Key Points
Major bleeding, thrombosis, and postpartum hemorrhage are frequent in propositi and relatives with congenital dysfibrinogenemia.
Hotspot mutations were not predictive of either phenotype or outcome.
Abstract
We conducted a multicenter study of 101 patients with congenital dysfibrinogenemia (CD) to characterize the incidence of hemorrhagic and thrombotic events as well as complications of pregnancy and surgery. At the time of diagnosis, 10.9% and 13.9% had experienced major bleeding and thrombotic events, respectively. During a mean follow-up of 8.8 years after CD diagnosis, the incidence of major bleeding and thrombotic events was 2.5 and 18.7 per 1000 patient-years, respectively, with estimated cumulative incidences at age 50 years of 19.2% and 30.1%. We identified 111 pregnancies with an overall incidence of spontaneous abortions and postpartum hemorrhage of 19.8% and 21.4%, respectively. The risk of postpartum hemorrhage was associated with a previously identified bleeding phenotype (odds ratio, 5.8; 95% CI, 1.2 to 28.0). Among 137 surgical procedures analyzed, 9 (6.5%) were complicated by abnormal bleeding. Propositi vs relatives, sex, mutation hotspots, fibrinogen levels, and activity:antigen ratios were not associated with the risk of thrombotic or bleeding outcomes. In conclusion, the results of our study, the largest in genotyped CD and the first including long-term history, indicate that propositi with CD and their relatives carry not only a high risk of major bleeding, including postpartum hemorrhage, but also of thrombotic event.
Medscape Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint providership of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 581.
Disclosures
The authors, Associate Editor José A. López, and CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Describe complications of major bleeding and thrombosis in persons with congenital dysfibrinogenemia (CD) and their affected relatives.
Identify complications of pregnancy and surgery in persons with CD and their affected relatives.
Distinguish the effect of clinical, laboratory, and genetic factors on the risk for thrombotic or bleeding outcomes in persons with CD and their affected relatives.
Release date: January 15, 2015; Expiration date: January 15, 2016
Introduction
Congenital dysfibrinogenemia (CD) is a qualitative congenital fibrinogen disorder characterized by normal fibrinogen antigen levels associated with low functional activity.1 The vast majority of cases are the result of heterozygous missense mutations in the coding region of one of the three fibrinogen genes, FGA, FGB, or FGG.2 CD is the most frequent type of congenital fibrinogen disorder, but its exact prevalence is difficult to establish because of the large number of unreported asymptomatic cases.3
The clinical management of patients with CD is challenging4 because the precise risks of bleeding or thrombotic events (either arterial or venous) and of pregnancy-related complications for the majority of patients with CD remain to be determined. A large study of the Scientific and Standardization Committee Subcommittee on Fibrinogen evaluated 125 patients with CD or hypodysfibrinogenemia and suggested high risks of bleeding, thrombosis, and pregnancy-related complications.5 However, these data were limited by the incomplete genotyping of patients and the heterogeneity of the sample with regard to the fibrinogen defect. Furthermore, although biological, functional, and molecular information is often reported in studies of CD, clinical information is often limited with no follow-up. In this respect, several patients initially classified as asymptomatic may in fact become symptomatic a few years later.
Thus, our aim was to characterize the thrombotic, hemorrhagic, pregnancy, and surgery-related risks in patients with genetically confirmed CD and to explore whether these risks were associated with specific demographic factors or mutations.
Patients and methods
This study was performed with institutional review board approval and with written informed consent from all patients, in accordance with the Declaration of Helsinki.
Study population and case definition
We conducted a retrospective study of all consecutive CD cases genotyped in our laboratory from March 2005 to January 2014. Patients with suspected CD on the basis of discrepant fibrinogen activity and antigen levels were referred from 21 European or American hematological centers. CD was defined by low functional fibrinogen levels with normal or increased antigen levels, combined with molecular defects identified in 1 of the 3 fibrinogen genes. The inclusion of affected relatives of the propositus (the family member who was first referred for diagnostic work-up) was performed at the discretion of the clinician in charge of the propositus.
Data collection and variable and outcome definition
A standardized case report form was designed to assess the circumstances of diagnosis, coagulation test values, past medical histories (including bleeding and thrombotic events), obstetrical history, surgeries, long-term prophylaxis, and family history. Referring clinicians completed the case report form based on their most recent medical files, once the diagnosis of CD was genetically confirmed. Patients were contacted by phone by their physician or invited for a follow-up visit if any information was outdated or incomplete.
The clinical diagnosis was defined as the time when the CD was diagnosed on the basis of the discrepancy between functional and antigenic fibrinogen. A ratio of functional activity:antigen lower than 0.7 was considered suggestive of CD.6 The molecular diagnosis was defined as the time when the genetic defect was identified.
The lifelong history of bleeding was quantified using the International Society on Thrombosis and Haemostasis/Scientific and Standardization Committee bleeding assessment tool (BAT).7 Menorrhagia was evaluated by means of a standardized pictogram.8 Minor bleeding episodes were self-reported by patients.
Major bleeding was defined as bleeding that requires blood transfusion, surgical hemostasis, or fibrinogen replacement therapy (FRT), or bleeding in a critical area (intracranial, intraspinal, intraocular, retroperitoneal, pericardial, or intramuscular with compartment syndrome). All medical records of major bleeding were centrally adjudicated. All FRTs were performed with fibrinogen concentrates. Postpartum hemorrhage (PPH) was defined as an estimated blood loss of more than 500 mL after vaginal delivery or more than 1000 mL after cesarean delivery. In addition to major bleeding, postsurgery bleeding was defined as any overt hemorrhage more than would be expected requiring nonsurgical medical attention.
Thrombotic events included ischemic stroke, cerebral transient ischemic attack, acute myocardial infarction, peripheral arterial thrombosis, renal or mesenteric artery occlusion, deep venous thrombosis, thrombosis of cerebral or splanchnic veins, pulmonary embolism (PE), and superficial venous thrombosis. Venous thromboembolism (VTE) was stratified into provoked or unprovoked according to recent guidelines.9 Referring clinicians reported only objectively documented clinical events. VTE was considered temporally associated with FRT if it occurred within 1 week of its use.
Early spontaneous abortion was defined as a pregnancy loss before 13 weeks of gestation, late spontaneous abortion as pregnancy loss between 13 and 20 weeks of gestation, and stillbirth as pregnancy loss after 20 weeks of gestation.10
Major surgery was defined as any orthopedic, abdominal, gynecologic, urologic, neurologic, thoracic, or otolaryngological surgery. All other surgical procedures, including multiple dental extractions, were considered minor.
Laboratory analyses and genotyping
Fibrinogen was measured in local laboratories by using various assays, including the Clauss assay, prothrombin time (PT)-derived, and immunologic and precipitation methods. PT, activated partial thromboplastin time, reptilase time (RT), and thrombin time (TT) were also performed and were considered normal or abnormal on the basis of local reference ranges.
After isolation of genomic DNA by using standard protocols, exons and intron-exon junctions of the fibrinogen genes were amplified by polymerase chain reaction and sequenced as previously described,11 starting with the analysis of exon 2 of FGA and exon 8 of FGG, according to the flow chart for mutation detection used in our laboratory.12 Mutations were described according to the Human Genome Variation Society guidelines.13 Nucleotide numbering was based on the complementary DNA sequences from GenBank: entry #M64982 for FGA encoding the α-chain, #M64983 for FGB encoding the fibrinogen β-chain, and #M10014 for FGG encoding the γ-chain. Amino acid residues and substitutions are numbered from the initiator methionine. Corresponding amino acid substitutions in the mature chain lacking the signal peptide14 are indicated in Table 1.
