Abstract
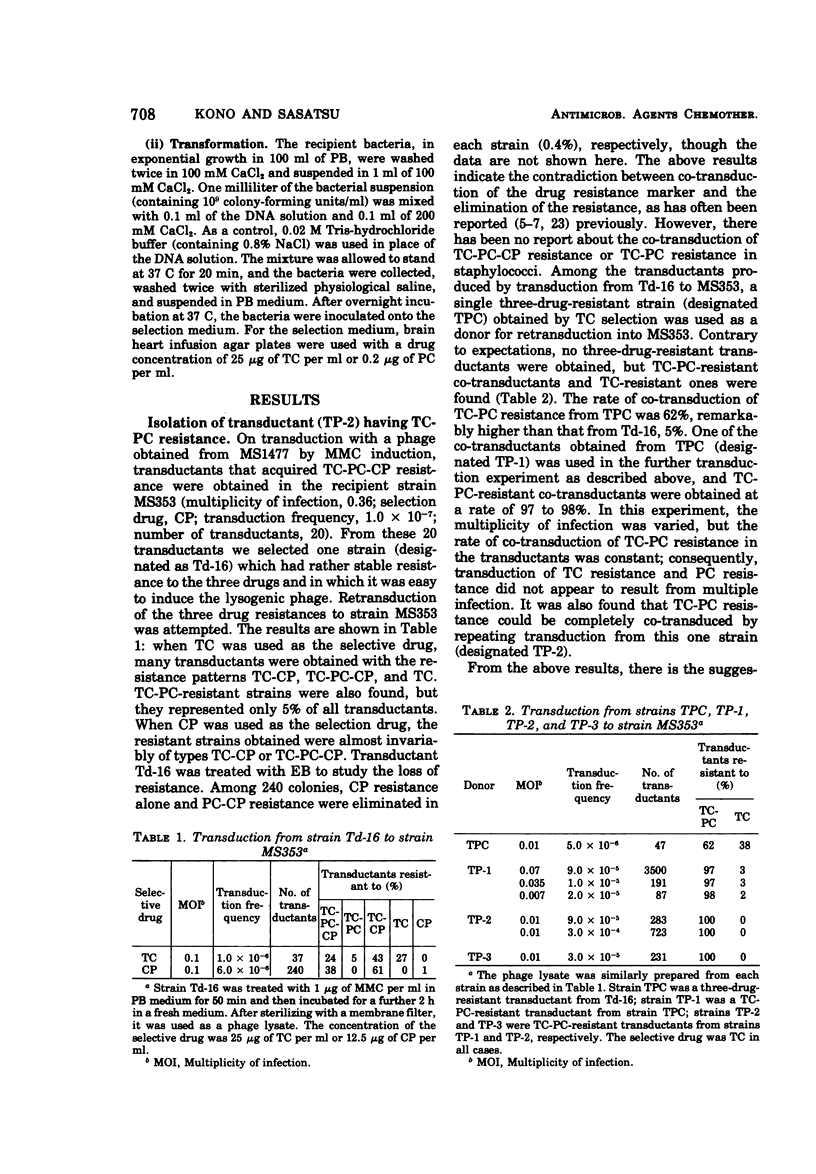
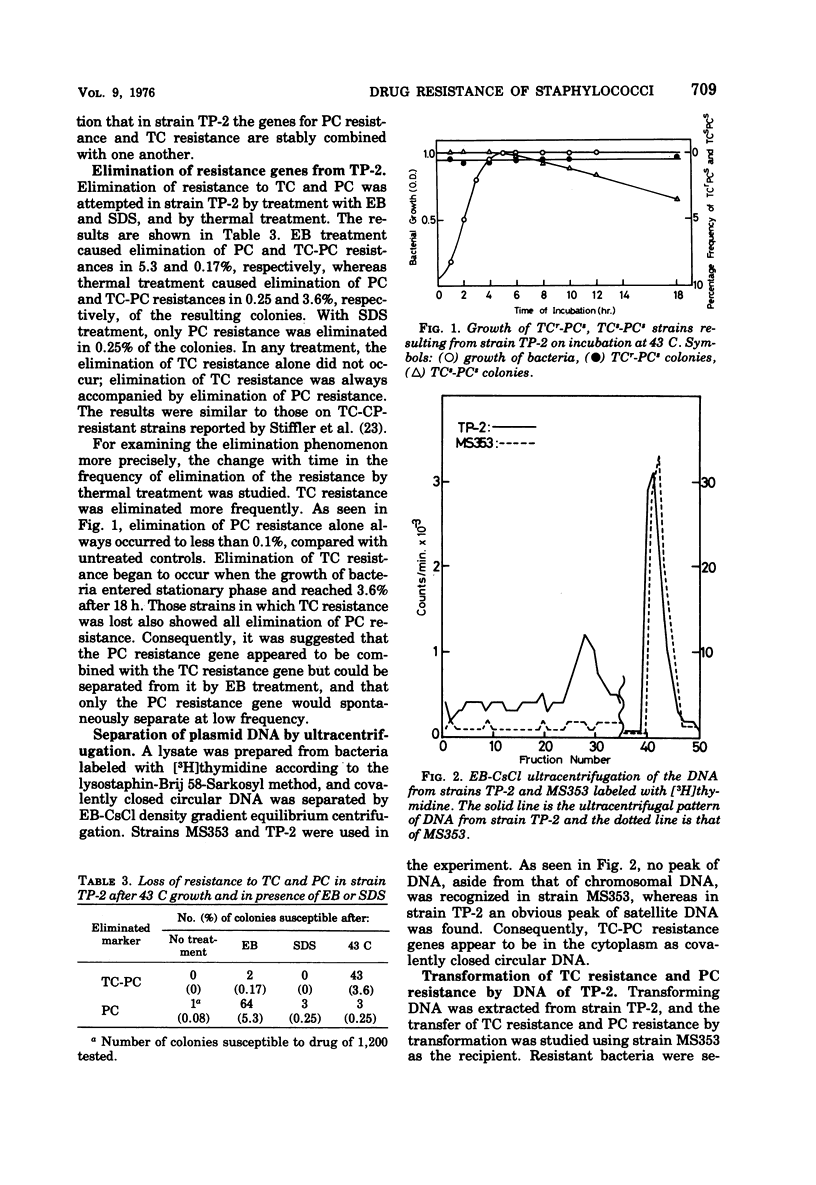
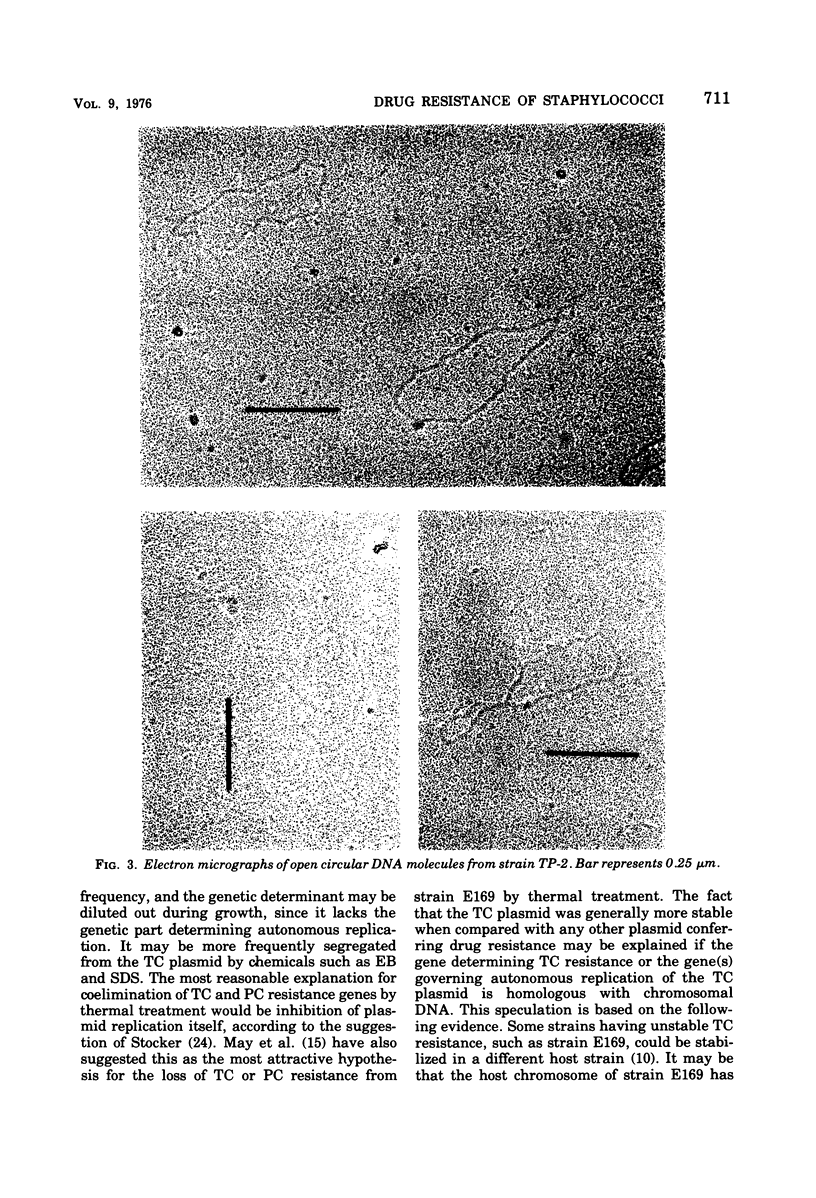
On transduction with a lysogenic strain of Straphylococcus aureus isolated from a clinical specimen and having tetracycline (TC)-penicillin (PC)-chloramphenicol (CP)-resistant plasmids, the three-drug-resistant strain was frequently obtained. By repeatedly transducing from this strain, a strain (TP-2) having stable resistance to TC and PC could be obtained. In transformation with the deoxyribonucleic acid (DNA) of TP-2 as donor, all of the transformants obtained by selecting with either TC or PC were both TC and PC resistant. According to electron microscopy study of the covalently closed circular DNA of TP-2, the plasmid DNA size was 1.37 ± 0.03 μm (2.84 × 106 daltons). The plasmid (PTP-2) is presumed to be a new plasmid in which the PC resistance gene was integrated into the TC-resistant plasmid.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asheshov E. H. The genetics of penicillinase production in Staphylococcus aureus strain PS80. J Gen Microbiol. 1969 Dec;59(3):289–301. doi: 10.1099/00221287-59-3-289. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Multiple molecular species of circular R-factor DNA isolated from Escherichia coli. Nature. 1969 Dec 27;224(5226):1273–1277. doi: 10.1038/2241273a0. [DOI] [PubMed] [Google Scholar]
