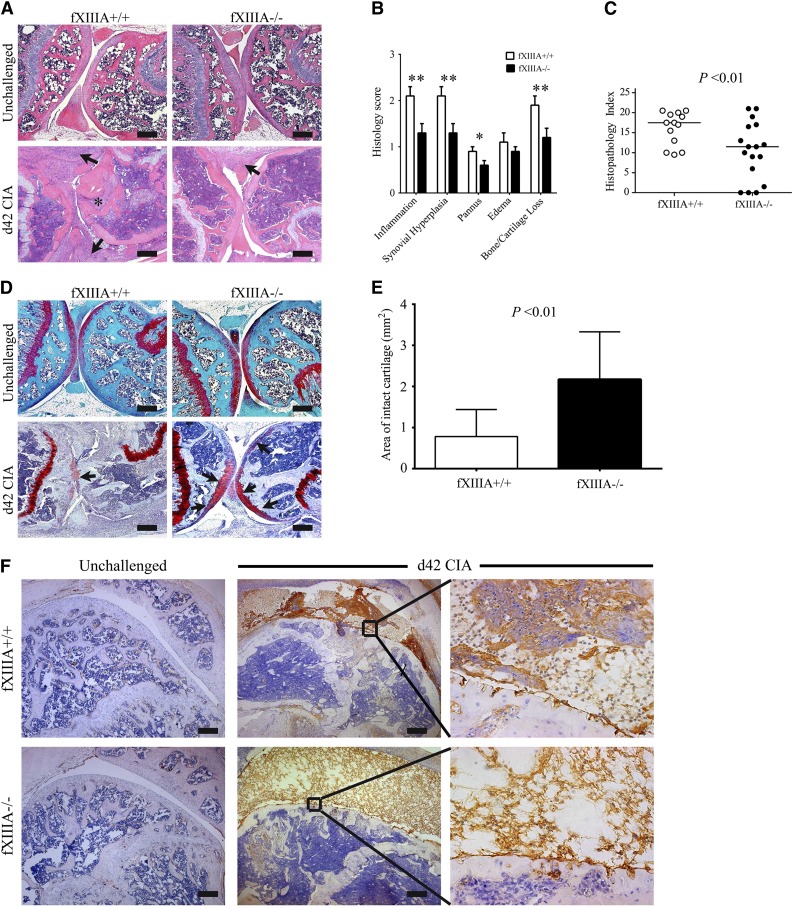
Knee joints harvested at day 42 of the CIA protocol were evaluated for histologic signs of arthritis. The pattern and severity of joint disease mirrored the macroscopic clinical scores in that fXIIIA deficiency resulted in markedly diminished disease severity in the knee joints relative to wild-type mice (Figure 2A). Comparative, semiquantitative analyses of CIA pathological features, revealed that inflammation, synovial hyperplasia, pannus, and cartilage/bone loss was significantly lower in the knee joints of fXIIIA−/− mice (Figure 2B), leading to an overall significant reduction in the composite histopathology index score relative to wild-type mice (Figure 2C). Notably, even in knee joints from fXIIIA−/− mice that displayed robust inflammation and hyperplasia, cartilage degradation was significantly diminished relative to fXIIIA+/+ mice. A formal assessment of cartilage degradation by safranin-O staining indicated preservation of articular cartilage within the knee joints of fXIIIA−/− mice compared with wild-type mice (Figure 2D-E). Similar results were observed in histologic analyses of female mice (supplemental Figure 1). These data indicate that fXIIIA is an important modifier of arthritis, advancing key pathological metrics of disease, including the most devastating aspects of cartilage degradation and bone erosion.
Figure 2.
Elimination of fXIIIA results in diminished CIA joint pathology and destruction. (A) Representative hematoxylin and eosin–stained knee joint sections from unchallenged and CIA-challenged fXIIIA+/+ and fXIIIA−/− cohorts. Upon CIA challenge, significant inflammation, synovial hyperplasia (arrows), and erosive pannus (asterisk) are apparent in fXIIIA+/+ mice, whereas knee joints of fXIIIA−/− mice display markedly attenuated pathological features. (B) Semiquantitative microscopic analysis knee joint pathological features from fXIIIA+/+ (n = 13, white bars) and fXIIIA−/− (n = 17, black bars) male mice. Student t test. (C) Scatter plot of composite histopathology index analysis (see Methods) of hematoxylin and eosin–stained knee joint sections. Each symbol represents the composite score for individual mice and bars denote median values for each genotype. (D) Representative safranin-O stained knee joint sections from unchallenged and CIA-challenged fXIIIA+/+ and fXIIIA−/− mice. (E) Quantification of area of intact/preserved articular cartilage per knee based on safranin-O stain. Data are mean ± standard error of the mean with n = 5 mice per genotype and analyzed using Student t test. (F) Representative images of immunohistochemical fibrin(ogen) staining within the knee joints of (left) unchallenged and (middle and right) CIA-challenged mice. Bars represent 200 µm. *P < .05; **P < .01 (B).
Given that the most well-characterized function of fXIII is to crosslink fibrin, we analyzed local fibrin deposition at day 42 within the knee joints of CIA-challenged mice. Fibrin(ogen)-deposits were never observed in the knee joint of unchallenged mice, regardless of the presence or absence of fXIIIA (Figure 2F). Consistent with retained thrombin generation potential, retained fibrinogen availability, and normal thrombin clotting times in fXIIIA−/− mice, fibrin(ogen) deposition within the knees joints was observed in CIA-challenged fXIIIA−/− animals. However, the patterns of fibrin staining in CIA-challenged fXIIIA+/+ and fXIIIA−/− mice were qualitatively distinct (Figure 2F). Fibrin appeared as thick layers along the inflamed synovium, dispersed through the hyperplastic granulation tissue, and as dense deposits within the synovial space in wild-type mice. However, knee joints from CIA-challenged fXIIIA−/− mice displayed more diffuse fibrin deposits that were generally limited to the joint space (Figure 2F). Notably, qualitatively fewer inflammatory cells were associated with the fibrin matrix in CIA-challenged fXIIIA−/− mice (Figure 2F). Immunohistochemical staining for neutrophils (supplemental Figure 2A-B) and macrophages (supplemental Figure 2C-D) supported the conclusion that fewer inflammatory cells were present in the joint space of fXIIIA−/− mice compared with fXIIIA+/+ mice at day 42 of the CIA protocol.