Table 1.
List of mutations
| cDNA | Gene | Exon | Nascent chain | Mature chain | Total patients | Propositus | Relatives | Family* | Thrombosis | Bleeding phenotype/major bleeding |
|---|---|---|---|---|---|---|---|---|---|---|
| c.92C>T | FGA | 2 | Gly31Val | Gly12Val | 1 | 1 | 0 | 0 | 0 | 1/0 |
| c.103C>T | FGA | 2 | Arg35Cys | Arg16Cys | 9 | 7 | 2 | 1 | 2 | 5/1 |
| c.103C>A | FGA | 2 | Arg35Ser | Arg16Ser | 1 | 1 | 0 | 0 | 0 | 1/0 |
| c.104G>A | FGA | 2 | Arg35His | Arg16His | 14 | 11 | 3 | 1 | 3 | 6/1 |
| c.107G>A | FGA | 2 | Gly36Asp | Gly17Asp | 2 | 1 | 1 | 1 | 0 | 2/0 |
| c.112A>G | FGA | 2 | Arg38Gly | Arg19Gly | 4 | 2 | 2 | 2 | 2 | 1/0 |
| c.550T>C | FGA | 5 | Cys184Arg | Cys165Arg | 1 | 1 | 0 | 0 | 1 | 1/1 |
| c.1410_1411insT | FGA | 5 | Gly471Trpfs | Gly452Trpfs | 1 | 1 | 0 | 0 | 0 | 1/1 |
| c.1482-1495del | FGA | 5 | Met495Hisfs | Met476Hisfs | 1 | 1 | 0 | 0 | 1 | 0/0 |
| c.1717G>C | FGA | 5 | Arg573Cys | Arg554Cys | 1 | 1 | 0 | 0 | 0 | 0/0 |
| c.130C>T | FGB | 2 | Arg44Cys | Arg14Cys | 1 | 1 | 0 | 0 | 1 | 0/0 |
| c.586C>T | FGB | 4 | Arg196Cys | Arg166Cys | 1 | 1 | 0 | 0 | 0 | 1/0 |
| c.620A>G | FGG | 6 | Tyr207Cys | Tyr181Cys | 3 | 1 | 2 | 1 | 1 | 2/2 |
| c.837G>C | FGG | 7 | Trp279Cys | Trp253Cys | 1 | 1 | 0 | 0 | 1 | 0/0 |
| c.902G>A | FGG | 8 | Arg301His | Arg275His | 19 | 11 | 8 | 4 | 5 | 7/2 |
| c.902G>C | FGG | 8 | Arg301Cys | Arg275Cys | 33 | 20 | 13 | 8 | 9 | 14/5 |
| c.917A>G | FGG | 8 | Tyr306Cys | Tyr280Cys | 2 | 2 | 0 | 2 | 1 | 0/0 |
| c.1067A>T | FGG | 8 | Asp356Val | Asp330Val | 3 | 1 | 2 | 1 | 0 | 3/0 |
| c.1073C>G | FGG | 8 | Ser358Cys | Ser332Cys | 2 | 1 | 1 | 1 | 1 | 2/0 |
| c.1173T>A | FGG | 9 | Asn391Lys | Asn365Lys | 1 | 1 | 0 | 0 | 0 | 1/0 |
Previously unreported mutations are indicated in bold.
cDNA, complementary DNA.
Family with 1 or more relatives.
Statistical methods
No participants were excluded from the primary analyses. Characteristics of propositi and relatives at the time of CD diagnosis were compared by using the χ2 test (binary variables) or Student t test with unequal variance (continuous variables). We used time-to-event methods to estimate thrombotic and major bleeding risks from birth and from the time of CD diagnosis. In the latter analysis, patients with events before CD diagnosis were excluded. Thrombotic outcomes were assessed as VTE or arterial events, whichever came first, and in secondary analyses, they were considered separately as venous events and arterial events. Participants were censored at the time of the last clinical encounter or death (n = 1). Incidence rates (IRs) and Kaplan-Meier estimated cumulative incidences were calculated with their 95% confidence intervals (95% CIs). We used Cox proportional hazards regression models to estimate associations between biological, clinical, and genotypic factors (sex, propositus vs relative status) and the risk of thrombosis or major bleeding from birth. Modifications of the risk of clinical events between the periods before and after the diagnosis of CD were evaluated by P values of a time-varying variable representing the time after the diagnosis of CD. For pregnancies, we compared the risk of pregnancy-related complications between groups of women (according to the propositus status, the group of mutations, and the bleeding phenotype) by using logistic regression with standard errors accounting for correlation between pregnancies of individual women. Analyses were adjusted for age and parity.
The proportional hazards assumption was assessed by use of tests based on Schoenfeld residuals; no evidence of a violation of the assumption was found for any of the analyses. Apart from reptilase and thrombin times, which were not measured for all participants, there were no missing data. All statistical tests were 2-sided with a significance level of 0.05. We used STATA statistical software version 11.2 (STATA, College Station, TX) for all analyses.
Results
Our cohort included 101 consecutive patients from 67 families (67 propositi and 34 relatives) from Belgium, Finland, France, Switzerland, and the United States. Their mean age at CD diagnosis was 29.2 years (standard deviation [SD], 16.8 years; 2971.9 person-years of observation prior to diagnosis). They were followed up for a mean of 8.8 years (SD, 9.5 years; 829.9 person-years).
Characteristics of participants
Almost all patients (96%) were white and two-thirds were women (Table 2). Propositi were diagnosed with CD at an older age than their relatives (32.7 years compared with 22.3 years; P = .03). The majority of propositi had an incidental diagnosis of CD, either during routine laboratory testing or before surgery, and less than 20% were diagnosed after a bleeding or thrombotic event.
Table 2.
Patients’ characteristics
| Characteristic | All patients (n = 101) | Propositi (n = 67) | Relatives (n = 34) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | Mean | SD | No. | % | Mean | SD | No. | % | Mean | SD | |
| Demographics | ||||||||||||
| Males | 33 | 32.7 | 22 | 32.8 | 11 | 32.4 | ||||||
| Age at clinical diagnosis, y | 29.2 | 16.8 | 32.7 | 15.6 | 22.3 | 17.1 | ||||||
| Age at molecular diagnosis, y | 37.1 | 17.7 | 41.4 | 16.8 | 27.7 | 16.1 | ||||||
| Circumstances of diagnosis | ||||||||||||
| Incidental findings | 18 | 17.8 | 18 | 26.8 | NA | |||||||
| Before surgery | 21 | 20.8 | 21 | 31.3 | NA | |||||||
| Pregnancy | 10 | 9.9 | 10 | 14.9 | NA | |||||||
| Thrombosis | 7 | 6.9 | 7 | 10.4 | NA | |||||||
| Bleeding | 12 | 11.9 | 12 | 17.9 | NA | |||||||
| Familial screening | 34 | 33.7 | NA | 34 | 100 | |||||||
| Laboratory values | ||||||||||||
| Fibrinogen activity, g/L | 0.80 | 0.4 | 0.79 | 0.4 | 0.82 | 0.4 | ||||||
| Fibrinogen antigen, g/L | 2.86 | 1.0 | 2.90 | 1.0 | 2.77 | 1.0 | ||||||
| Prolonged TT* | 78 | 87.6 | 53 | 89.8 | 25 | 83.3 | ||||||
| Prolonged RT† | 52 | 89.7 | 37 | 92.5 | 15 | 83.3 | ||||||
| Platelets, g/L | 271.9 | 67.2 | 265.8 | 67.1 | 283.1 | 67 | ||||||
| Bleeding phenotype | 48 | 47.5 | 34 | 50.8 | 14 | 41.2 | ||||||
| Cutaneous | 21 | 20.8 | 12 | 17.9 | 9 | 26.5 | ||||||
| Epistaxis | 8 | 7.9 | 6 | 9.0 | 2 | 5.9 | ||||||
| Minor wound | 7 | 6.9 | 4 | 6.0 | 3 | 9.9 | ||||||
| Oral cavity | 7 | 6.9 | 3 | 4.5 | 4 | 11.8 | ||||||
| Gastrointestinal | 4 | 4.0 | 4 | 6.0 | 0 | 0 | ||||||
| Hemarthrosis | 1 | 1.0 | 1 | 1.5 | 0 | 0 | ||||||
| Postsurgery | 9 | 8.9 | 9 | 13.4 | 0 | 0 | ||||||
| Menorrhagia‡ | 20 | 29.4 | 16 | 35.6 | 4 | 17.4 | ||||||
NA, not applicable.
