Abstract
Objective
Rifampin mono-resistant tuberculosis (RMR-TB) is increasingly identified due to scale-up of rapid molecular tests. The longitudinal association of RMR-TB, multidrug-resistant TB (MDR-TB), and HIV/AIDS is incompletely described.
Methods
We examined clinical characteristics and treatment outcomes of patients with RMR-TB, isoniazid mono-resistant TB (IMR-TB), MDR-TB, and drug-susceptible TB during a sixteen year period (1993–2008) in California. TB cases were cross-matched with the state HIV/AIDS registry, and HIV prevalence denominators modeled using non-parametric backcalculation.
Results
Of 42,582 TB cases, 178 (0.4%), 3,469 (8.1%), 635 (1.5%) were RMR-TB, IMR-TB, and MDR-TB, respectively. From the pre-HAART (1993–1996) to HAART (2005–2008) era, RMR-TB rates declined rapidly (12.0 vs. 0.5 per 100,000) among patients with HIV infection. The proportion of patients for whom rifampin resistance indicated RMR-TB (rather than MDR-TB) decreased from 31% (95% CI 26%–38%) to 11% (95% CI 5%–19%). In multivariate analysis controlling for HIV co-infection and other covariates, patients with RMR-TB were twice as likely to die as patients with drug sensitive TB (RR 1.94, 95% CI 1.40–2.69).
Conclusions
RMR-TB/HIV rates declined substantially over time in association with improved TB control and HIV control in California. Mortality among patients with RMR-TB was high, even after adjusting for HIV status.
Keywords: drug-resistant tuberculosis, human immunodeficiency virus (HIV), rifampin-monoresistant (RMR) tuberculosis, multidrug-resistant (MDR) tuberculosis
INTRODUCTION
Rifamycins are critical to the success of current tuberculosis (TB) chemotherapy regimens. The development of resistance to rifampin (RMP) necessitates the extension of treatment, is associated with poor treatment outcomes, [1, 2] and may lead sequentially to multidrug-resistant disease (MDR-TB). [3] Thus, the loss of RMP from treatment regimens has serious implications for individual patients and TB control programs.
Spontaneously occurring resistance-conferring mutations to RMP (more than 95% of which occur in an 81-bp region of rpoB) are less common than to all other first-line anti-TB drugs.[4] Global data on RMP mono-resistance (RMR) is scarce, though most national drug resistance surveys have found it to occur in fewer than 1% of TB cases.[5] RMR-TB presents substantial challenges for TB programs, however, and is increasingly identified in high TB incidence settings due to scale-up of rapid molecular tests.[6] Further, RMR-TB is associated with human immunodeficiency virus (HIV) co-morbidity, especially in the setting of intermittent TB treatment regimens, [7–10] and increasingly identified in high HIV-burden settings.[11, 12] Yet, the longitudinal relationship of incident drug-resistant TB (including RMR- and MDR-TB) and HIV burden has been infrequently examined on a population level.
California has the largest number of TB cases and the second highest number of persons living with HIV/AIDS in the United States. We describe trends, sociodemographic and clinical characteristics, and treatment outcomes of RMR-TB in California in relation to INH mono-resistant TB (IMR-TB), MDR-TB, and drug-susceptible TB during an era of dynamic change in population-level HIV burden.
METHODS
The study population included tuberculosis cases reported to the California Department of Public Health TB registry between January 1, 1993 and December 31, 2008. We excluded culture-negative cases and culture-positive cases without initial drug susceptibility testing (DST) results for at least isoniazid (INH), RMP, ethambutol (EMB), and pyrazinamide (PZA). DST was performed at local laboratories or at the California Microbial Diseases Laboratory using the BACTEC 460 (Becton Dickinson Diagnostic Instruments, Sparks, MD, USA), MGIT 960 (Becton Dickinson), or the agar proportion method.
TB patients with HIV co-infection were identified through a state-wide registry match with the California Office of AIDS using Registry Plus™ Link Plus software,[13] a probabilistic record linkage program developed by the U.S. Centers for Disease Control and Prevention (CDC). Registry cross-match criteria included name, sex, race/ethnicity, date and place of birth. Manual review of all matched cases was performed, with only those matches above a pre-determined priority threshold considered to represent a co-infected case.[14]
Overall state population denominators were based on California Department of Finance estimates.[15] California HIV prevalence estimates were obtained through nonparametric backcalculation of HIV incidence rates based on racial/ethnic-group-specific incidence counts of reported AIDS cases and reported AIDS deaths from 1981 to 2008 (see Supplementary text). This analysis was determined to be a public health analysis, which used de-identified and routinely collected surveillance and treatment data, and not subject to human subjects review (according to the U.S. Code of Federal Regulations, 45 CFR 46.101) by the Tuberculosis Control Branch, Division of Communicable Disease Control.