Reduced local proinflammatory cytokine expression in CIA-challenged fXIIIA−/− mice
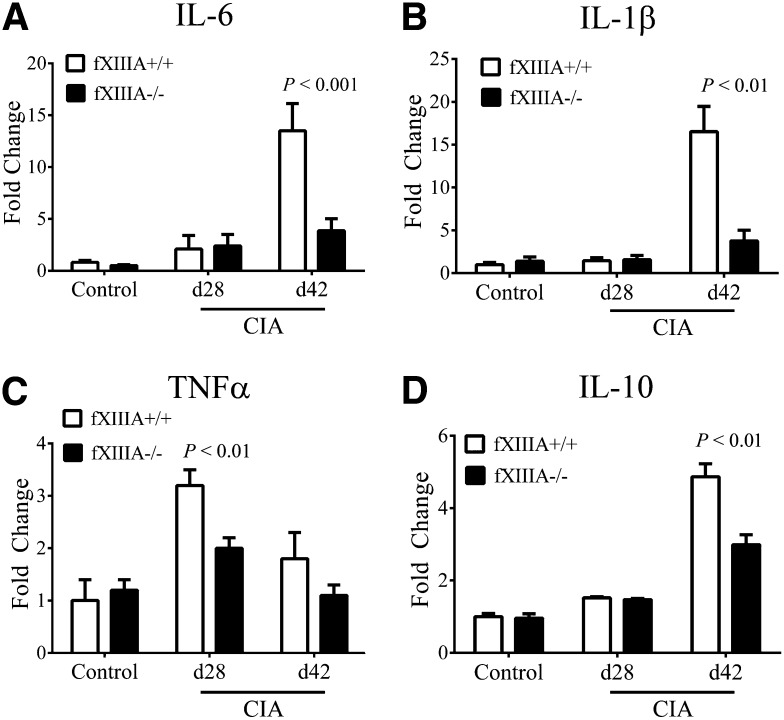
Expression levels of key proinflammatory and antiinflammatory cytokines (eg, IL-6, TNFα, IL-1β, IL-10) measured by quantitative reverse-transcription polymerase chain reaction (qRT-PCR) in hind paw extracts were comparable in unchallenged fXIIIA+/+ and fXIIIA−/− mice, and predictably, the levels of each of these cytokines were significantly increased in CIA-challenged animals (Figure 3). However, consistent with the observed differences in disease severity, mRNA levels of IL-6 and IL-1β were markedly diminished in joints of fXIIIA−/− mice relative to wild-type mice at day 42 of CIA (Figure 3A-B, respectively). Local mRNA levels of TNFα were significantly elevated in the early effector phase of CIA (ie, day 28) for wild-type mice, as previously documented,8 but significantly reduced in CIA-challenged fXIIIA−/− mice (Figure 3C). Similarly, mRNA levels of anti-inflammatory cytokines, IL-10 (Figure 3D) and TGF-β (data not shown), were also elevated at day 42 of CIA in fXIIIA+/+ mice relative to fXIIIA−/− mice. These results suggest that the reduction in CIA severity observed in fXIIIA−/− mice is due to an attenuated proinflammatory response rather than an enhanced local antiinflammatory cytokine response. Interestingly, the pattern of inflammatory cytokine expression in fXIIIA−/− mice was identical to that reported in CIA-challenged fibrinogen-deficient mice.8 These findings support the concept that one mechanism by which fXIIIA promotes local inflammation is through its primary substrate and an established proinflammatory mediator, fibrinogen.
Figure 3.
Reduced inflammatory cytokine expression in CIA-challenged fXIIIA-deficient mice. Quantitative RT-PCR analysis of messenger RNA levels for inflammatory cytokines IL-6 (A), IL-1β (B), TNFα (C), and IL-10 (D) in the hind paws obtained from unchallenged (control, n = 6 per genotype), day 28 (n = 9 per genotype), and day 42 (n = 10 per genotype) of fXIIIA+/+ and fXIIIA−/− mice. Data are expressed as average fold change over unchallenged fXIIIA+/+ group with error bars denoting standard error of the mean. P < .001 (A); Data analyzed by 2-way analysis of variance, followed by Student-Newman-Keuls posthoc test.
FXIIIA deficiency does not alter peripheral T- and B-cell responses after CII immunization
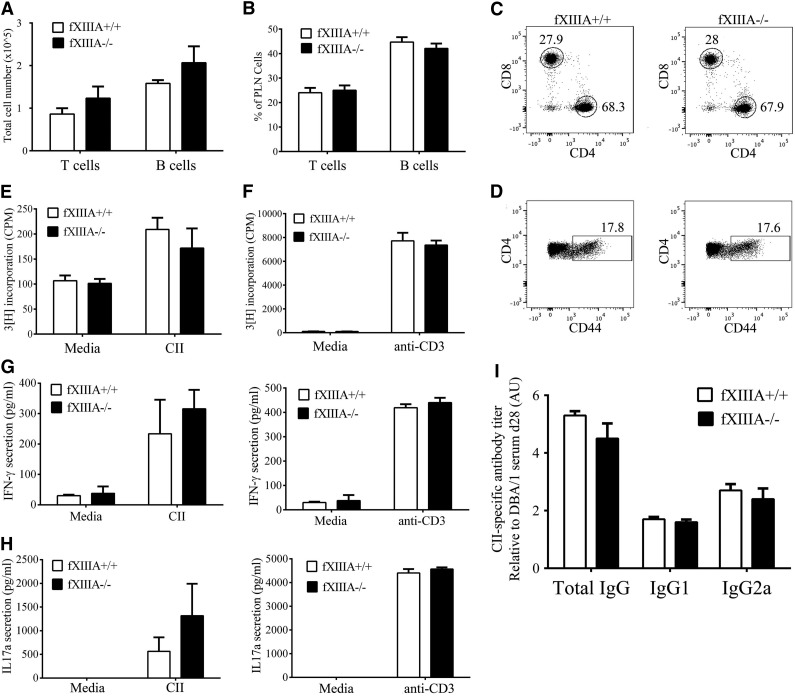
To determine if the reduced CIA severity observed in fXIIIA−/− mice was secondary to an impaired adaptive immune response after collagen immunization, cells isolated from the draining lymph nodes or spleen, and the plasma were analyzed. No genotype-dependent difference in numbers (Figure 4A) or percentages (Figure 4B) of total T and B cells were observed within the draining popliteal lymph node (PLN) 10 days after immunization with CII in the footpad. Similarly, no genotype-dependent differences were observed in numbers and percentages of CD4+ and CD8+ T-cell subsets (Figure 4C) and activated CD44hi CD4+ T cells from the PLN of mice with (Figure 4D) or without CII immunization (data not shown). Furthermore, no genotype-dependent differences were observed in the proliferative response of cells isolated from the spleens of CII-immunized mice after either re-stimulation with CII (Figure 4E) or direct antibody-mediated T-cell receptor activation (Figure 4F). Secretion of IFN-γ (Figure 4G) and IL-17A (Figure 4H) by PLN cells after restimulation was also comparable between fXIIIA−/− and fXIIIA+/+ mice. Finally, analysis of CII-specific total IgG, Th1-dependent IgG2a and the complement-fixing IgG1 antibodies in the plasma revealed similar anti-CII antibody titers between the 2 genotypes (Figure 4I). Together these data suggest that the reduced CIA severity in fXIIIA−/− mice is not due to impaired peripheral T- and B-cell responses after CII-immunization.
Figure 4.