Data missing for 12 patients.
Data missing for 43 patients.
Among women.
The most frequently used fibrinogen activity assay was the Clauss assay (83.2%). For antigen level measurement, assays included radial immunodiffusion or turbidimetric latex immunoassay (30.9%), enzyme-linked immunosorbent assay (24.7%), and heat precipitation (18.9%). Functional fibrinogen and antigenic fibrinogen values ranged from 0.1 g/L to 3.72 g/L and from 1.4 g/L to 6.2 g/L, respectively, with mean ratios of functional and antigenic fibrinogen of 0.3 g/L (range, 0.1 to 0.7 g/L).
Eight patients (7.9%) had normal functional fibrinogen values but abnormal functional:antigenic ratios, including 5 measured with a PT-derived test. One patient with high fibrinogen functional level (3.72 g/L by the Clauss method) and high antigenic fibrinogen level (6.2 g/L by heat precipitation) had a concomitantly important inflammatory activity and was diagnosed after a postpartum PE. Successive measurements showed a decrease in the functional and antigenic levels (2.1 g/L by the Clauss method and 3.9 g/L by the immunologic method) but similar ratios. She was found to be heterozygous for a missense mutation in FGB: Arg44Cys. Among those with available RT and/or TT measurements, RT and TT were abnormally prolonged in 87.6% and 89.7% of cases, respectively. Four individuals with normal RT and normal TT were identified, all heterozygous for novel missense mutations: FGG Tyr207Cys (3 patients from the same family) and FGA Cys184Arg (1 patient with slightly increased PT and activated partial thromboplastin time). No patients suffered from amyloidosis, hepatopathy, or pulmonary hypertension.
Bleeding
At least 1 bleeding symptom was reported in 48 patients (47.5%), with mean BAT scores of 1.6 (SD, 2.3). The prevalence of symptoms (50.8% vs 41.2%; P = .36) and mean BAT scores (1.9 vs 1.1; P = .1) were not different between propositi and relatives. The most common bleeding symptoms were menorrhagia (n = 20 [29.4%] of 68 women), cutaneous bleeding (n = 21 [20.8%]), and bleeding after surgery (n = 9 [8.9%]; Table 2). Four patients had suffered from gastrointestinal bleeding: 1 child with posttraumatic liver hematoma, 1 man with recurrent lower gastrointestinal bleeding under antiplatelet therapy, 1 woman with lower gastrointestinal bleeding resulting from an anal fistula, and 1 woman as a result of a colorectal polyp. Only 1 patient had a spontaneous hemarthrosis.
A total of 13 patients (12.8%) experienced major bleeding, 11 before or at the time of diagnosis and 2 during the follow-up (Table 3): 7 PPHs requiring blood transfusion or FRT, 2 cerebral bleeding, 1 retroperitoneal bleeding after gastric surgery, 1 blood transfusion after ovarian cyst surgery, 1 surgical hemostasis after a polyp biopsy, and 1 massive postoperative hemothorax requiring surgery. None were fatal. Excluding pregnancy and surgical management, no patients received fibrinogen substitution in prophylaxis. Both cases of intracerebral hemorrhage, 1 in a 20-year-old woman and 1 in a child at birth, did not require any blood product, and the clinical course was uneventful. In addition, 9 patients received tranexamic acid, mostly because of menorrhagia (n = 3) and multiple tooth extractions (n = 4). The introduction of a hormonal substitution decreased bleeding associated with menorrhagia in 7 of 20 women.
Table 3.
Major bleeding and thrombosis at the time of diagnosis and during follow-up
| At time of diagnosis | During follow-up | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All patients (n = 101) | Propositi (n = 67) | Relatives (n = 34) | All patients (n = 101) | Propositi (n = 67) | Relatives (n = 34) | |||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Major bleeding | 11 | 10.9 | 9 | 13.4 | 2 | 5.9 | 2 | 2.0 | 2 | 3.0 | 0 | 0 |
| Critical area* | 3 | 3.0 | 3 | 4.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Requiring blood or fibrinogen transfusion | 6 | 5.9 | 4 | 6.0 | 2 | 5.9 | 2 | 2.0 | 2 | 3.0 | 0 | 0 |
| Requiring surgery intervention | 2 | 2.0 | 2 | 3.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total thrombosis† | 14 | 13.9 | 11 | 16.4 | 3 | 8.8 | 14 | 13.8 | 11 | 16.4 | 3 | 12.5 |
| Venous thrombosis | 10 | 9.9 | 7 | 10.5 | 3 | 8.8 | 11 | 10.9 | 9 | 13.4 | 2 | 5.9 |
| Deep venous thrombosis | 8 | 7.9 | 6 | 9.0 | 2 | 5.9 | 3 | 3.0 | 2 | 3.0 | 1 | 2.9 |
| Provoked | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2.0 | 1 | 1.6 | 1 | 2.9 |
| Recurrent | 3 | 3.0 | 2 | 3.0 | 1 | 3.0 | 1 | 1.0 | 0 | 0 | 1 | 2.9 |
| PE | 2 | 2.0 | 1 | 1.5 | 1 | 2.9 | 2 | 2.0 | 2 | 3.0 | 0 | 0 |
| Provoked | 1 | 1.0 | 1 | 1.5 | 0 | 0 | 2 | 2.0 | 2 | 3.0 | 0 | 0 |
| Superficial thrombophlebitis | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2.0 | 2 | 3.0 | 0 | 0 |
| Other‡ | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 4.0 | 3 | 4.5 | 1 | 2.9 |
| Recurrent | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1.0 | 1 | 1.6 | 0 | 0 |
| Arterial thrombosis | 4 | 4.0 | 4 | 6.0 | 0 | 0 | 6 | 5.9 | 4 | 6.4 | 2 | 5.9 |
| Stroke | 3 | 3.0 | 3 | 4.5 | 0 | 0 | 2 | 2.0 | 1 | 1.6 | 1 | 2.9 |
| Acute myocardial infarction | 1 | 1.0 | 1 | 1.5 | 0 | 0 | 2 | 2.0 | 1 | 1.6 | 1 | 2.9 |
| Recurrent | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2.0 | 1 | 1.6 | 1 | 2.9 |
| Peripheral thrombosis | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1.0 | 1 | 1.6 | 0 | 0 |
| Recurrent | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1.0 | 1 | 1.6 | 0 | 0 |
| Other§ | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1.0 | 1 | 1.6 | 0 | 0 |
Retroperitoneal, n = 1; intracranial, n = 2.