Definitions
A RMR-TB was a patient with a M. tuberculosis isolate from any anatomic site with resistance to RMP, with documented sensitivity to INH and without documented resistance to EMB or PZA. Similarly, an INH mono-resistant TB case (IMR-TB) was a patient with a M. tuberculosis isolate from any site with resistance to INH, with documented sensitivity to RIF and without documented resistance to EMB or PZA. A multidrug-resistant TB (MDR-TB) case was a patient with a M. tuberculosis isolate from any site with resistance to at least RMP and INH, regardless of additional drug resistance. We defined a drug-susceptible TB case as a patient with a M. tuberculosis isolate from any site with documented sensitivity to INH and RMP and no documented resistance to PZA or EMB. We defined acquired drug resistance as an initially drug-susceptible isolate that demonstrated drug resistance at the final reported drug susceptibility test within a single TB treatment course. Primary drug resistance was defined as patients with isolates having drug resistance at the reported initial drug susceptibility test. Among retreatment TB cases, lack of genotypic data precluded accurate differentiation between acquired drug resistance and reinfection with a drug resistant strain. Because expanded drug resistance in a subsequent TB episode is rare in California, [16] patients with drug resistance noted at initial drug susceptibility testing were considered to have “primary” drug resistance, regardless of history of prior TB diagnosis. Patients with both primary and acquired drug resistance were combined to assess trends in drug resistance. Timeframes for comparison were chosen to represent a “pre-Highly Active Antiretroviral Therapy (HAART)” (i.e., 1993–1996, prior to wide availability of HAART) and “HAART” era (i.e., 2005–2008, following the widespread availability of HAART).
Statistical analysis
Categorical data were analyzed by the χ2 test, or by calculating prevalence ratios (PRs), 95% confidence intervals (CIs), and P values for the comparison of RMR-, IMR- and MDR-with drug-susceptible TB. Differences in prevalence of binary covariates throughout the study period (1993–2008) were determined using logistic regression with robust standard errors. Associations with absolute mortality (death at diagnosis or at any time following diagnosis) adjusted for covariates based on a priori subject-matter knowledge (including age, sex, race/ethnicity, HIV status, foreign birth, self-administered treatment, and year of report) were examined using generalized linear models with a log link and robust standard errors. Differences in distribution of continuous variables were determined using the Wilcoxon rank sum test. All analyses were performed with Stata 12.1 (StataCorp., College Station, TX, USA).
RESULTS
Frequency and trends of drug-resistant TB in California, 1993–2008
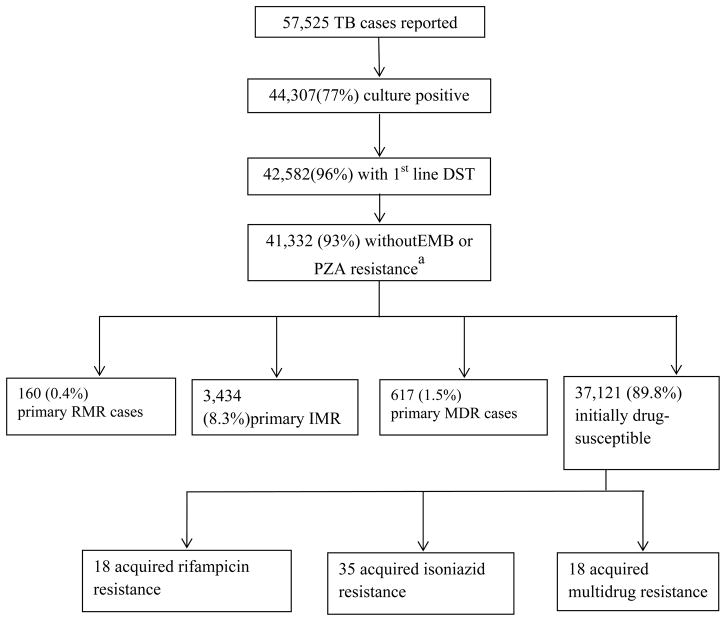
A total of 57,525 cases of TB were reported in California between 1993 and 2008, of which 44,307 (77%) were culture-confirmed. Of these, 42,582 (96%) had first-line DST results available. Characteristics of patients were similar for those with and without culture and DST performed (data not shown). Of isolates with available DST, 178 (0.4%) were RMR, 3,469 (8.0%) were IMR, and 635 (1.5%) were MDR (Figure 1). Acquired drug resistance was strikingly more common among individuals with RMR-TB (18%, n=18/178) than either IMR-TB (1.0%, n=35/3,469) or MDR-TB (2.8%, n=18/635) (p<0.001).
Figure 1. Study flow diagram.
Definition of Abbreviations: DST, drug susceptibility test; EMB, ethambutol; HIV, human immunodeficiency virus; IMR, isoniazid-resistant; IQR, interquartile range; MDR, multi drug-resistant; PZA, pyrazinamide; RIF, rifampicin; RMR, rifampicin mono-resistant; TB, tuberculosis.
a 1,250 TB cases with EMB or PZA resistance included 18 cases with rifampin resistance and 346 cases with isoniazid resistance
Among 3,254 (7.5% of total) culture-confirmed TB patients with HIV co-morbidity, 74 (2.3%) had RMR-TB/HIV, 172 (5.3%) had IMR-TB/HIV and 35 (1.1%) had MDR-TB/HIV. There was a greater decline in the incidence of RMR-TB/HIV from the pre-HAART era to the HAART era (12.0 per 100,000 vs. 0.5 per 100,000) than IMR-TB/HIV (18.9 per 100,000 vs. 8.9 per 100,000; p<0.001), MDR-TB/HIV (3.5 per 100,000 vs. 0.5 per 100,000; p<0.01), or RMR-TB among patients without HIV co-infection (0.038 per 100,000 vs. 0.009 per 100,000; p=0.02).
The proportion of all TB patients with RMP resistance who had RMR-TB rather than MDR-TB decreased from 31% (95% CI 26%–38%) in the pre-HAART era to 11% (95% CI 5%–19%) in the HAART era (p<0.001). This proportion decreased at a faster rate among HIV co-infected patients (−13% per year, 95% CI −18% to −7%) than among those without known HIV co-infection (−6% per year, 95% CI −10% to −2%). Seventy-eight percent (n=18/23) of rifampin-resistant isolates concurrently tested for rifabutin susceptibility were also rifabutin-resistant.