CII-specific T- and B-cell responses are similar in fXIIIA+/+ and fXIIIA−/− mice. Flow cytometry-based analyses of CD3+/CD19− total T-cell and CD3−/CD19+ total B-cell (A) numbers and (B) percentages within the draining popliteal lymph nodes of fXIIIA+/+ and fXIIIA−/− mice 10 days after immunization with CII in the footpad. Representative scatter dot plots highlight both similar (C) percentages of CD4+ and CD8+ T cells, and (D) CD4+ T-cell activation based on surface expression of CD44 in PLN of CII-immunized mice of both genotypes. Proliferation of splenocytes harvested from CIA-challenged fXIIIA+/+ and fXIIIA−/− after stimulation with either (E) heat-inactivated CII or (F) direct T-cell receptor stimulation using anti-CD3 antibody as evaluated by [3H] thymidine incorporation. Measurement of (G) IFN-γ and (H) IL-17A secretion by popliteal lymph node cells harvested from CII-immunized fXIIIA+/+ and fXIIIA−/− mice after ex vivo re-stimulation with CII (left panel) or anti-CD3 (right panel) antibody. Note that for all T-cell analyses (n = 4 mice per genotype). (I) CII-specific antibody titers in the plasma of CIA-challenged fXIIIA+/+ and fXIIIA−/− mice (n = 9 mice per genotype). All data are presented as mean ± standard error of the mean. CPM, counts per minute.
FXIIIA deficiency impairs osteoclast differentiation in vivo
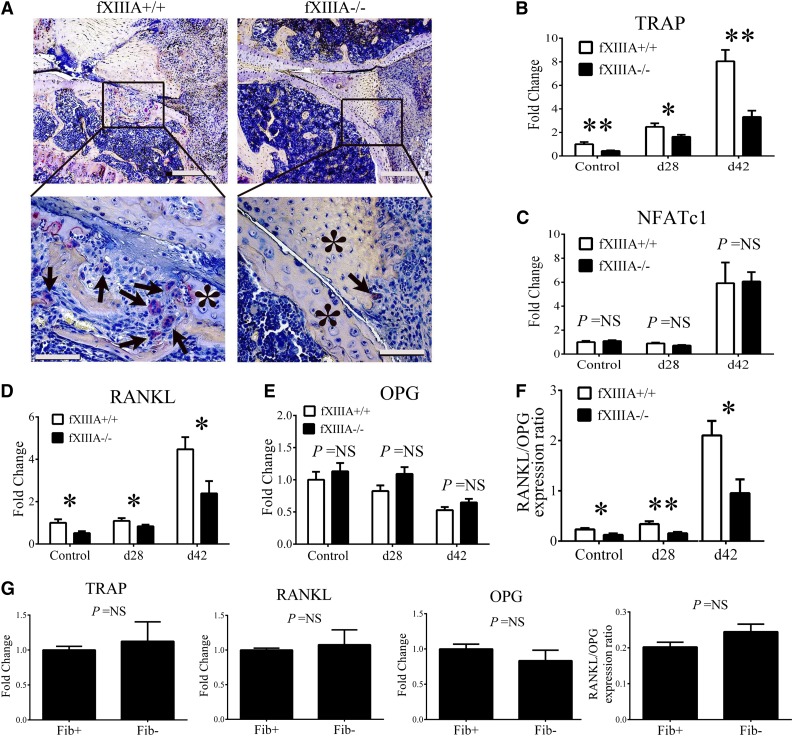
Cartilage and bone resorption are mediated, in part, by osteoclasts derived from the monocyte and macrophage lineage.16-18 We analyzed knee joints of CIA-challenged fXIIIA+/+ and fXIIIA−/− mice harvested at day 42 for the presence of osteoclasts by TRAP staining. Whereas large, multinucleated, TRAP+ osteoclasts were readily observed within focal lesions of synovial and granulation tissue invasion into the bone of fXIIIA+/+ mice, similar TRAP+ cells and erosive lesions were largely absent in knees of fXIIIA−/− mice (Figure 5A). Furthermore, qRT-PCR analysis revealed a significant diminution in local mRNA expression of TRAP gene (Acp5) in CIA-challenged fXIIIA−/− mice compared with controls (Figure 5B). Of note, TRAP expression was significantly reduced, even in unchallenged fXIIIA−/− mice compared with fXIIIA+/+ mice (Figure 5B). Despite the observed quantitative differences in TRAP gene expression, similar mRNA levels of the transcription factor associated with differentiation of osteoclasts, NFATc1, were observed between the 2 genotypes with or without CIA challenge (Figure 5C).
Figure 5.
Impaired osteoclast differentiation/activation within the joints of fXIIIA-deficient mice. (A) Representative images of knee joint sections harvested from CIA-challenged fXIIIA+/+ and fXIIIA−/− mice stained for TRAP. Note numerous TRAP+, multinucleated osteoclasts (arrows) within erosive lesions of fXIIIA+/+ knee joints, but largely absent in sections from fXIII−/− mice. The * indicates area of intact bone. Quantitative RT-PCR analysis mRNA for (B) TRAP gene expression, (C) NFATc1, (D) RANKL gene expression, and (E) OPG gene expression within hind paw joints harvested from unchallenged and CIA-challenged fXIIIA+/+ and fXIIIA−/− mice. Data are expressed as average fold change relative to unchallenged fXIIIA+/+ group. (F) Ratio of RANKL and OPG relative expression. (G) Quantitative RT-PCR analysis of local mRNA levels of TRAP, RANKL, OPG, and the ratio of RANKL/OPG in the hind paws of unchallenged Fib+ and Fib− mice (n = 6 per genotype). All data are expressed as mean ± standard error of the mean. Data were analyzed by 2-way analysis of variance followed by Student-Newman-Keuls posthoc test in which *P < .05; **P < .01. NS = not significant (B-G). Scale bar represents 200 μm (top panels) and 10 μm (bottom panels) (A).
Local mRNA levels of the key osteoclast regulators RANKL and OPG were evaluated by qRT-PCR. As shown in Figure 5D, RANKL expression was significantly reduced in the paws of fXIIIA−/− mice relative to wild-type mice both at days 28 and 42 of CIA. Interestingly, a significant reduction in RANKL expression was also observed in unchallenged fXIIIA−/− mice compared with controls (Figure 5D), whereas OPG mRNA levels were similar between the 2 genotypes at the different time points (Figure 5E). Consistent with reduced disease activity in CIA-challenged fXIIIA−/− mice, the ratio of RANKL/OPG mRNA levels was significantly decreased relative to CIA-challenged fXIIIA+/+ mice, both prior to disease induction and during the effector phase of CIA (Figure 5F), corresponding with the reduction in osteoclasts (ie, TRAP+ cells) in fXIIIA-deficient mice.
To determine whether changes in local osteological activity observed in fXIIIA−/− mice were replicated in animals deficient in fibrin(ogen), local levels of RANKL, OPG, and TRAP were evaluated in fibrinogen-deficient mice. As shown in Figure 5G, no genotype-dependent differences in mRNA levels of RANKL, OPG, RANKL/OPG ratio, or TRAP within the hind paws of unchallenged fibrinogen-sufficient and fibrinogen-deficient mice were observed. These results indicate that unlike fXIIIA deficiency, fibrinogen deficiency does not impact baseline expression of important osteoclast effectors in the paws of mice. Together, these data support the novel concept that fXIIIA mediates local tissue destruction through a potential fibrinogen-independent mechanism.