Total of first arterial or venous thrombosis; 3 patients had both arterial and venous thromboses.
Three splanchnic and 1 retinal thrombosis.
Mesenteric artery thrombosis.
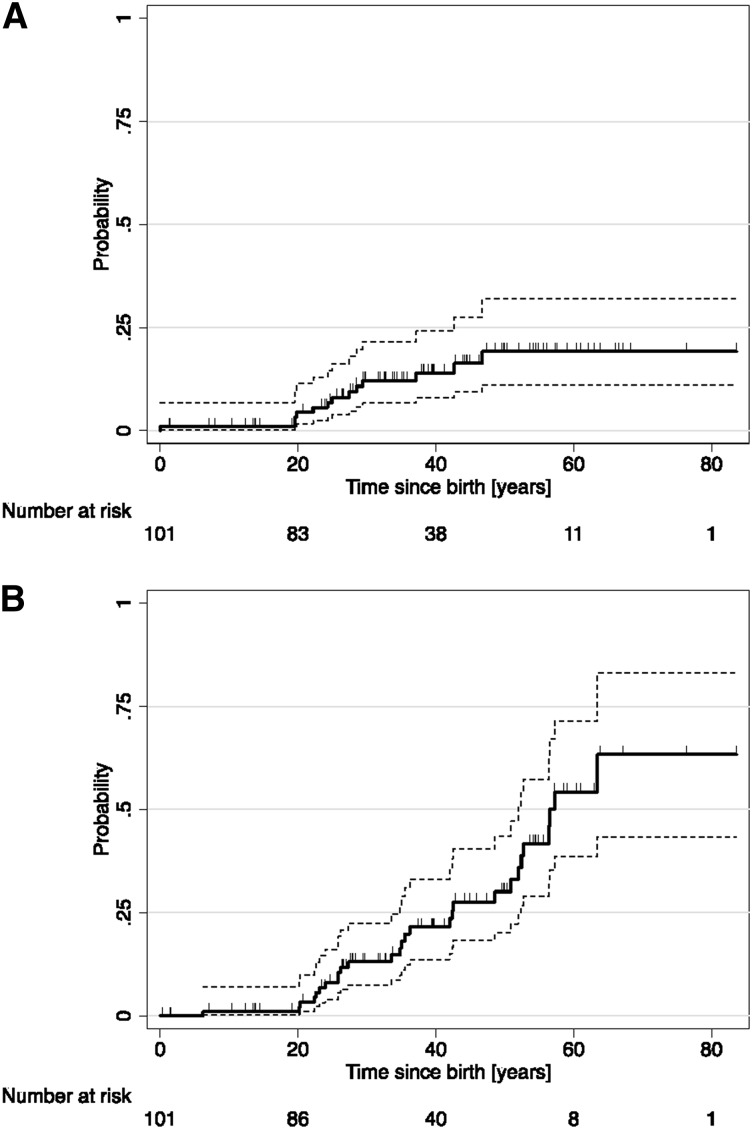
The overall IR of major bleeding was 3.5/1000 patient-years (6.0/1000 patient-years for adults age >18 years). The IR before the clinical CD diagnosis (3.8/1000; 95% CI, 2.1/1000 to 6.9/1000 patient-years) decreased somewhat during the follow-up (2.5/1000; 95% CI, 0.6/1000 to 10/1000 patient-years) without strong evidence (P = .45) (Table 4). The estimated cumulative incidence of major bleeding at age 50 years was 19.2% (95% CI, 11.1% to 31.9%) with Kaplan-Meier curves suggesting a prominent risk at age 20 through 40 years (Figure 1A). In age- and sex-adjusted analyses, the risk of major bleeding appeared somewhat higher in propositi than in relatives and in women than in men, but estimates remained imprecise (hazard ratio [HR], 2.1 [95% CI, 0.5 to 9.6] and HR, 1.8 [95% CI, 0.5 to 6.5]).
Table 4.
IRs and HRs for major bleeding and thrombosis
| Bleeding events | Thrombotic events | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of events | Person-years | IR* | 95% CI | HR† | 95% CI | P | No. of events | Person-years | IR* | 95%CI | HR† | 95%CI | P | |
| Overall | 13 | 3699.9 | 3.5 | 2.0-6.1 | 28 | 3663.8 | 7.6 | 5.3-11.1 | ||||||
| Restricted to adults (≥18 y) | 12 | 1984 | 6.0 | 3.4-10.7 | 27 | 1949.4 | 13.9 | 9.5-2.2 | ||||||
| Relatives | 2 | 1064.5 | 1.9 | 0.5-7.5 | 1.0 (ref) | 6 | 1038.8 | 5.8 | 2.6-12.9 | 1.0 (ref) | ||||
| Propositi | 11 | 2635.4 | 4.2 | 2.3-7.5 | 2.1 | 0.5-9.6 | .33 | 22 | 2625 | 8.4 | 5.5-12.7 | 1.1 | 0.4-2.7 | .86 |
| Men | 3 | 1281.9 | 2.3 | 0.8-7.3 | 1.0 (ref) | 11 | 1192.3 | 9.2 | 5.1-16.7 | 1.0 (ref) | ||||
| Women | 10 | 2418 | 4.1 | 0.2-7.7 | 1.8 | 0.5-6.5 | .39 | 17 | 2471.5 | 6.9 | 4.3-11.1 | .9 | 0.4-1.9 | .72 |
| Nonhotspot mutations‡ | 4 | 1085.8 | 3.7 | 1.4-9.8 | 1.0 (ref) | 9 | 1090.8 | 8.3 | 4.3-15.9 | 1.0 (ref) | ||||
| FGA Arg35 | 2 | 818.2 | 2.4 | 0.6-9.8 | 0.8 | 0.1-4.1 | .74 | 5 | 809 | 6.2 | 2.6-14.8 | .8 | 0.3-2.4 | .68 |
| FGG Arg301 | 7 | 1795.9 | 3.9 | 1.9-8.2 | 1.2 | 0.4-4.1 | .79 | 14 | 1763.9 | 7.9 | 4.7-13.4 | 1.1 | 0.5-2.6 | .83 |
| Until diagnosis of CD | 11 | 2896.9 | 3.8 | 2.1-6.9 | 1.0 (ref) | 14 | 2955.6 | 4.7 | 2.8-8.0 | 1.0 (ref) | ||||
| After diagnosis of CD | 2 | 803.1 | 2.5 | 0.6-10.0 | 0.5 | 0.1-2.6 | .45 | 14 | 748 | 18.7 | 11.0-31.6 | 2.0 | 0.9-4.7 | .1 |
ref, reference.
Per 1000 patient-years.
HR adjusted for age (time variable) and sex.
Mutations other than FGA Arg35 and FGG Arg301.
Figure 1.
Kaplan-Meier estimated cumulative probability. (A) Incident bleeding events and (B) incident thrombotic events. Marks on failure curves represent censoring times. The 95% lower and upper confidence intervals are depicted by the dashed lines.
Levels of fibrinogen activity or ratios of fibrinogen activity:antigen were not associated with the major bleeding risk (HR, 1.2 [95% CI, 0.7 to 2.0] per 1-g/L increment in fibrinogen activity; P = .51; HR, 1.0 [95% CI, 0.7 to 1.4] per 0.1-unit decrement in fibrinogen activity:antigen ratio; P = .97).