Sociodemographic characteristics
Sociodemographic characteristics of the study population are shown in Table 1. In univariate analysis, patients with primary RMR-TB were more likely than patients with drug-susceptible TB to be of Hispanic ethnicity (PR 1.39, 95% CI 1.01–1.90), black race (PR 1.76, 95% CI 1.18–2.64), incarcerated within the prior year (PR 2.32, 95% CI 1.31–4.11), or injection drug users (PR 1.94, 95% CI 1.02–3.68). Patients with primary RMR-TB were less likely than patients with drug-susceptible TB to be foreign-born (PR 0.50, 95% CI 0.37–0.69), or Asian (PR 0.39 95% CI 0.27–0.57). In contrast, patients with primary MDR-TB were less likely than patients with drug-susceptible TB to be male (PR 0.75, 95% CI 0.64–0.88), and more likely to be foreign-born (PR 2.52, 95% CI 1.99–3.19) or Asian (PR 1.97, 95% CI 1.68–2.31). Patients with IMR-TB had similar sociodemographic characteristics as those with MDR-TB (see Table 1). The majority of RMR-TB cases born outside the United States (57% of total) were born in Mexico (42%), Central America (10%), the Philippines (13%), and mainland Southeast Asia (12%). Foreign-born MDR-TB cases most often originated from Mexico (25%), the Philippines (21%) and mainland Southeast Asia (26%).
Table 1.
Sociodemographic Characteristics of Drug-Resistant and Drug-Susceptible Tuberculosis Cases, California, 1993–2008
| Characteristic | RMR (N=160) | MDR (N=617) | IMR (N=3,434) | Drug-susceptible (N=37,121) |
|---|---|---|---|---|
| Age, median years (IQR) | 41 (31.5–55.5)* | 40 (28–57)* | 43 (30–58)* | 47 (32–65) |
| Male | 112 (70.0) 1.37 (0.98–1.93) |
346 (56.1) 0.75 (0.64–0.88) |
2,094 (61.0) 0.92 (0.86–0.99) |
23,371 (63.0) Ref |
| Race/Ethnicity | ||||
| White, non-Hispanic | 28 (17.6) 1.47 (0.97–2.20) |
47 (7.7) 0.57 (0.42–0.77) |
227 (6.7) 0.49 (0.43–0.56) |
4,687 (12.7) Ref |
| Black, non-Hispanic | 29 (18.2) 1.76 (1.18–2.64) |
23 (3.8) 0.31 (0.20–0.47) |
194 (5.7) 0.48 (0.41–0.55) |
4,140 (11.2) Ref |
| Hispanic | 68 (42.8) 1.39 (1.01–1.90) |
189 (30.8) 0.83 (0.69–0.98) |
997 (29.2) 0.77 (0.71–0.83) |
12,896 (35.0) Ref |
| Asian/Pacific Islander | 34 (21.4) 0.39 (0.27–0.57) |
355 (57.8) 1.97 (1.68–2.31) |
1,993 (58.4) 2.01 (1.88–2.17) |
15,123 (41.0) Ref |
| Foreign birth | 91 (56.9) 0.50 (0.37–0.69) |
535 (86.7) 2.52 (1.99–3.19) |
2,905 (84.6) 2.15 (1.95–2.37) |
26,717 (72.0) Ref |
| Country or region of origina | ||||
| Mexico | 38 (41.8) 1.65 (1.08–2.50) |
134 (25.1) 0.77 (0.63–0.93) |
675 (23.2) 0.69 (0.63–0.76) |
8,110 (30.4) Ref |
| Central America | 9 (9.9) 1.75 (0.88–3.49) |
20 (3.7) 0.62 (0.39–0.97) |
115 (4.0) 0.66 (0.54–0.80) |
1,578 (5.9) Ref |
| The Philippines | 12 (13.2) 0.62 (0.34–1.14) |
111 (20.8) 1.07 (0.86–1.32) |
775 (26.7) 1.48 (1.36–1.62) |
5,271 (19.7) Ref |
| China | 2 (2.2) 0.32 (0.08–1.29) |
35 (6.5) 0.99 (0.70–1.39) |
164 (5.6) 0.84 (0.72–0.99) |
1,770 (6.6) Ref |
| Mainland Southeast Asia | 11 (12.1) 0.65 (0.35–1.22) |
137 (25.6) 1.63 (1.33–1.99) |
742 (25.5) 1.62 (1.49–1.78) |
4,657 (17.4) Ref |
| Sub-Saharan Africa | 4 (4.4) 2.45 (0.89–6.69) |
6 (1.1) 0.60 (0.27–1.34) |
34 (1.2) 0.63 (0.44–0.90) |
493 (1.9) Ref |
| Other | 15 (16.5) 0.89 (0.51–1.55) |
92 (17.2) 0.94 (0.75–1.17) |
400 (13.8) 0.72 (0.65–0.81) |
4,838 (18.1) Ref |
| Time from U.S. entry to TB diagnosis, median years (IQR)a | 6.2 (1.5–16.1) | 3.3 (0.2–9.6)* | 7.7 (2.1–16.2)* | 9.6 (2.7–19.3) |
| Homeless | 13 (8.1) 1.04 (0.59–1.83) |
29 (4.7) 0.58 (0.40–0.84) |
147 (4.3) 0.52 (0.44–0.62) |
2,915 (7.9) Ref |
| Incarcerated | 13 (8.1) 2.32 (1.31–4.11) |
14 (2.3) 0.61 (0.36–1.04) |
125 (3.6) 0.99 (0.82–1.20) |
1,361 (3.7) Ref |
| Injection-drug user | 10 (6.3) 1.94 (1.02–3.68) |
8 (1.3) 0.38 (0.19–0.77) |
57 (1.7) 0.49 (0.38–0.64) |
1,235 (3.3) Ref |
| Excess alcohol use | 20 (12.5) 1.01 (0.63–1.62) |
46 (7.5) 0.57 (0.42–0.77) |
304 (8.9) 0.69 (0.61–0.77) |
4,589 (12.4) Ref |
| Time period | ||||
| 1993–1996 | 89 (55.6) 2.73 (1.99–3.73) |
202 (32.7) 1.06 (0.89–1.26) |
1,041 (30.3) 0.95 (0.88–1.02) |
11,683 (31.5) Ref |
| 1997–2008 | 71 (44.4) 0.37 (0.27–0.50) |
415 (67.3) 0.94 (0.80–1.12) |
2,393 (69.7) 1.06 (0.98–1.14) |
25,438 (68.5) Ref |
Definition of abbreviations: IMR, isoniazid-resistant; IQR, interquartile range; MDR, multi drug-resistant; Ref, reference group; RMR, rifampin mono-resistant; TB, tuberculosis.