In vitro differentiation of osteoclasts is significantly diminished in the absence of fXIIIA
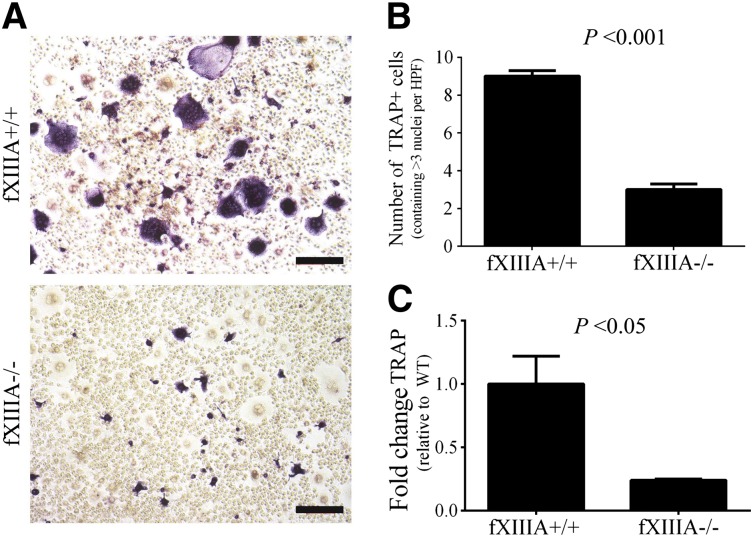
Next, we investigated whether the reduction in osteoclast activity observed in fXIIIA−/− mice was due to a direct effect of fXIIIA on osteoclasts, their progenitors, or simply a function of reduced RANKL availability. The numbers and percentages of CD11b+ cells (ie, representative of the myeloid compartment containing osteoclast precursor cells) within the spleen were similar in unchallenged fXIIIA+/+ and fXIIIA−/− mice (data not shown). Splenocytes grown in culture with M-CSF, the growth factor driving the proliferation and survival of myeloid-derived cells, revealed similar numbers of monocytoid cells in both fXIIIA+/+ and fXIIIA−/− cultures (data not shown). Osteoclastogenesis was driven by stimulation of splenocytes with both M-CSF and RANKL, which produced large, TRAP+ multinucleated cells readily apparent in preparations derived from fXIIIA+/+ mice (Figure 6A). TRAP+ cells were fewer in number, smaller, and rarely multinucleated in cultures derived from fXIIIA−/− mice (Figure 6A). A significant reduction in the number of TRAP+ cells containing at least 3 nuclei was seen in these cultures (Figure 6B). Moreover, diminution in TRAP gene expression was observed in cultures derived from fXIIIA−/− mice compared with those from fXIIIA+/+ mice (Figure 6C). Thus, the defect in osteoclastogenesis in cultures from fXIIIA−/− spleens is linked to diminished RANKL-induced osteoclast formation, and not due to quantitative differences in precursor cells or their proliferative and survival capacity in vitro.
Figure 6.
FXIIIA deficiency results in significantly reduced osteoclastogenesis in vitro. (A) Representative images of TRAP staining of splenocyte cultures harvested from fXIIIA+/+ and fXIIIA−/− mice and cultured in the presence of 30 ng/mL monocyte colony stimulating factor (M-CSF) and 100 ng/mL RANKL for 8 days. Note the presence of numerous large, multinucleated, TRAP+ (purple) osteoclasts in fXIIIA+/+ cultures, whereas fXIIIA−/− splenocyte cultures produced visibly fewer TRAP+/multinucleated cells. (B) Quantification of osteoclast number in 10 random high-powered fields per culture from each of 3 mice. TRAP+ cells that contained >3 nuclei were counted as osteoclasts. Data are represented as mean ± standard error of the mean. (C) Quantitative RT-PCR analysis of TRAP gene expression in osteoclast cultures (n = 3 mice per genotype). Data are expressed as average fold change over wild-type with error bars denoting standard error of the mean. Data were analyzed by the Student t test.
Pharmacologic inhibition of transglutaminase activity reduces CIA incidence and severity
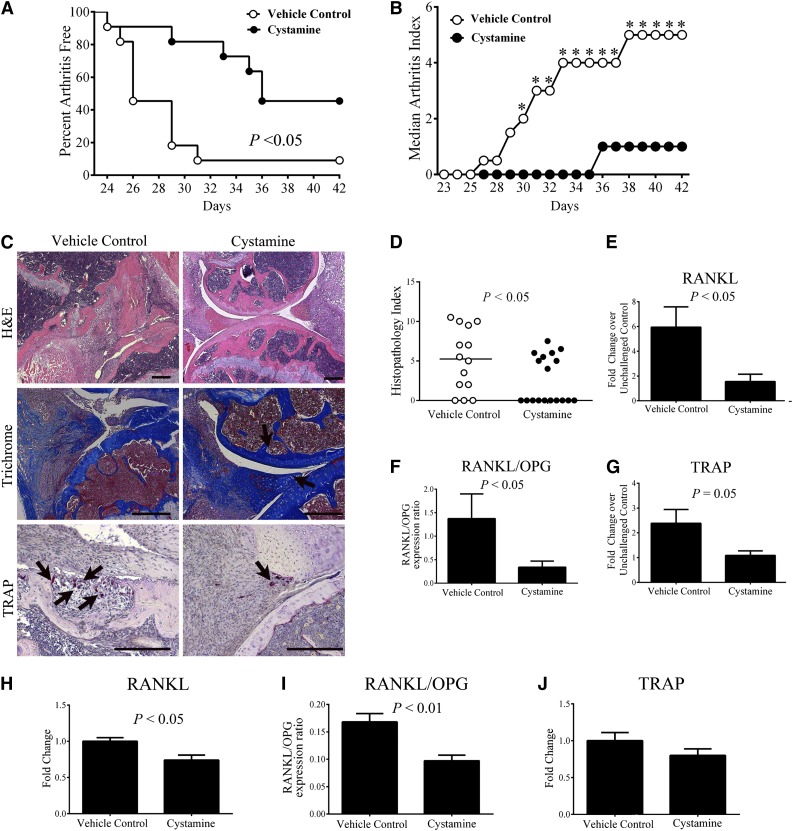
To complement the genetic studies, we determined whether pharmacologic treatment with a transglutaminase inhibitor was similarly effective in limiting CIA development. Approximately 50% of mice treated with the pan-transglutaminase inhibitor cystamine showed no evidence of arthritis in the paws compared with 90% of vehicle-treated control mice developing at least 1 joint with obvious arthritis after CIA induction (Figure 7A). Accordingly, the arthritis index was significantly reduced in cystamine-treated mice compared with vehicle-treated controls (Figure 7B). Histologic evaluation of hematoxylin and eosin–stained knee joints revealed that cystamine-treated mice had reduced pathological signs of inflammatory arthritis relative to vehicle-treated mice (Figure 7C), which was supported by a significantly lower histopathology index score (Figure 7D). Importantly, cartilage degradation and bone loss was significantly attenuated, even among those cystamine-treated mice that showed signs of inflammation and synovial hyperplasia compared with vehicle treatment (data not shown). Knee joints of cystamine-treated mice had less severe cartilage degradation compared with vehicle-treated mice (Figure 7C). Furthermore, TRAP+ osteoclast-rich lesions were readily apparent within the bone of vehicle-treated control mice, whereas TRAP+ cells were fewer in number and appeared dispersed in the synovial tissue of cystamine-treated animals (Figure 7C). Of note, significant diminutions in (1) RANKL mRNA; (2) ratio of RANKL/OPG mRNA, and (3) TRAP mRNA were found in the paws of CIA-challenged mice treated with cystamine as compared with CIA-challenged vehicle-treated (ie, simple drinking water) controls (Figures 7E-G). Even in otherwise unchallenged animals (ie, without any CIA challenge), cystamine treatment significantly reduced RANKL mRNA levels and the ratio of RANKL/OPG mRNA compared controls (Figure 7H-I, respectively), findings similar to what was observed in fXIIIA−/− animals. Local expression of TRAP in the paws of cystamine-treated mice was consistently diminished relative to vehicle-treated animals, although the changes did not reach statistical significance (Figure 7J). Together, these data suggest that treatment with a transglutaminase inhibitor limits arthritis pathogenesis and osteoclast function in a manner similar to genetic fXIIIA deficiency.