Thrombotic events
A thrombotic event led to the diagnosis of CD in 7 (6.9%) cases. The 28 first thrombotic events included 20 venous events (11 deep venous thromboses, 3 PEs, 2 superficial vein thrombophlebitis events, and 4 thromboses at unusual sites) and 8 arterial events (4 strokes, 2 acute myocardial infarctions, 1 peripheral artery occlusion, and 1 mesenteric artery thrombosis; Table 3). The majority of VTEs were idiopathic (71.4%). All were treated by anticoagulation from 4 weeks (superficial venous thrombosis) to 1 year. All arterial events were treated by antiplatelet agents. Only 1 event may have been secondary to FRT: a woman developed symptoms leading to a later diagnosis of PE a few days after a gastric surgery performed under a substitution of 3 g of a fibrinogen concentrate, Clottagen (LFB Biomanufacturing, Alès, France). Eight patients had recurrent arterial (n = 3) or VTE (n = 5) events. Recurrences tended to occur in the same vascular territory (venous or arterial) as the initial event. No patient had recurrent thrombosis while taking anticoagulant therapy.
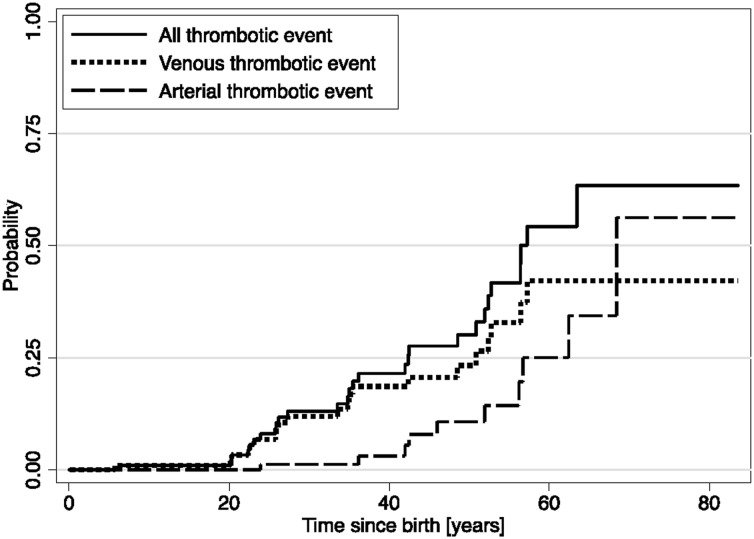
Half the thrombotic events occurred before or at the time of CD diagnosis and half during the clinical follow-up. The overall IR of incident arterial or VTE events was 7.6/1000 patient-years (13.9/1000 patient-years for adults >18 years of age). We observed a statistically nonsignificant increase of the age- and sex-adjusted risk of thrombotic events after the CD diagnosis (IR, 4.7/1000 and 18.7/1000 patient-years before and after CD diagnosis, respectively) (Table 4). Participants appeared at greater risk of VTE than of arterial thrombotic events (5.7/1000 vs 2.6/1000 patient-years). The estimated cumulative incidence of thrombotic events at age 50 years was 30.1% (95% CI, 20.1% to 43.5%), with Kaplan-Meier curves suggesting a gradual rise from age 20 years (Figure 1B), and estimated cumulative incidences at age 50 years greater for VTE (23.3%) than arterial (10.8%) events (Figure 2). In age- and sex-adjusted Cox regression analyses, there was no difference in the risk of overall thrombotic events between women and men (HR, 0.9; 95% CI, 0.4 to 1.9) or between propositi and relatives (HR, 1.1; 95% CI, 0.4 to 2.7) (Table 4).
Figure 2.
Kaplan-Meier estimated cumulative probability of all thrombotic outcomes, venous thrombotic outcomes and arterial thrombotic outcomes.
The levels of fibrinogen activity or of the ratios of fibrinogen activity:antigen were not associated with the thrombotic risk (HR, 1.2; 95% CI, 0.6 to 2.6 per 1-g/L increment in fibrinogen activity; P = .63; HR, 0.9; 95% CI, 0.9 to 1.4 per 0.1-unit decrement in fibrinogen activity:antigen ratio; P = .46).
Pregnancies
Among 64 women older than age 15 years, 48 (75%) had at least 1 pregnancy for a total of 111 pregnancies (mean of 2.3 per woman) at a median age of 28 years (interquartile range, 20 to 36 years). These resulted in 84 live births (75.7%), 22 spontaneous abortions (19.8%; 21 early and 1 late), and 5 stillbirths (4.5%). The mean gestational age of fetal loss in case of early spontaneous abortion was 9 weeks gestation (range, 4 to 12 weeks). Eighty-one pregnancies were reported at the time of clinical CD diagnosis and 30 pregnancies were reported after clinical CD diagnosis. Four women (8.3%) had recurrent pregnancy losses. Nineteen (21.4%) live or still births were complicated by PPH, 7 of which required blood transfusion and 3 of which were secondary PPH with associated placenta retention.
FRT was rarely used at delivery in only 4 women (9 pregnancies [8.1%]), including 2 women with a prior bleeding phenotype. The mean amount of fibrinogen concentrate administered was 4 g (range, 2 to 8 g). A PPH was observed in 5 (44.4%) of 9 deliveries, despite the fibrinogen substitution. Furthermore, 1 woman was treated with tranexamic acid during 3 of her pregnancies, which were nevertheless all complicated by PPH.
In parity- and age-adjusted logistic regression analyses (Table 5), propositi had a statistically borderline greater risk of miscarriage or stillbirth (odds ratio [OR], 3.6; 95% CI, 0.8 to 15.7; P = .09) but not of PPH (OR, 0.7; 95% CI, 0.2 to 3.0; P = .62). Women with a bleeding phenotype, defined as having at least 1 bleeding symptom other than obstetrical, were at increased risk of PPH (OR, 5.8; 95% CI, 1.2 to 28.0; P = .03) but decreased risk of spontaneous abortion (OR, 0.4; 95% CI, 0.1 to 1.0; P = .05). Similarly, fibrinogen activity levels or fibrinogen activity:antigen ratios were not associated with these risks.
Table 5.
Multivariate analysis of risk factors for obstetrical complications
| Miscarriage or still birth (n = 27/111 pregnancies) | Postpartum hemorrhage* (n = 19/89 pregnancies) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | OR† | 95% CI | P | No. | % | OR† | 95% CI | P | |
| Relatives | 2/24 | 8.3 | 1.0 (ref) | 5/23 | 21.7 | 1.0 (ref) | ||||
| Propositi | 25/87 | 28.7 | 3.6 | 0.8-15.7 | .09 | 14/66 | 21.2 | 0.7 | 0.2-3.0 | .65 |
| No bleeding phenotype | 17/54 | 31.5 | 1.0 (ref) | 3/39 | 7.7 | 1.0 (ref) | ||||
| Bleeding phenotype | 10/57 | 17.5 | 0.4 | 0.1-1.0 | .05 | 16/34 | 32.0 | 5.8 | 1.2-28.0 | .03 |
| Nonhotspot mutations | 15/48 | 31.3 | 1.0 (ref) | 11/36 | 30.6 | 1.0 (ref) | ||||
| FGA Arg35 mutation | 3/15 | 12.0 | 0.6 | 0.1-3.3 | .60 | 1/12 | 8.3 | 0.3 | 0.1-2.4 | .24 |
| FGG Arg301 mutation | 9/48 | 18.8 | 0.6 | 0.2-1.6 | .29 | 7/41 | 17.1 | 0.6 | 0.1-2.2 | .39 |
Adjusted for age (at pregnancy) and parity.