All values are No. (%) / Prevalence Ratio (95% CI), unless otherwise noted. All categories of drug resistance exclude those who acquired drug resistance during therapy. The denominator for each characteristic excludes missing or unknown values.
p <0.001 difference in medians relative to drug-susceptible.
Calculated for foreign-born individuals only.
Clinical and treatment characteristics
In univariate analysis, patients with primary RMR-TB were seven times more likely than drug-susceptible TB patients to be co-infected with HIV (PR 7.23, 95% CI 5.23–9.99), while patients with primary MDR-TB (PR 0.54, 95% CI 0.37–0.81) or primary IMR-TB (PR 0.63, 95% CI 0.54–0.74) were less likely to be co-infected with HIV (Table 1). Of 160 primary RMR-TB patients, 23 (14.5%) reported a previous diagnosis of TB occurring a median of 3.5 years (IQR 2.4–6.2 years) prior to the index diagnosis. In comparison, prior TB diagnoses were noted among 186 (30.4%) of primary MDR-TB and 279 (8.2%) of primary IMR-TB patients. Among patients with a documented culture conversion, the median time to conversion was longer for RMR-TB and MDR-TB (94 days and 80 days, respectively) than for IMR-TB and drug-susceptible TB (48 days and 50 days, respectively). Patients with MDR-TB (PR 2.02, 95% CI 1.71–2.40) were more likely to have cavitary lung disease than patients with drug-susceptible TB, a finding not demonstrated among those with RMR-TB (PR 1.04, 95% CI 0.70–1.53). Patients with MDR-TB (PR 0.30, 95% CI 0.23–0.39) and IMR-TB (PR 0.83, 95% CI 0.77–0.90) were less likely to have received self-administered therapy (SAT) throughout their treatment course relative to patients with drug-susceptible TB; patients with RMR-TB had a similar probability as those with drug-susceptible TB to have self-administered therapy (PR 0.92, 95% CI 0.66–1.27) (Table 2).
Table 2.
Clinical Characteristics Drug-Resistant and Drug-Susceptible Tuberculosis, California, 1993–2008
| Characteristic | RMR (N=160) | MDR (N=617) | IMR (N=3,434) | Drug-susceptible (N=37,121) |
|---|---|---|---|---|
| HIV co-infection | 59 (36.9) 7.23 (5.23–9.99) |
26 (4.2) 0.54 (0.37–0.81) |
167 (4.9) 0.63 (0.54–0.74) |
2,775 (7.5) Ref |
| Sputum AFB smear-positive | 74 (68.5) 1.35 (0.95–1.91) |
359 (67.8) 1.67 (1.41–1.99) |
1,535 (58.8) 1.08 (1.01–1.17) |
15,131 (57.9) Ref |
| Cavitary disease | 32 (29.1) 1.04 (0.70–1.53) |
198 (36.4) 2.02 (1.71–2.40) |
707 (25.9) 1.12 (1.02–1.22) |
6,909 (24.9) Ref |
| Extrapulmonary TB Disease Site | 50 (31.3) 1.35 (0.96–1.88) |
73 (11.8) 0.40 (0.31–0.51) |
704 (20.5) 0.76 (0.70–0.83) |
9,363 (25.2) Ref |
| Previous TB diagnosis | 23 (14.5) 2.75 (1.77–4.29) |
186 (30.4) 7.11 (5.95–8.49) |
279 (8.2) 1.45 (1.27–1.65) |
2,133 (5.8) Ref |
| Culture conversion | 91 (77.1) 0.91 (0.59–1.40) |
397 (84.8) 1.51 (1.17–1.95) |
2,189 (82.9) 1.32 (1.18–1.40) |
21,190 (78.7) Ref |
| Time to culture conversion, median days (IQR)a | 93.5 (42.0–162.0)* | 79.5 (38.0–144.0)* | 48 (26.0–84.0) | 50 (27.0–81.0) |
| Self-administered therapy | 51 (31.5) 0.92 (0.66–1.27) |
71 (13.6) 0.30 (0.23–0.39) |
974 (30.1) 0.83 (0.77–0.90) |
11,678 (34.1) Ref |
| Duration of therapy, median days (IQR)b | 531 (371.0–582.0)* | 749 (606.0–834.0)* | 289 (223.0–375.5)* | 252 (192.0–316.0) |
| Time to culture conversion, median days (IQR)a | 93.5 (42.0–162.0)* | 79.5 (38.0–144.0)* | 48 (26.0–84.0) | 50 (27.0–81.0) |
| Reason therapy stopped | ||||
| Completed treatment | 97 (65.5) 0.40 (0.28–0.55) |
354 (68.5) 0.47 (0.39–0.57) |
2,765 (84.6) 1.18 (1.07–1.30) |
28,867 (82.5) Ref |
| Died | 33 (22.53) 2.82 (1.92–4.15) |
72 (14.13.9) 1.58 (1.23–2.03) |
223 (6.98) 0.73 (0.63–0.83) |
3,196 (9.21) Ref |
| Default | 7 (4.7) 1.79 (0.83–3.81) |
20 (3.9) 1.47 (0.93–2.30) |
83 (2.5) 0.94 (0.75–1.18) |
940 (2.7) Ref |
| Transferred | 10 (6.8) 1.32 (0.69–2.51) |
63 (12.2) 2.57 (1.97–3.36) |
178 (5.5) 1.05 (0.90–1.23) |
1,811 (5.2) Ref |
Definition of abbreviations: IMR, isoniazid-resistant; IQR, interquartile range; MDR, multi drug-resistant; Ref, reference group; RMR, rifampin mono-resistant; TB, tuberculosis.