- Follett E. A., Crawford L. V. Electron microscope study of the denaturation of human papilloma virus DNA. I. Loss and reversal of supercoiling turns. J Mol Biol. 1967 Sep 28;28(3):455–459. doi: 10.1016/s0022-2836(67)80095-0. [DOI] [PubMed] [Google Scholar]
- Grubb W. B., O'Reilly R. J. Joint transduction of separate extrachromosomal drug resistance determinants in Staphylococcus aureus E169. Biochem Biophys Res Commun. 1971 Feb 5;42(3):377–383. doi: 10.1016/0006-291x(71)90381-0. [DOI] [PubMed] [Google Scholar]
- Grubb W. B., O'Reilly R. J., May J. W. Segregation o co-transduced streptomycin and tetracycline resistance in Staphylococcus aureus. Genet Res. 1972 Aug;20(1):43–50. doi: 10.1017/s0016672300013574. [DOI] [PubMed] [Google Scholar]
- Inoue M., Hashimoto H., Yamagishi S., Mitsuhashi S. Transduction analysis of the genetic determinants for chloramphenicol resistance in Staphylococci. Jpn J Microbiol. 1970 Jul;14(4):261–268. doi: 10.1111/j.1348-0421.1970.tb00523.x. [DOI] [PubMed] [Google Scholar]
- Kasuga T., Hashimoto H., Mitsuhashi S. Drug resistance of staphylococci. VII. Genetic determinants responsible for the resistance to tetracycline, streptomycin, sulfanilamide, and penicillin. J Bacteriol. 1968 May;95(5):1764–1766. doi: 10.1128/jb.95.5.1764-1766.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga T., Mitsuhashi S. Drug resistance of staphylococci. 8. Genetic properties of resistance to tetracycline in Staphylococcus aureus E169. Jpn J Microbiol. 1968 Sep;12(3):269–273. doi: 10.1111/j.1348-0421.1968.tb00395.x. [DOI] [PubMed] [Google Scholar]