Among live and still births.
Surgeries
Throughout the study period, 62 patients underwent 35 minor and 102 major surgeries, of which 76 (55.5%) occurred before the diagnosis of dysfibrinogenemia (supplemental Table 1 available on the Blood Web site). Orthopedic and abdominal procedures were most common, especially osteosynthesis following trauma and appendectomy. Nine surgeries were complicated by abnormal bleeding exclusively among propositi, including 4 major bleeding events: 2 minor surgeries (multiple tooth extractions) and 7 major surgeries (ovarian cyst removal, aortic aneurism repair, adenoidectomy, mastoidectomy, gastroplasty, and orthopedic surgeries). FRT was used in 3 minor procedures (multiple tooth extractions) and 16 major (mainly orthopedic) procedures, in which no bleeding occurred. Postsurgery FRT was not given. Tranexamic acid was administered before 5 minor (dental) and 1 major surgery, with no reports of bleeding. In univariate analyses, the use of FRT or tranexamic acid in any surgery and of FRT in major surgeries was not associated with a decreased risk of bleeding (Fisher’s exact test P = .21 and P = .59, respectively).
Genotype
Screening for mutations was performed according to the flow chart for mutation detection used in our laboratory,12 starting with the analysis of FGA exon 2 and FGG exon 8. Once the causative mutation was identified, either because the mutation was previously reported15 or based on SIFT analysis16 and, when possible, familial segregation, sequencing was interrupted. Causative mutations are listed in Table 1. We identified 20 different causative mutations in the 101 participants, including 6 novel missense mutations (one published separately as a case report).17 Almost all CD mutations included have already been reported in studies assessing their causative function,15 and causative role of new mutations was mainly assumed on the basis of SIFT analysis.16
All patients were heterozygous for a missense mutation except 2 for which FGA frameshift mutations were identified: 1410_1411insT18 (in heterozyosity) and 1482_1495del19 (in homozygosity). FGA mutations affecting residue Arg35 (ie, Arg35His and Arg35Cys) at the fibrinopeptide A thrombin cleavage site were frequent in 13.9% and 8.9% of patients, respectively. Mutations identified in FGG included the common Arg301Cys and Arg301His mutations, identified in 32.7% and 18.9% of patients, respectively. Altogether, mutations affecting exon 2 of FGA and exon 8 of FGG were found to be responsible for 86.9% of CDs. Only 2 mutations were identified in FGB.
Clinical phenotypes were heterogeneous (Table 1). In our study, 11 patients with thrombosis also had a bleeding phenotype. The propositus with FGA Cys184Arg reported major bleeding (PPH with blood transfusion) and ischemic stroke. The propositus with mutation FGG Ser358Cys had both a thrombotic and a severe bleeding phenotype, characterized by a splanchnic thrombosis as well as PPH, epistaxis, and menorrhagia, whereas her son presented with a moderate bleeding phenotype. Two family members heterozygous for FGG Tyr207Cys had a severe bleeding phenotype. Our patient with the well-known thrombotic mutation FGA Arg573Cys20 was placed on prophylactic oral anticoagulant treatment because of a family history of recurrent thrombotic events. In one woman, the common FGA Arg35Cys mutation was associated with severe bleeding.
Because of the high allelic heterogeneity of CD, genotype/phenotype correlations are very difficult to establish. In our study, only the common substitutions affecting the mutation hotspots FGA Arg35 and FGG Arg301 were frequent enough to explore their association with a clinical outcome. In age- and sex-adjusted Cox analyses, compared with other mutations, mutations of FGA Arg35 and FGG Arg301 were not significantly associated with either the risk of thrombotic events (HR, 0.8 [95% CI, 0.3 to 2.4] and HR, 1.1 [95% CI, 0.5 to 2.6], respectively) or with the risk of major bleeding (HR, 0.8 [95% CI, 0.1 to 4.1] and HR, 1.2 [95% CI, 0.4 to 4.1]) (Table 4). In addition, these mutations were not associated with pregnancy complications (Table 5).
Discussion
This cohort study is the largest study of genotyped CD and the first to describe its natural history. Our results suggest high risks of bleeding, thrombosis, and pregnancy complications, regardless of the propositus or relative status.
In our series, 58% of propositi were identified incidentally, similar to the 55% reported by Haverkate and Samama5 and the 48% reported by Shapiro et al.21 Thus, most patients appear to be asymptomatic at the moment of diagnosis. Because the diagnosis is often made at a young age, we can assume that some patients did not have time to develop thrombotic or bleeding symptoms. Conversely, asymptomatic patients for whom the diagnosis is made in advanced age may never become symptomatic.
Bleeding is a highly variable feature of CD. In our series, the bleeding phenotype was generally mild, and spontaneous life-threatening bleeds were rare. In women, menorrhagia was common and was often the presenting symptom of CD. Similar results were reported by Miesbach et al22: among 21 propositi (57%) with at least 1 episode of hemorrhage, easy bruising was by far the most common (16 [76%] of 21). Among 35 patients with CD reported by Shapiro et al,21 menorrhagia (7 [20%] of 35 patients) and easy bruising (6 [17%] of 35 patients) were also frequently reported. Our results suggest that the risk of major bleeding occurred primarily between the ages of 20 and 40 years, in part as a result of the increased hemostatic challenges faced by childbearing women. We did not observe a statistically significant decrease in the risk of major bleeding after the diagnosis of CD because we were underpowered to do so, but we did observe a trend toward less bleeding after diagnosis. Clearly, the influence of hemostatic support with FRT and tranexamic acid needs to be further explored in future studies.
Different mechanisms may explain the risk of thrombosis in CD,23 including elevated levels of thrombin resulting from the defect in binding fibrinogen,24 and altered strength, structure, and stability of the fibrin clot,25 as well as impaired fibrinolysis of abnormal fibrinogen.26 Compared with recent smaller retrospective series of genotyped CD,21,22 our study found a high prevalence of VTE at the time of diagnosis of CD. We observed an incidence of incident VTE of 5.58/1000 patient-years, which is greater than that reported in the general population (1.5/1000 patient-years)27 and similar to that of carriers of severe thrombophilia such as homozygous factor V G1691A Leiden and prothrombin G20210A.28 Furthermore, the mean age at incident venous (34 years) and arterial thrombotic (49 years) events was younger than that reported in the general population.29,30 Interestingly, relatives were not at lower risk of thrombotic events than propositi. This supports the necessity of family screening in case of a CD diagnosis to optimize the management of thrombotic risk factors.