Figure 7.
Treatment of wild-type mice with the transglutaminase inhibitor cystamine limits CIA incidence and progression and osteoclastogenesis. (A) Percentage of mice free from any evidence of macroscopic arthritis in the paws of DBA/1 male mice administered cystamine or vehicle control starting 14 days prior to first CII immunization (n = 10 per treatment group). Data were analyzed by Kaplan-Meier log rank analysis. (B) Median arthritis index analysis. (C) Representative images of knee joint histology from mice harvested at day 42 of CIA. Hematoxylin and eosin (H&E) staining (top panel); trichrome staining (arrows: intact cartilage, middle panel) and TRAP staining for visualizing osteoclasts (arrows, bottom panel). (D) Scatter plot of composite histopathology index analysis of hematoxylin and eosin–stained knee joint sections. Symbols represent composite score for individual mice and bars denote median values for each genotype. Data were analyzed by the Mann-Whitney U test. Quantitative RT-PCR analysis of mRNA levels of RANKL (E), RANKL/OPG ratio (F), and TRAP (G). Data are expressed as average fold change over unchallenged control group; Quantitative RT-PCR analysis of mRNA levels of RANKL (H), ratio of RANKL/OPG (I), and TRAP (J) within the hind paws of otherwise unchallenged mice administered cystamine or vehicle control. Data are representative of 2 independent experiments, presented as mean ± standard error of the mean, and analyzed by the Student t test. Scale bars represent 200 µm (C). *P < .05 Mann-Whitney U test.
Discussion
Previous studies suggested that fXIII may play a role in RA, a disease associated with pathological coagulation system activity. Plasma from RA patients produces altered fibrin-clot structures in vitro that were postulated to be associated with excessive fXIII activity.19 Levels of D-dimer, a degradation product derived from fXIII-crosslinked fibrin, are increased in the plasma and synovial fluid from RA patients and experimental animals with inflammatory arthritis.4,20 The studies presented here definitively establish for the first time that fXIIIA is a crucial molecular determinant of inflammatory arthritis progression and joint tissue destruction. Multiple studies have directly implicated the primary fXIIIA substrate fibrin(ogen) in the pathogenesis of inflammatory arthritis.8,21-24 These data also support the concept that fXIIIA contributes to arthritis pathogenesis through distinct fibrinogen-dependent inflammatory processes and through altered osteoclast function-mediated mechanisms.
FXIIIA is not essential for initial fibrin clot formation, and consistent with exuberant fibrin within human arthritic joints, appreciable fibrin(ogen) deposition was consistently observed in CIA-challenged mice, regardless of the presence or absence of fXIIIA. However, the present studies revealed that CIA-challenged fXIIIA−/− mice displayed a qualitatively distinct pattern of fibrin deposition associated with fewer inflammatory cells within the knee joints relative to CIA-challenged wild-type mice. Noncrosslinked fibrin matrices have structural and functional properties distinct from stable crosslinked fibrin matrices.25-27 Differential inflammatory properties would be anticipated because stable fibrin matrices appear to support increased inflammatory cell adhesion, migration, and cytokine production relative to monomeric fibrinogen.28-30 Interestingly, the common V34L polymorphism in the fXIIIA gene, which is associated with more rapid fXIII activation, correlates with higher levels of the proinflammatory cytokine IL-6.31 Indeed, the observed diminution of CIA severity in fXIIIA−/− mice reported here is in-line with previous studies indicating that fibrinogen-deficient mice develop diminished CIA with a corresponding reduction in local proinflammatory cytokine expression.8 Furthermore, our studies are in agreement with the notion that noncrosslinked fibrin deposits exhibit a reduced capacity to drive proarthritic and proinflammatory leukocyte activation and cytokine production. Thus, at least 1 mechanism by which fXIIIA deficiency limits arthritis progression and severity appears to be by altering local properties of fibrin within arthritic joints rendering the provisional matrix protein less proinflammatory.
It is possible that extracellular substrates of fXIII other than fibrin contribute to fXIII-mediated CIA. Indeed, fXIIIA is a promiscuous transglutaminase capable of crosslinking multiple nonfibrin substrates in the joint microenvironment relevant to inflammatory arthritis including additional coagulation system components (eg, α2-antiplasmin, thrombin activatable fibrinolysis inhibitor, and plasminogen), extracellular matrix components (eg, fibronectin, laminin, and collagen), and cell surface receptors (eg, αvβ3,).11,32 The glycoprotein osteopontin (OPN) has been identified as a fXIIIA substrate that is of particular interest in connection to cartilage and bone remodeling and inflammatory joint disease.33 OPN is believed to promote joint destruction during RA in part through engagement of αVβ3 and αVβ5 on osteoclasts.34,35 Antibody-mediated blockade of OPN function has been reported to be protective in murine inflammatory arthritis models.36 Thus, an emerging hypothesis from these findings is that extracellular fXIIIA could contribute to arthritis pathogenesis through both fibrinogen-dependent and fibrinogen-independent mechanisms, but further studies are required to elucidate the relative contribution of the multiple pathways to disease.
In RA and CIA, bone erosion is predominantly mediated by osteoclasts, which are myeloid-derived multinucleated cells. Osteoclastogenesis and osteoclast activation are primarily driven by the RANKL/RANK receptor signaling system and tightly controlled and countered by OPG, the soluble decoy receptor of RANKL.37-39 Ultimately, the degree of osteoclast differentiation and activation is dictated by the balance between OPG and RANKL within the bone microenvironment.40 Here we demonstrate that whereas CIA-challenged, and even naïve, fXIIIA−/− mice displayed molecular changes consistent with reduced osteoclast differentiation and osteoclast numbers (ie, reduced mRNA levels of RANKL and TRAP), fibrinogen-deficient mice did not show the same molecular changes. Additionally, inflammatory cytokines, such as TNFα, IL-6, and IL-1β, synergize with RANKL to promote osteoclast differentiation and activation.41-43 Multiple lines of evidence have demonstrated that RANKL plays a dominant role in arthritis-associated bone loss in that inhibiting RANKL signaling directly (by genetic elimination or antibody-mediated blockade) or indirectly (by regulating the local balance of RANKL/OPG) significantly protects from bone and cartilage loss, but has little-to-no effect on inflammation during arthritis progression.44-48 The in vivo analyses and in vitro osteoclastogenesis assays performed here suggest that fXIIIA is critical for RANKL-mediated osteoclast differentiation and function. Even under conditions in which RANKL is not limiting, fXIIIA−/− splenocytes have a reduced capacity to form TRAP+ osteoclasts. Thus, collectively our findings support the novel concept that targeting fXIIIA transglutaminase could be a powerful new avenue to mitigate multiple pathogenic arms of inflammatory arthritis including synovial inflammation and devastating cartilage and bone loss.