All values are No. (%) / Prevalence Ratio (95% CI), unless otherwise noted. All categories of drug resistance exclude those who acquired drug resistance during therapy. The denominator for each characteristic excludes missing or unknown values.
p <0.001 difference in medians relative to drug-susceptible.
Calculated for individuals who cultured-converted only.
Calculated for individuals who completed therapy only.
HIV co-infection
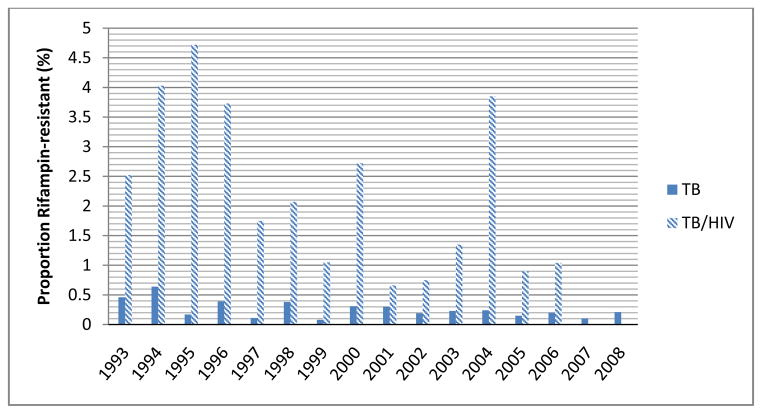
The percentage of RMR-TB patients with HIV co-infection decreased from 1993 to 2008, while the percentage of IMR-, MDR-, and drug susceptible-TB patients with known HIV co-infection, and RMR-TB without HIV did not substantially change (p<0.05; Figure 2). RMR-TB/HIV patients were more likely to have had a prior TB diagnosis (PR 3.06, 95% CI 1.48–6.33), to be male (PR 1.48, 95% CI 1.07–2.05), and to have died during anti-TB therapy (PR 3.03, 95% CI 1.90–4.81) relative to patients with drug-susceptible TB/HIV (See Supplementary Table 1). Among 74 patients with RMR-TB/HIV, 31 (42%) died while on anti-TB therapy, and 2 (3%) died prior to initiating treatment.
Figure 2.
Proportion of TB cases with Rifampin Resistance, California, 1993–2008
Treatment outcomes
Treatment outcomes were available for 92% (n=147/160) of primary RMR-TB, 82% (n=509/617) of primary MDR-TB, 95% (n=3249/3434) of primary IMR-TB, and 94% (n=34,814/37,121) of drug-susceptible TB cases. Among those with complete data, RMR-TB patients and MDR-TB patients were significantly less likely to complete treatment than drug-susceptible cases (PR 0.40, 95% CI 0.28–0.55 and PR 0.47, 95% CI 0.39–0.57, respectively). Unadjusted mortality during TB treatment was higher for RMR-TB (22.5%, n=33/147) than MDR-TB (14.1%, n=72/509; p=0.01), IMR-TB (6.9%, n=223/3249; p<0.001), or drug-susceptible TB (9.2%, n=3196/34814; p<0.001). Unadjusted RMR-TB mortality was substantially higher in the pre-HAART (36.2%; n=34/94) relative to HAART era (12.5%; n=9/72; p<0.001). In multivariate analysis adjusting for age, sex, race/ethnicity, foreign birth, HIV status, AFB smear status, therapy administration, and year of diagnosis, death during treatment was more likely among patients with RMR-TB (RR 1.94, 95% CI 1.40–2.69) and MDR-TB (RR 2.24 95% CI 1.78–2.82) compared with drug-susceptible TB (Table 3). Death was similar among patients with IMR-TB (RR 1.01, 95% CI 0.88–1.15) and drug-susceptible TB.
Table 3.