Obstetric complications of dysfibrinogenemia are frequent and include pregnancy loss, hemorrhage, placental abruption, and thrombosis.31 The importance of fibrinogen in pregnancy has been demonstrated in studies with fibrinogen knockout mice in which gestation cannot be maintained to term32 and in in vitro models of human trophoblast proliferation.33 In CD, the physiological increase of fibrinogen levels is observed throughout the pregnancy.34 However, a concomitant increase of abnormal fibrinogen is also observed. Additional structural changes could lead to alterations in fibrinolytic activity and thus contribute to the adverse outcomes.35 Compared with reports of risks in the general population, we did not observe a greater risk of spontaneous abortions36 but we did find a substantial risk of PPH that appears greater.37 No recommendations exist for the management of pregnant women with CD, which is challenging, especially around delivery, because of the high risks of bleeding and thrombosis. Our data suggest that PPH was mostly observed in women with a prior bleeding phenotype, which emphasizes the importance of personal and family history. These women should be carefully followed during the peripartum and postpartum periods.38 Whereas some authors have advocated for continuous FRT starting in early pregnancy,39 the hypercoagulable state of pregnancy should also be taken into account, and the use of postpartum thromboprophylaxis should be discussed with individual patients.34
The laboratory diagnosis of CD is difficult. Reported overestimations of functional and antigenic fibrinogen values depend on the type of assay.40 The most sensitive tests are the TT and RT,21 although in some exceptional cases most coagulation parameters are normal and only genotyping can confirm the diagnosis. To date about 100 mutations resulting in CD are documented. In our study, the majority of mutations were identified in exon 2 of FGA and exon 8 of the FGG, including the 2 mutation hotspots FGA Arg35 and FGG Arg301, confirming previous data.3 In total, screening only FGA exon 2 and FGG exon 8 identify around 85% of CD causative mutations. We were not able to identify associations between genotypes and clinical phenotypes. This may be a result of the low statistical power to do so, even for the most common hotspot mutations.5
Our study has limitations. First, selection bias cannot be excluded, because no screening for CD exists; therefore, CD may have been diagnosed in patients with more severe phenotypes. Second, our large cohort still suffers from low statistical power to detect associations between phenotypes and genotypes, especially for the risk of major bleeding. Third, the influence of antithrombotic therapies on the risk of thrombotic events could not be analyzed because of heterogeneity in treatment in the absence of guidelines. Fourth, the influence of fibrinogen supplementation on bleeding outcomes during surgery or delivery could not be reliably estimated because of the rarity of its use and the high potential for confounding by indication. Fifth, we had no control group and thus were only able to indirectly compare the risk of bleeding or thrombotic risks of patients with and without CD.
In conclusion, we found that propositi and their affected relatives carry a high risk of bleeding, thrombotic events, and postpartum hemorrhage but not of spontaneous abortion. Results from ongoing prospective studies such as the proRBDD project (http://eu.rbdd.org/) may improve our knowledge of the risk of bleeding and thrombosis in specific situations such as surgery and pregnancy and may evaluate the risk and benefit of FRT.
Acknowledgments
The authors thank Severine Nolli for expert technical assistance and all of the participants who provided patients for inclusion in this study: C. Barro (Grenoble, France), M. Bertrand (Besançon, France), E. Bianchi (Lausanne, Switzerland), J. Borg (Rouen, France), M. Briquel (Nancy, France), B. Chatelain (Yvoir, Belgium), M. Chirila (Valence, France), M. Daskalakis (Berne, Switzerland), B. Delahousse (Tours, France), B. Devalet (Yvoir, Belgium), K. Devreese (Gand, Belgium), R. d’Oiron (Paris, France), S. Eeckhoudt (Bruxelles, Belgium), B. Fimbel (Tours, France), N. Franciane (Montpellier, France), F. Grandoni (Lausanne, Switzerland), C. Hermans (Bruxelles, Belgium), N. Hezard (Reims, France), A. Kutila (Helsinki, Finland), V. Le Cam Duchez (Rouen, France), A. Marques Verdier (Clermont Ferrand, France), N. Trillot (Lille, France), F. Volot (Dijon, France), A. Zimrin (Baltimore, MD).
This work was supported by an unrestricted grant from the ISTH2007 Presidential Fund and by the Swiss National Science Foundation.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.C., A.L., M.B., P.d.M., and M.N.-A. designed the research and wrote the paper; M.B. and A.C. performed the statistical analyses; A.C., J.K., and M.N.-A. performed the research and contributed to the analysis of the data; and A.L., V.T., E.d.M., P.G., and C.B. contributed analytical tools.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Philippe de Moerloose, University Hospitals of Geneva and Faculty of Medicine, Hemostasis Unit, 1211 Geneva, Switzerland; e-mail: philippe.demoerloose@hcuge.ch.
References
- 1.Neerman-Arbez M, de Moerloose P. Hereditary fibrinogen abnormalities. In: Kaushansky K, Lichtman M, Beutler E, Kipps T, Prchal J, Seligsohn U, editors. Williams Hematology. New York: McGraw-Hill; 2010:2051-2068. [Google Scholar]
- 2.Galanakis DK. Afibrinogenemias and dysfibrinogenemias. In: Marder V, White GC, Aird WC, editors. Hemostasis and Thrombosis: Basic Principles and Clinical Practice. Philadelphia: Lippincott Williams & Wilkins; 2012. pp. 693–708. [Google Scholar]
- 3.de Moerloose P, Casini A, Neerman-Arbez M. Congenital fibrinogen disorders: an update. Semin Thromb Hemost. 2013;39(6):585–595. doi: 10.1055/s-0033-1349222. [DOI] [PubMed] [Google Scholar]
- 4.Peyvandi F. Epidemiology and treatment of congenital fibrinogen deficiency. Thromb Res. 2012;130(Suppl 2):S7–S11. doi: 10.1016/S0049-3848(13)70004-5. [DOI] [PubMed] [Google Scholar]
- 5.Haverkate F, Samama M. Familial dysfibrinogenemia and thrombophilia. Report on a study of the SSC Subcommittee on Fibrinogen. Thromb Haemost. 1995;73(1):151–161. [PubMed] [Google Scholar]
- 6.Krammer B, Anders O, Nagel HR, Burstein C, Steiner M. Screening of dysfibrinogenaemia using the fibrinogen function versus antigen concentration ratio. Thromb Res. 1994;76(6):577–579. doi: 10.1016/0049-3848(94)90287-9. [DOI] [PubMed] [Google Scholar]
- 7.Rodeghiero F, Tosetto A, Abshire T, et al. ISTH/SSC joint VWF and Perinatal/Pediatric Hemostasis Subcommittees Working Group. ISTH/SSC bleeding assessment tool: a standardized questionnaire and a proposal for a new bleeding score for inherited bleeding disorders. J Thromb Haemost. 2010;8(9):2063–2065. doi: 10.1111/j.1538-7836.2010.03975.x. [DOI] [PubMed] [Google Scholar]
- 8.Janssen CA, Scholten PC, Heintz AP. A simple visual assessment technique to discriminate between menorrhagia and normal menstrual blood loss. Obstet Gynecol. 1995;85(6):977–982. doi: 10.1016/0029-7844(95)00062-V. [DOI] [PubMed] [Google Scholar]