During skeletal homeostasis, osteoblasts are the chief source of RANKL, whereas under pathological conditions such as RA, cell types such as activated T cells and synovial fibroblasts also express RANKL.41,49,50 Transglutaminase activity in general has been shown to promote osteoblast adhesion and differentiation.51 Osteoblasts and osteoblastic cell lines have been reported to express both fXIIIA and tissue transglutaminase 2 (TG2).52,53 Despite the local expression differences in crucial osteologic effectors observed in fXIIIA−/− mice, no overt skeletal abnormalities have been reported for fXIIIA−/− mice. Additionally, studies have reported that baseline skeletal properties of TG2-deficient mice are unaltered.54-56 Such findings suggest that fXIIIA and TG2 are independently not required for either skeletal development or physiological skeletal homeostasis. Our studies suggest that at least for some aspects of bone remodeling, loss of fXIII transglutaminase activity alone is sufficient to mitigate altered function (ie, osteoclastogenesis). Specific contributions of fXIIIA and TG2, either alone or in combination, to bone-remodeling activities, including osteoblast function, in the setting of arthritis or other bone degenerative pathological contexts remains to be established.
A central consideration in determining the mechanisms by which fXIIIA influences arthritis pathogenesis is that fXIII is present both in circulation and as an intracellular dimeric form (cfXIIIA) within various cell types, including platelets, monocytes and macrophages, osteoblasts, and chondrocytes.32 The presence of cfXIIIA in the monocyte and macrophage lineage appears to be of particular functional importance. For instance, monocytes isolated from fXIIIA-deficient patients have impaired Fcγ- and complement receptor-mediated phagocytic abilities.57 The chemotactic responses of fXIIIA-deficient dendritic cells to CCL19, a chemokine required for dendritic cell maturation, were also significantly impaired.58 Additionally, fXIIIA-mediated crosslinking of AT1 receptors in human monocytes correlated with enhanced adhesiveness to endothelial cells.59 Given that fXIII appears to influence multiple processes (eg, differentiation, migration and activation and function of monocytic lineages), cfXIIIA could contribute to arthritis pathogenesis through distinct mechanisms. The precise impact of fXIIIA-mediated extracellular crosslinking and intracellular cfXIIIA activity on synovial fibroblast proliferation, inflammatory cytokine production, monocytes and macrophage infiltration, and osteoclastogenesis within the arthritic joint constitutes an exciting area for more detailed study.
The pharmacalogic inhibition studies presented here suggest that intervention at the level of limiting fXIII transglutaminase activity could provide significant benefit in the context of inflammatory arthritis. These findings are consistent with previous reports wherein inhibition of transglutaminase activity has been found beneficial in the context of various other inflammatory pathologies. For instance, using 2,4,6-trinitrobenzenesulfonic acid-induced colitis as a model of inflammatory bowel disease in rats, it was demonstrated that cystamine treatment markedly diminished colitis-associated inflammation including reduced mucosal proinflammatory cytokine expression.60 Likewise, cysteamine-mediated inhibition of transglutaminase activity was shown to limit the severity and progression of experimental autoimmune encephalomyelitis, a model of multiple sclerosis in mice.61 Thus, inhibiting transglutaminase function may be beneficial in limiting a wide array of inflammatory diseases. In summary, the findings presented here provide valuable proof-of-concept that intervention at the level of fXIII transglutaminase activity is an attractive therapeutic strategy to limit both inflammation and cartilage and bone loss associated with RA and potentially other destructive bone diseases.
Acknowledgments
We wish to thank Alice Jone, Ariel Hall, Leah Rosenfeldt, Maureen Shaw, and Kathryn Talmage-McElhinney for their excellent technical assistance. We would also like to thank Dr Joseph Palumbo, Dr William Ridgway, Dr Alisa Wolberg, and Dr James Luyendyk for their helpful suggestions in the preparation of this manuscript.
This work was supported by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute (HL096126) (J.L.D.) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR056990) (M.J.F.); and from the Animal Models of Inflammatory Diseases Core, Cincinnati Rheumatic Diseases Core Center, National Institute of Arthritis and Musculoskeletal and Skin Diseases (P30 AR47363) (M.J.F.).
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.R., S.T., J.L.D., and M.J.F. designed the research, performed experiments, and wrote the manuscript; C.C., C.L.R., and M.D.F. conducted experiments; H.R., S.T., J.L.D., M.J.F., C.C., C.L.R., and M.D.F. analyzed data; and E.S.M. and J.G.S. provided critical guidance on experimental procedures and helped write the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Matthew J. Flick, Children's Hospital Research Foundation, Division of Experimental Hematology and Cancer Biology - ML7015, 3333 Burnet Ave, Cincinnati, OH 45229-3039; e-mail: matthew.flick@cchmc.org.