- Kono M., O'Hara K., Nagawa M., Mitsuhashi S. Antibacterial activity of chloramphenicol-related compounds toward a chloramphenicol-resistant strains of Staphylococcus aureus. Jpn J Microbiol. 1972 Nov;16(6):461–467. doi: 10.1111/j.1348-0421.1972.tb00685.x. [DOI] [PubMed] [Google Scholar]
- Lacey R. W. Antibiotic resistance plasmids of Staphylococcus aureus and their clinical importance. Bacteriol Rev. 1975 Mar;39(1):1–32. doi: 10.1128/br.39.1.1-32.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg M., Sjöström J. E., Johansson T. Transformation of chromosomal and plasmid characters in Staphylococcus aureus. J Bacteriol. 1972 Feb;109(2):844–847. doi: 10.1128/jb.109.2.844-847.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAY J. W., HOUGHTON R. H., PERRET C. J. THE EFFECT OF GROWTH AT ELEVATED TEMPERATURES ON SOME HERITABLE PROPERTIES OF STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1964 Nov;37:157–169. doi: 10.1099/00221287-37-2-157. [DOI] [PubMed] [Google Scholar]
- MITSUHASHI S., HASHIMOTO H., KONO M., MORIMURA M. DRUG RESISTANCE OF STAPHYLOCOCCI. II. JOINT ELIMINATION AND JOINT TRANSDUCTION OF THE DETERMINANTS OF PENICILLINASE PRODUCTION AND RESISTANCE TO MACROLIDE ANTIBIOTICS. J Bacteriol. 1965 Apr;89:988–992. doi: 10.1128/jb.89.4.988-992.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi S., Inoue M., Kawabe H., Oshima H., Okubo T. Genetic and biochemical studies of drug resistance in staphylococci. Contrib Microbiol Immunol. 1973;1:144–165. [PubMed] [Google Scholar]