Drug-Resistance and Other Factors Associated With Mortality among Tuberculosis Patients, California, 1993–2008
| Characteristic | Adjusted Relative Risk (95% CI) |
|---|---|
| Drug-resistance pattern | |
| RMR | 1.94 (1.40–2.69) |
| MDR | 2.24 (1.78–2.82) |
| IMR | 1.01 (0.88–1.15) |
| Drug-susceptible | Ref |
| Known HIV co-infection | 4.19 (3.76–4.65) |
| Year a | 0.99 (0.98–0.99) |
| Age b | 1.68 (1.65–1.72) |
| Male | 1.14 (1.06–1.23) |
| Race/ethnicity | |
| White/non-Hispanic | Ref |
| Black/non-Hispanic | 1.14 (1.01–1.29) |
| Hispanic | 1.09 (0.97–1.22) |
| Asian/Pacific Islander | 0.97 (0.86–1.10) |
| Foreign birth | 0.63 (0.57–0.70) |
| Self-administered treatment c | 0.94 (0.86–1.02) |
| Sputum smear-positive | 1.26 (1.18–1.36) |
Definition of abbreviations: IMR, isoniazid-resistant; HIV, human immunodeficiency virus; IQR, interquartile range; MDR, multi drug-resistant; Ref, reference group; RMR, rifampin mono-resistant; TB, tuberculosis.
All categories of drug resistance exclude those who acquired drug resistance during therapy.
Per year for time interval 1993–2008.
Per 10 year increase in age.
Reference group included individuals with DOT of any duration during treatment.
DISCUSSION
We analyzed patients with TB and TB/HIV in a population of approximately 38 million over a 16-year period to describe demographic and clinical trends associated with drug-resistant TB and its intersection with California’s HIV epidemic. We found a striking decline of RMR-TB among HIV co-infected patients that was more prominent than the decline of drug-susceptible TB and all other forms of drug-resistant TB. Death was a frequent outcome for patients with RMR-TB, especially prior to the availability of HAART.
We document a decreasing incidence in RMR-TB temporally associated with population-level improvements in HIV care (e.g., availability of HAART, improved HIV testing/awareness),[17] and improvements in programmatic TB control.[18] TB treatment and control interventions may have differentially influenced RMR-TB over other forms of drug-resistant TB because of increased use of directly observed therapy and daily, rather than intermittent, anti-TB treatment regimens among patients with HIV co-infection.[19] Two trials examining rifapentine and rifabutin (Tuberculosis Trials Consortium (TBTC)/US Public Health Service Study 22 and Study 23) recruited patients with TB/HIV co-morbidity in California and were associated with acquired rifamycin resistance.[8, 20, 21] Genotyping data from the period in question is unavailable, however, and the contribution of these cases to downstream RMR-TB incidence is unknown. While particular rifampin-resistance conferring mutations predict the likelihood of rifampin-resistant/rifabutin-sensitive strains [22] or low-level rifampin resistance, [23] the role of rifabutin or high-dose rifampin in clinical management remains unclear.
As in previous studies, [9, 10, 20, 24] we found a strong association of RMR with HIV co-infection in California. The causal determinants of this relationship likely include host factors (e.g., malabsorption, immunosuppression leading to higher disease burden), intermittent treatment regimens, and/or drug interactions leading to functional monotherapy. [20, 25–27] That the propensity for acquired rifampin resistance is high is supported by our data; such acquired resistance would be preferentially transmitted among persons at high risk for early progression to active disease. An additional hypothesis that relatively less fit organisms are able to propagate in the setting of severe immunocompromise [3] is less persuasive given the lack of fitness cost associated with the rpoB S531L mutation, [28, 29] worldwide the most common RMP resistance-conferring mutation. Within the subgroup of patients with HIV co-infection in California, those with RMR-TB differed from those with drug-susceptible disease in being more likely to have had a previous diagnosis of TB, less likely to have been foreign-born or female, and more likely to have had poor treatment outcomes.
The ongoing scale-up of rapid molecular drug-susceptibility tests provides an opportunity to better determine the burden of RMR-TB in diverse settings, including sub-Saharan Africa where rates may be increasing due to expansion of continuation-phase rifamycins and high HIV prevalence.[11, 12] GeneXpert MTB/RIF® (Cepheid Diagnostics, Sunnyvale, CA), an automated nucleic acid amplification assay, has been implemented in 67 low- and middle-income countries since WHO endorsement in 2010, identifying more than 4,500 additional rifampin-resistant cases in 2011 alone.[6] The WHO recommendation for immediate initiation of MDR-TB regimens including INH for patients at moderate or high risk of drug resistance detected as RMP-resistant [30] is supported by our finding of similar adverse treatment outcome among patients with RMR- and MDR-TB. [31] Cohort studies examining clinical outcomes of patients with rifampin resistance detected by rapid molecular tests are needed.
Initial rifampin resistance as identified by rapid molecular tests is a surrogate marker for MDR-TB in 73–90% of individuals, depending on regional prevalence and pre-test probability.[32] In California, the proportion of RMP-resistant INH-susceptible isolates decreased substantially during an era when the MDR-TB prevalence remained low, in particular among those with HIV co-morbidity. Population-level changes in the proportion of HIV-infected individuals who have AIDS or are being treated with antiretroviral medications may be an additional consideration in determining the predictive value of Xpert® MTB/RIF-detected RMP-resistance for MDR-TB, and RMR-TB rates may be an indicator of the success of TB and HIV control activities.
Our study has some potential limitations. HIV infection was determined via a match between California Department of Public Health HIV/AIDS and TB surveillance data; misclassification may have occurred but likely declined in a time-dependent fashion.[33] Level of immunosuppression (e.g., CD4+ T-lymphocyte count) and antiretroviral treatment data were unavailable. Since any death that occurred in a TB patient was reported, an unknown but likely small proportion of deaths included in the analysis were unrelated to TB. Additionally, since treatment outcomes data were incomplete, particularly for those with MDR-TB, we may have differentially underestimated the frequency of death for MDR-TB patients on anti-TB therapy.