References
- 1.Choi Y, Arron JR, Townsend MJ. Promising bone-related therapeutic targets for rheumatoid arthritis. Nat Rev Rheumatol. 2009;5(10):543–548. doi: 10.1038/nrrheum.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal SK. Biologic agents in rheumatoid arthritis: an update for managed care professionals. J Manag Care Pharm. 2011;17(9 Suppl B):S14–S18. doi: 10.18553/jmcp.2011.17.s9-b.S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zacharski LR, Brown FE, Memoli VA, et al. Pathways of coagulation activation in situ in rheumatoid synovial tissue. Clin Immunol Immunopathol. 1992;63(2):155–162. doi: 10.1016/0090-1229(92)90008-c. [DOI] [PubMed] [Google Scholar]
- 4.Busso N, Hamilton JA. Extravascular coagulation and the plasminogen activator/plasmin system in rheumatoid arthritis. Arthritis Rheum. 2002;46(9):2268–2279. doi: 10.1002/art.10498. [DOI] [PubMed] [Google Scholar]
- 5.Hoppe B, Dörner T. Coagulation and the fibrin network in rheumatic disease: a role beyond haemostasis. Nat Rev Rheumatol. 2012;8(12):738–746. doi: 10.1038/nrrheum.2012.184. [DOI] [PubMed] [Google Scholar]
- 6.Marty I, Péclat V, Kirdaite G, Salvi R, So A, Busso N. Amelioration of collagen-induced arthritis by thrombin inhibition. J Clin Invest. 2001;107(5):631–640. doi: 10.1172/JCI11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang YH, Hall P, Little CB, et al. Reduction of arthritis severity in protease-activated receptor-deficient mice. Arthritis Rheum. 2005;52(4):1325–1332. doi: 10.1002/art.21001. [DOI] [PubMed] [Google Scholar]
- 8.Flick MJ, LaJeunesse CM, Talmage KE, et al. Fibrin(ogen) exacerbates inflammatory joint disease through a mechanism linked to the integrin alphaMbeta2 binding motif. J Clin Invest. 2007;117(11):3224–3235. doi: 10.1172/JCI30134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komáromi I, Bagoly Z, Muszbek L. Factor XIII: novel structural and functional aspects. J Thromb Haemost. 2011;9(1):9–20. doi: 10.1111/j.1538-7836.2010.04070.x. [DOI] [PubMed] [Google Scholar]
- 10.Bagoly Z, Koncz Z, Hársfalvi J, Muszbek L. Factor XIII, clot structure, thrombosis. Thromb Res. 2012;129(3):382–387. doi: 10.1016/j.thromres.2011.11.040. [DOI] [PubMed] [Google Scholar]
- 11.Richardson VR, Cordell P, Standeven KF, Carter AM. Substrates of Factor XIII-A: roles in thrombosis and wound healing. Clin Sci (Lond) 2013;124(3):123–137. doi: 10.1042/CS20120233. [DOI] [PubMed] [Google Scholar]
- 12.Inbal A, Lubetsky A, Krapp T, et al. Impaired wound healing in factor XIII deficient mice. Thromb Haemost. 2005;94(2):432–437. doi: 10.1160/TH05-04-0291. [DOI] [PubMed] [Google Scholar]
- 13.Karimi M, Bereczky Z, Cohan N, Muszbek L. Factor XIII Deficiency. Semin Thromb Hemost. 2009;35(4):426–438. doi: 10.1055/s-0029-1225765. [DOI] [PubMed] [Google Scholar]
- 14.Palumbo JS, Barney KA, Blevins EA, et al. Factor XIII transglutaminase supports hematogenous tumor cell metastasis through a mechanism dependent on natural killer cell function. J Thromb Haemost. 2008;6(5):812–819. doi: 10.1111/j.1538-7836.2008.02938.x. [DOI] [PubMed] [Google Scholar]
- 15.Suh TT, Holmbäck K, Jensen NJ, et al. Resolution of spontaneous bleeding events but failure of pregnancy in fibrinogen-deficient mice. Genes Dev. 1995;9(16):2020–2033. doi: 10.1101/gad.9.16.2020. [DOI] [PubMed] [Google Scholar]
- 16.Schett G. Cells of the synovium in rheumatoid arthritis. Osteoclasts. Arthritis Res Ther. 2007;9(1):203. doi: 10.1186/ar2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medicherla S, Ma JY, Mangadu R, et al. A selective p38 alpha mitogen-activated protein kinase inhibitor reverses cartilage and bone destruction in mice with collagen-induced arthritis. J Pharmacol Exp Ther. 2006;318(1):132–141. doi: 10.1124/jpet.105.098020. [DOI] [PubMed] [Google Scholar]
- 18.Touaitahuata H, Cres G, de Rossi S, Vives V, Blangy A. The mineral dissolution function of osteoclasts is dispensable for hypertrophic cartilage degradation during long bone development and growth. Dev Biol. 2014;393(1):57–70. doi: 10.1016/j.ydbio.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Kwasny-Krochin B, Gluszko P, Undas A. Unfavorably altered fibrin clot properties in patients with active rheumatoid arthritis. Thromb Res. 2010;126(1):e11–e16. doi: 10.1016/j.thromres.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 20.So AK, Varisco PA, Kemkes-Matthes B, et al. Arthritis is linked to local and systemic activation of coagulation and fibrinolysis pathways. J Thromb Haemost. 2003;1(12):2510–2515. doi: 10.1111/j.1538-7836.2003.00462.x. [DOI] [PubMed] [Google Scholar]
- 21.Busso N, Péclat V, Van Ness K, et al. Exacerbation of antigen-induced arthritis in urokinase-deficient mice. J Clin Invest. 1998;102(1):41–50. doi: 10.1172/JCI2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raghu H, Flick MJ. Targeting the coagulation factor fibrinogen for arthritis therapy. Curr Pharm Biotechnol. 2011;12(9):1497–1506. doi: 10.2174/138920111798281144. [DOI] [PubMed] [Google Scholar]
- 23.Ho PP, Lee LY, Zhao X, et al. Autoimmunity against fibrinogen mediates inflammatory arthritis in mice. J Immunol. 2010;184(1):379–390. doi: 10.4049/jimmunol.0901639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sánchez-Pernaute O, López-Armada MJ, Calvo E, et al. Fibrin generated in the synovial fluid activates intimal cells from their apical surface: a sequential morphological study in antigen-induced arthritis. Rheumatology (Oxford) 2003;42(1):19–25. doi: 10.1093/rheumatology/keg021. [DOI] [PubMed] [Google Scholar]
- 25.Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3(8):1894–1904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 26.Mosesson MW. The roles of fibrinogen and fibrin in hemostasis and thrombosis. Semin Hematol. 1992;29(3):177–188. [PubMed] [Google Scholar]
- 27.Hethershaw EL, Cilia La Corte AL, Duval C, et al. The effect of blood coagulation factor XIII on fibrin clot structure and fibrinolysis. J Thromb Haemost. 2014;12(2):197–205. doi: 10.1111/jth.12455. [DOI] [PubMed] [Google Scholar]
- 28.Flick MJ, Du X, Degen JL. Fibrin(ogen)-alpha M beta 2 interactions regulate leukocyte function and innate immunity in vivo. Exp Biol Med (Maywood) 2004;229(11):1105–1110. doi: 10.1177/153537020422901104. [DOI] [PubMed] [Google Scholar]
- 29.Flick MJ, Du X, Witte DP, et al. Leukocyte engagement of fibrin(ogen) via the integrin receptor alphaMbeta2/Mac-1 is critical for host inflammatory response in vivo. J Clin Invest. 2004;113(11):1596–1606. doi: 10.1172/JCI20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez RL, Roman J. Fibrin enhances the expression of IL-1 beta by human peripheral blood mononuclear cells. Implications in pulmonary inflammation. J Immunol. 1995;154(4):1879–1887. [PubMed] [Google Scholar]
- 31.Marín F, Corral J, Roldán V, et al. Factor XIII Val34Leu polymorphism modulates the prothrombotic and inflammatory state associated with atrial fibrillation. J Mol Cell Cardiol. 2004;37(3):699–704. doi: 10.1016/j.yjmcc.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Muszbek L, Bereczky Z, Bagoly Z, Komáromi I, Katona É. Factor XIII: a coagulation factor with multiple plasmatic and cellular functions. Physiol Rev. 2011;91(3):931–972. doi: 10.1152/physrev.00016.2010. [DOI] [PubMed] [Google Scholar]