- 9.Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuunemann HJ. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):7S-47S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark CA, Davidovits J, Spitzer KA, Laskin CA. The lupus anticoagulant: results from 2257 patients attending a high-risk pregnancy clinic. Blood. 2013;122(3):341–347. doi: 10.1182/blood-2013-02-485839. [DOI] [PubMed] [Google Scholar]
- 11.Neerman-Arbez M, de Moerloose P, Bridel C, et al. Mutations in the fibrinogen aalpha gene account for the majority of cases of congenital afibrinogenemia. Blood. 2000;96(1):149–152. [PubMed] [Google Scholar]
- 12.de Moerloose P, Neerman-Arbez M. Congenital fibrinogen disorders. Semin Thromb Hemost. 2009;35(4):356–366. doi: 10.1055/s-0029-1225758. [DOI] [PubMed] [Google Scholar]
- 13.den Dunnen JT, Antonarakis SE. Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum Mutat. 2000;15(1):7–12. doi: 10.1002/(SICI)1098-1004(200001)15:1<7::AID-HUMU4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 14.Medved L, Weisel JW Fibrinogen and Factor XIII Subcommittee of Scientific Standardization Committee of International Society on Thrombosis and Haemostasis. Recommendations for nomenclature on fibrinogen and fibrin. J Thromb Haemost. 2009;7(2):355–359. doi: 10.1111/j.1538-7836.2008.03242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanss M, Biot F. A database for human fibrinogen variants. Ann N Y Acad Sci. 2001;936:89–90. doi: 10.1111/j.1749-6632.2001.tb03495.x. [DOI] [PubMed] [Google Scholar]
- 16.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4(7):1073–1081. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 17.Casini A, De Maistre E, Casini-Stuppi V, Fontana P, Neerman-Arbez M, de Moerloose P. Fibrinogen geneva II: a new congenitally abnormal fibrinogen alpha chain (Gly17Asp) with a review of similar mutations resulting in abnormal knob A. Blood Coagul Fibrinolysis. 2014;25(3):280–282. doi: 10.1097/MBC.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 18.Furlan M, Steinmann C, Jungo M, et al. A frameshift mutation in Exon V of the A alpha-chain gene leading to truncated A alpha-chains in the homozygous dysfibrinogen Milano III. J Biol Chem. 1994;269(52):33129–33134. [PubMed] [Google Scholar]
- 19.Brennan SO, Homer VM, Ockelford P, George PM. Low expression of truncated Aalpha chain variant in circulating fibrinogen. Thromb Haemost. 2002;88(3):533–534. [PubMed] [Google Scholar]
- 20.Soria J, Soria C, Caen P. A new type of congenital dysfibrinogenaemia with defective fibrin lysis—Dusard syndrome: possible relation to thrombosis. Br J Haematol. 1983;53(4):575–586. doi: 10.1111/j.1365-2141.1983.tb07309.x. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro SE, Phillips E, Manning RA, et al. Clinical phenotype, laboratory features and genotype of 35 patients with heritable dysfibrinogenaemia. Br J Haematol. 2013;160(2):220–227. doi: 10.1111/bjh.12085. [DOI] [PubMed] [Google Scholar]
- 22.Miesbach W, Scharrer I, Henschen A, Neerman-Arbez M, Spitzer S, Galanakis D. Inherited dysfibrinogenemia: clinical phenotypes associated with five different fibrinogen structure defects. Blood Coagul Fibrinolysis. 2010;21(1):35–40. doi: 10.1097/MBC.0b013e328331e6db. [DOI] [PubMed] [Google Scholar]
- 23.Weisel JW, Litvinov RI. Mechanisms of fibrin polymerization and clinical implications. Blood. 2013;121(10):1712–1719. doi: 10.1182/blood-2012-09-306639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Moerloose P, Boehlen F, Neerman-Arbez M. Fibrinogen and the risk of thrombosis. Semin Thromb Hemost. 2010;36(1):7–17. doi: 10.1055/s-0030-1248720. [DOI] [PubMed] [Google Scholar]
- 25.Ariëns RA. Fibrin(ogen) and thrombotic disease. J Thromb Haemost. 2013;11(Suppl 1):294–305. doi: 10.1111/jth.12229. [DOI] [PubMed] [Google Scholar]
- 26.Koopman J, Haverkate F, Grimbergen J, et al. Molecular basis for fibrinogen Dusart (A alpha 554 Arg—>Cys) and its association with abnormal fibrin polymerization and thrombophilia. J Clin Invest. 1993;91(4):1637–1643. doi: 10.1172/JCI116371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrøm J. Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost. 2007;5(4):692–699. doi: 10.1111/j.1538-7836.2007.02450.x. [DOI] [PubMed] [Google Scholar]
- 28.Martinelli I, De Stefano V, Mannucci PM. Inherited risk factors for venous thromboembolism. Nat Rev Cardiol. 2014;11(3):140–156. doi: 10.1038/nrcardio.2013.211. [DOI] [PubMed] [Google Scholar]
- 29.Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117(1):19–25. doi: 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 30.Savji N, Rockman CB, Skolnick AH, et al. Association between advanced age and vascular disease in different arterial territories: a population database of over 3.6 million subjects. J Am Coll Cardiol. 2013;61(16):1736–1743. doi: 10.1016/j.jacc.2013.01.054. [DOI] [PubMed] [Google Scholar]
- 31.Bornikova L, Peyvandi F, Allen G, Bernstein J, Manco-Johnson MJ. Fibrinogen replacement therapy for congenital fibrinogen deficiency. J Thromb Haemost. 2011;9(9):1687–1704. doi: 10.1111/j.1538-7836.2011.04424.x. [DOI] [PubMed] [Google Scholar]
- 32.Iwaki T, Sandoval-Cooper MJ, Paiva M, Kobayashi T, Ploplis VA, Castellino FJ. Fibrinogen stabilizes placental-maternal attachment during embryonic development in the mouse. Am J Pathol. 2002;160(3):1021–1034. doi: 10.1016/S0002-9440(10)64923-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snir A, Brenner B, Paz B, Ohel G, Lanir N. The role of fibrin matrices and tissue factor in early-term trophoblast proliferation and spreading. Thromb Res. 2013;132(4):477–483. doi: 10.1016/j.thromres.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 34.Franchini M, Raffaelli R, Musola M, et al. Management of inherited dysfibrinogenemia during pregnancy: a description of four consecutive cases. Ann Hematol. 2007;86(9):693–694. doi: 10.1007/s00277-007-0307-5. [DOI] [PubMed] [Google Scholar]
- 35.Pretorius E, Bronkhorst P, Briedenhann S, Smit E, Franz RC. Comparisons of the fibrin networks during pregnancy, nonpregnancy and pregnancy during dysfibrinogenaemia using the scanning electron microscope. Blood Coagul Fibrinolysis. 2009;20(1):12–16. doi: 10.1097/mbc.0b013e328322b429. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Chen C, Wang L, Chen D, Guang W, French J. Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil Steril. 2003;79(3):577–584. doi: 10.1016/s0015-0282(02)04694-0. [DOI] [PubMed] [Google Scholar]
- 37.Lu MC, Fridman M, Korst LM, et al. Variations in the incidence of postpartum hemorrhage across hospitals in California. Matern Child Health J. 2005;9(3):297–306. doi: 10.1007/s10995-005-0009-3. [DOI] [PubMed] [Google Scholar]
- 38.Peyvandi F, Bidlingmaier C, Garagiola I. Management of pregnancy and delivery in women with inherited bleeding disorders. Semin Fetal Neonatal Med. 2011;16(6):311–317. doi: 10.1016/j.siny.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Miesbach W, Galanakis D, Scharrer I. Treatment of patients with dysfibrinogenemia and a history of abortions during pregnancy. Blood Coagul Fibrinolysis. 2009;20(5):366–370. doi: 10.1097/MBC.0b013e32832aec2b. [DOI] [PubMed] [Google Scholar]
- 40.Miesbach W, Schenk J, Alesci S, Lindhoff-Last E. Comparison of the fibrinogen Clauss assay and the fibrinogen PT derived method in patients with dysfibrinogenemia. Thromb Res. 2010;126(6):e428–e433. doi: 10.1016/j.thromres.2010.09.004. [DOI] [PubMed] [Google Scholar]