In conclusion, in a large, ethnically diverse population in a low-TB-incidence setting, we found a striking decline of RMR-TB temporally associated with improvements in care of TB-HIV patients and declines in incident AIDS cases. Although poor treatment outcomes, particularly the increased risk of death, suggests potential missed prevention opportunities, the risk of death was high regardless of case management techniques (i.e. use of directly observed therapy) and presence of HIV co-infection. Current investigation of TB deaths on a national level may provide further insight into prevention of these deaths. [34] These findings serve as a reminder that rifamycin-resistant TB requires effective treatment with rigorous monitoring.
Supplementary Material
Acknowledgments
This work was supported in part by the National Institutes of Health (K23 AI094251 to J.Z.M., R24TW008822 to L.M.P., and R01 AI076476 to P.C.H.) and the Robert Wood Johnson Foundation (AMFDP Medical Faculty Development Award to J.Z.M.).
We thank the public health departments in California who reported the data used in this analysis.
Footnotes
Potential conflicts of interest: no reported conflicts (all authors).
References
- 1.Mitchison DA, Nunn AJ. Influence of initial drug resistance on the response to short-course chemotherapy of pulmonary tuberculosis. Am Rev Respir Dis. 1986;133:423–430. doi: 10.1164/arrd.1986.133.3.423. [DOI] [PubMed] [Google Scholar]
- 2.Espinal MA, Kim SJ, Suarez PG, Kam KM, Khomenko AG, Migliori GB, et al. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. JAMA. 2000;283:2537–2545. doi: 10.1001/jama.283.19.2537. [DOI] [PubMed] [Google Scholar]
- 3.Bifani P, Mathema B, Kurepina N, Shashkina E, Bertout J, Blanchis AS, et al. The evolution of drug resistance in Mycobacterium tuberculosis: from a mono-rifampin-resistant cluster into increasingly multidrug-resistant variants in an HIV-seropositive population. J Infect Dis. 2008;198:90–94. doi: 10.1086/588822. [DOI] [PubMed] [Google Scholar]
- 4.David HL. Probability distribution of drug-resistant mutants in unselected populations of Mycobacterium tuberculosis. Appl Microbiol. 1970;20:810–814. doi: 10.1128/am.20.5.810-814.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. Anti-tuberculosis drug resistance in the world 4th global report. Geneva, Switzerland: WHO; 2008. p. 152. [Google Scholar]
- 6.World Health Organization. [November 28, 2012];Global Tuberculosis Report. 2012 Accessed at http://www.who.int/tb/publications/global_report/en/ on.
- 7.Ridzon R, Whitney CG, McKenna MT, Taylor JP, Ashkar SH, Nitta AT, et al. Risk factors for rifampin mono-resistant tuberculosis. Am J Respir Crit Care Med. 1998;157:1881–1884. doi: 10.1164/ajrccm.157.6.9712009. [DOI] [PubMed] [Google Scholar]
- 8.Vernon A, Burman W, Benator D, Khan A, Bozeman L. Acquired rifamycin monoresistance in patients with HIV-related tuberculosis treated with once-weekly rifapentine and isoniazid. Tuberculosis Trials Consortium. Lancet. 1999;353:1843–1847. doi: 10.1016/s0140-6736(98)11467-8. [DOI] [PubMed] [Google Scholar]
- 9.Munsiff SS, Joseph S, Ebrahimzadeh A, Frieden TR. Rifampin-monoresistant tuberculosis in New York City, 1993–1994. Clin Infect Dis. 1997;25:1465–1467. doi: 10.1086/516146. [DOI] [PubMed] [Google Scholar]
- 10.Sandman L, Schluger NW, Davidow AL, Bonk S. Risk factors for rifampin-monoresistant tuberculosis: A case-control study. Am J Respir Crit Care Med. 1999;159:468–472. doi: 10.1164/ajrccm.159.2.9805097. [DOI] [PubMed] [Google Scholar]
- 11.Mukinda FK, Theron D, van der Spuy GD, Jacobson KR, Roscher M, Streicher EM, et al. Rise in rifampicin-monoresistant tuberculosis in Western Cape, South Africa. Int J Tuberc Lung Dis. 2012;16:196–202. doi: 10.5588/ijtld.11.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dramowski A, Morsheimer MM, Jordaan AM, Victor TC, Donald PR, Schaaf HS. Rifampicin-monoresistant Mycobacterium tuberculosis disease among children in Cape Town, South Africa. Int J Tuberc Lung Dis. 2012;16:76–81. doi: 10.5588/ijtld.11.0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Link Plus Version 2.10 probabilistic record linkage software. Atlanta, GA: Centers for Disease Control and Prevention; 2006. [Google Scholar]
- 14.Xia QWJ, Schultz A, Nonoyama A, Elms W, Wu N, Tabshouri L, Ruiz J, Flood J. Matching AIDS and tuberculosis registry data to identify AIDS/tuberculosis comorbidity cases in California. Health Informatics Journal. 2011;17:41–50. doi: 10.1177/1460458210380524. [DOI] [PubMed] [Google Scholar]
- 15.State of California, Department of Finance, Race/Ethnic Population with Age and Sex Detail, 1990–1999 and 2000–2050. Sacramento, CA: May, 2009. [Google Scholar]