- 33.Prince CW, Dickie D, Krumdieck CL. Osteopontin, a substrate for transglutaminase and factor XIII activity. Biochem Biophys Res Commun. 1991;177(3):1205–1210. doi: 10.1016/0006-291x(91)90669-x. [DOI] [PubMed] [Google Scholar]
- 34.Ishii T, Ohshima S, Ishida T, et al. Osteopontin as a positive regulator in the osteoclastogenesis of arthritis. Biochem Biophys Res Commun. 2004;316(3):809–815. doi: 10.1016/j.bbrc.2004.02.124. [DOI] [PubMed] [Google Scholar]
- 35.Ohshima S, Kobayashi H, Yamaguchi N, et al. Expression of osteopontin at sites of bone erosion in a murine experimental arthritis model of collagen-induced arthritis: possible involvement of osteopontin in bone destruction in arthritis. Arthritis Rheum. 2002;46(4):1094–1101. doi: 10.1002/art.10143. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto N, Sakai F, Kon S, et al. Essential role of the cryptic epitope SLAYGLR within osteopontin in a murine model of rheumatoid arthritis. J Clin Invest. 2003;112(2):181–188. doi: 10.1172/JCI17778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ainola M, Mandelin J, Liljeström M, Konttinen YT, Salo J. Imbalanced expression of RANKL and osteoprotegerin mRNA in pannus tissue of rheumatoid arthritis. Clin Exp Rheumatol. 2008;26(2):240–246. [PubMed] [Google Scholar]
- 38.Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40(2):251–264. doi: 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 39.Bezerra MC, Carvalho JF, Prokopowitsch AS, Pereira RM. RANK, RANKL and osteoprotegerin in arthritic bone loss. Braz J Med Biol Res. 2005;38(2):161–170. doi: 10.1590/s0100-879x2005000200004. [DOI] [PubMed] [Google Scholar]
- 40.Geusens P. The role of RANK ligand/osteoprotegerin in rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2012;4(4):225–233. doi: 10.1177/1759720X12438080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hashizume M, Hayakawa N, Mihara M. IL-6 trans-signalling directly induces RANKL on fibroblast-like synovial cells and is involved in RANKL induction by TNF-alpha and IL-17. Rheumatology (Oxford) 2008;47(11):1635–1640. doi: 10.1093/rheumatology/ken363. [DOI] [PubMed] [Google Scholar]
- 42.Walsh MC, Kim N, Kadono Y, et al. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol. 2006;24:33–63. doi: 10.1146/annurev.immunol.24.021605.090646. [DOI] [PubMed] [Google Scholar]
- 43.Romas E, Gillespie MT, Martin TJ. Involvement of receptor activator of NFkappaB ligand and tumor necrosis factor-alpha in bone destruction in rheumatoid arthritis. Bone. 2002;30(2):340–346. doi: 10.1016/s8756-3282(01)00682-2. [DOI] [PubMed] [Google Scholar]
- 44.Romas E, Sims NA, Hards DK, et al. Osteoprotegerin reduces osteoclast numbers and prevents bone erosion in collagen-induced arthritis. Am J Pathol. 2002;161(4):1419–1427. doi: 10.1016/S0002-9440(10)64417-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kamijo S, Nakajima A, Ikeda K, et al. Amelioration of bone loss in collagen-induced arthritis by neutralizing anti-RANKL monoclonal antibody. Biochem Biophys Res Commun. 2006;347(1):124–132. doi: 10.1016/j.bbrc.2006.06.098. [DOI] [PubMed] [Google Scholar]
- 46.Pettit AR, Ji H, von Stechow D, et al. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Pathol. 2001;159(5):1689–1699. doi: 10.1016/S0002-9440(10)63016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stolina M, Schett G, Dwyer D, et al. RANKL inhibition by osteoprotegerin prevents bone loss without affecting local or systemic inflammation parameters in two rat arthritis models: comparison with anti-TNFalpha or anti-IL-1 therapies. Arthritis Res Ther. 2009;11(6):R187. doi: 10.1186/ar2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hofbauer LC, Zeitz U, Schoppet M, et al. Prevention of glucocorticoid-induced bone loss in mice by inhibition of RANKL. Arthritis Rheum. 2009;60(5):1427–1437. doi: 10.1002/art.24445. [DOI] [PubMed] [Google Scholar]
- 49.Kong YY, Feige U, Sarosi I, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402(6759):304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 50.Sato K, Suematsu A, Okamoto K, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203(12):2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aeschlimann D, Mosher D, Paulsson M. Tissue transglutaminase and factor XIII in cartilage and bone remodeling. Semin Thromb Hemost. 1996;22(5):437–443. doi: 10.1055/s-2007-999043. [DOI] [PubMed] [Google Scholar]
- 52.Al-Jallad HF, Nakano Y, Chen JL, McMillan E, Lefebvre C, Kaartinen MT. Transglutaminase activity regulates osteoblast differentiation and matrix mineralization in MC3T3-E1 osteoblast cultures. Matrix Biol. 2006;25(3):135–148. doi: 10.1016/j.matbio.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 53.Yin X, Chen Z, Liu Z, Song C. Tissue transglutaminase (TG2) activity regulates osteoblast differentiation and mineralization in the SAOS-2 cell line. Braz J Med Biol Res. 2012;45(8):693–700. doi: 10.1590/S0100-879X2012007500060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Laurenzi V, Melino G. Gene disruption of tissue transglutaminase. Mol Cell Biol. 2001;21(1):148–155. doi: 10.1128/MCB.21.1.148-155.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nanda N, Iismaa SE, Owens WA, Husain A, Mackay F, Graham RM. Targeted inactivation of Gh/tissue transglutaminase II. J Biol Chem. 2001;276(23):20673–20678. doi: 10.1074/jbc.M010846200. [DOI] [PubMed] [Google Scholar]
- 56.Nakano Y, Al-Jallad HF, Mousa A, Kaartinen MT. Expression and localization of plasma transglutaminase factor XIIIA in bone. J Histochem Cytochem. 2007;55(7):675–685. doi: 10.1369/jhc.6A7091.2007. [DOI] [PubMed] [Google Scholar]
- 57.Sárváry A, Szucs S, Balogh I, et al. Possible role of factor XIII subunit A in Fcgamma and complement receptor-mediated phagocytosis. Cell Immunol. 2004;228(2):81–90. doi: 10.1016/j.cellimm.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 58.Jayo A, Conde I, Lastres P, Jiménez-Yuste V, González-Manchón C. Possible role for cellular FXIII in monocyte-derived dendritic cell motility. Eur J Cell Biol. 2009;88(8):423–431. doi: 10.1016/j.ejcb.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 59.AbdAlla S, Lother H, Langer A, el Faramawy Y, Quitterer U. Factor XIIIA transglutaminase crosslinks AT1 receptor dimers of monocytes at the onset of atherosclerosis. Cell. 2004;119(3):343–354. doi: 10.1016/j.cell.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 60.Elli L, Ciulla MM, Busca G, et al. Beneficial effects of treatment with transglutaminase inhibitor cystamine on the severity of inflammation in a rat model of inflammatory bowel disease. Lab Invest. 2011;91(3):452–461. doi: 10.1038/labinvest.2010.186. [DOI] [PubMed] [Google Scholar]
- 61.Oh K, Park HB, Seo MW, Byoun OJ, Lee DS. Transglutaminase 2 exacerbates experimental autoimmune encephalomyelitis through positive regulation of encephalitogenic T cell differentiation and inflammation. Clin Immunol. 2012;145(2):122–132. doi: 10.1016/j.clim.2012.08.009. [DOI] [PubMed] [Google Scholar]