- Novick R. P., Bouanchaud D. The problems of drug-resistant pathogenic bacteria. Extrachromosomal nature of drug resistance in Staphylococcus aureus. Ann N Y Acad Sci. 1971 Jun 11;182:279–294. doi: 10.1111/j.1749-6632.1971.tb30664.x. [DOI] [PubMed] [Google Scholar]
- Odakura Y., Hashimoto H., Mitsuhashi S. R-factor mutant capable of specifying hypersynthesis of penicillinase. J Bacteriol. 1974 Dec;120(3):1260–1267. doi: 10.1128/jb.120.3.1260-1267.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush M. G., Gordon C. N., Novick R. P., Warner R. C. Penicillinase plasmid DNA from Staphylococcus aureus. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1304–1310. doi: 10.1073/pnas.63.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOCKER B. A. Abortive transduction of motility in Salmonella; a nonreplicated gene transmitted through many generations to a single descendant. J Gen Microbiol. 1956 Dec;15(3):575–598. doi: 10.1099/00221287-15-3-575. [DOI] [PubMed] [Google Scholar]
- Sjöström J. E., Lindberg M., Philipson L. Transfection of Staphylococcus aureus with bacteriophage deoxyribonucleic acid. J Bacteriol. 1972 Jan;109(1):285–291. doi: 10.1128/jb.109.1.285-291.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiffler P. W., Sweeney H. M., Cohen S. Co-transduction of plasmids mediating resistance to tetracycline and chloramphenicol in Staphylococcus aureus. J Bacteriol. 1974 Nov;120(2):934–934. doi: 10.1128/jb.120.2.934-944.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]