- 16.Pascopella L, Deriemer K, Watt JP, Flood JM. When tuberculosis comes back: who develops recurrent tuberculosis in california? PLoS One. 2011;6:e26541. doi: 10.1371/journal.pone.0026541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du P, Camacho F, Zurlo J, Lengerich EJ. Human immunodeficiency virus testing behaviors among US adults: the roles of individual factors, legislative status, and public health resources. Sex Transm Dis. 2011;38:858–864. doi: 10.1097/OLQ.0b013e31821a0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaulk CP, Moore-Rice K, Rizzo R, Chaisson RE. Eleven years of community-based directly observed therapy for tuberculosis. JAMA. 1995;274:945–951. [PubMed] [Google Scholar]
- 19.Acquired rifamycin resistance in persons with advanced HIV disease being treated for active tuberculosis with intermittent rifamycin-based regimens. MMWR Morb Mortal Wkly Rep. 2002;51:214–215. [PubMed] [Google Scholar]
- 20.Burman W, Benator D, Vernon A, Khan A, Jones B, Silva C, et al. Acquired rifamycin resistance with twice-weekly treatment of HIV-related tuberculosis. Am J Respir Crit Care Med. 2006;173:350–356. doi: 10.1164/rccm.200503-417OC. [DOI] [PubMed] [Google Scholar]
- 21.Benator D, Bhattacharya M, Bozeman L, Burman W, Cantazaro A, Chaisson R, et al. Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomised clinical trial. Lancet. 2002;360:528–534. doi: 10.1016/s0140-6736(02)09742-8. [DOI] [PubMed] [Google Scholar]
- 22.Tan Y, Hu Z, Zhao Y, Cai X, Luo C, Zou C, et al. The beginning of the rpoB gene in addition to the rifampin resistance determination region might be needed for identifying rifampin/rifabutin cross-resistance in multidrug-resistant Mycobacterium tuberculosis isolates from Southern China. J Clin Microbiol. 2012;50:81–85. doi: 10.1128/JCM.05092-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pang Y, Lu J, Wang Y, Song Y, Wang S, Zhao Y. Study of the rifampin monoresistance mechanism in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2013;57:893–900. doi: 10.1128/AAC.01024-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swaminathan S, Narendran G, Venkatesan P, Iliayas S, Santhanakrishnan R, Menon PA, et al. Efficacy of a 6-month versus 9-month intermittent treatment regimen in HIV-infected patients with tuberculosis: a randomized clinical trial. Am J Respir Crit Care Med. 2010;181:743–751. doi: 10.1164/rccm.200903-0439OC. [DOI] [PubMed] [Google Scholar]
- 25.Weiner M, Benator D, Burman W, Peloquin CA, Khan A, Vernon A, et al. Association between acquired rifamycin resistance and the pharmacokinetics of rifabutin and isoniazid among patients with HIV and tuberculosis. Clin Infect Dis. 2005;40:1481–1491. doi: 10.1086/429321. [DOI] [PubMed] [Google Scholar]
- 26.Chideya S, Winston CA, Peloquin CA, Bradford WZ, Hopewell PC, Wells CD, et al. Isoniazid, rifampin, ethambutol, and pyrazinamide pharmacokinetics and treatment outcomes among a predominantly HIV-infected cohort of adults with tuberculosis from Botswana. Clin Infect Dis. 2009;48:1685–1694. doi: 10.1086/599040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gurumurthy P, Ramachandran G, Hemanth Kumar AK, Rajasekaran S, Padmapriyadarsini C, Swaminathan S, et al. Decreased bioavailability of rifampin and other antituberculosis drugs in patients with advanced human immunodeficiency virus disease. Antimicrob Agents Chemother. 2004;48:4473–4475. doi: 10.1128/AAC.48.11.4473-4475.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gagneux S, Long CD, Small PM, Van T, Schoolnik GK, Bohannan BJ. The competitive cost of antibiotic resistance in Mycobacterium tuberculosis. Science. 2006;312:1944–1946. doi: 10.1126/science.1124410. [DOI] [PubMed] [Google Scholar]
- 29.Comas I, Borrell S, Roetzer A, Rose G, Malla B, Kato-Maeda M, et al. Whole-genome sequencing of rifampicin-resistant Mycobacterium tuberculosis strains identifies compensatory mutations in RNA polymerase genes. Nat Genet. 2012;44:106–110. doi: 10.1038/ng.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weyer K, Mirzayev F, Migliori G, Van Gemert W, D’Ambrosio L, Zignol M, et al. Rapid molecular TB diagnosis: evidence, policy-making and global implementation of Xpert(R)MTB/RIF. Eur Respir J. 2012 doi: 10.1183/09031936.00157212. [DOI] [PubMed] [Google Scholar]
- 31.Van Rie A, Mellet K, John MA, Scott L, Page-Shipp L, Dansey H, et al. False-positive rifampicin resistance on Xpert(R) MTB/RIF: case report and clinical implications. Int J Tuberc Lung Dis. 2012;16:206–208. doi: 10.5588/ijtld.11.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith SE, Kurbatova EV, Cavanaugh JS, Cegielski JP. Global isoniazid resistance patterns in rifampin-resistant and rifampin-susceptible tuberculosis. Int J Tuberc Lung Dis. 2011 doi: 10.5588/ijtld.11.0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vital signs: HIV testing and diagnosis among adults--United States, 2001–2009. MMWR. Morbidity and mortality weekly report. 2010;59:1550–1555. [PubMed] [Google Scholar]
- 34.Beavers S, Flood J, Weinfurter P, Davidow A, Hirsch-Moverman Y, Thickstun P, Munguia G, Kundapati S, Mangan J, Stein-Hart T, Belknap R, Graviss E. Tuberculosis mortality in the United States: how can it be prevented?. American Throacic Society 2012 International Conference; San Francisco, California. 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.