Abstract
DWP208 is a sodium succinate form of ZYM-201 which is a triterpenoid glycoside isolated from Sanguisorba officinalis, a medicinal plant prescribed for various diseases, such as duodenal ulcers and bleeding in East Asian counties. We demonstrated that this compound is able to normalize the altered lipid metabolism induced by hyperglycemia and a high fat diet. In this study, we determined whether hyperlipidemic conditions induced with chronically treated alcohol can also be restored by DWP208. Similar to our previous results, orally administered DWP208 (1 to 10 mg/kg) also ameliorated the hyperlipidemia that was induced by alcohol. This compound reversed the alcohol-induced hyperlipidemia including (i) up-regulated hyperlipidemic parameters such as low-density lipoprotein (LDL), very low-density lipoprotein (VLDL), atherosclerotic index (AI), triglyceride, and total cholesterol, and (ii) down-regulated hyperlipidemic parameters such as absolute body weight, superoxide dismutase (SOD) activity, and high-density lipoprotein (HDL) in serum and liver. According to our data, the ameliorative activity of DWP208 is due to its indirect anti-oxidative activity as a result of which lipid peroxide and hydroxyl radical levels were reduced and the activity of SOD was enhanced. Therefore, our data strongly suggest that DWP208 can be used as a remedy against alcohol-induced hyperlipidemia.
Keywords: Anti-hyperlipidemic activity, Chronic alcohol treatment, DWP208, Sanguisorba officinalis, Triterpenoid glycoside
INTRODUCTION
Chronic ethanol intake is a serious social problem since it increases social expenses in terms of health and family problems. Recently, a great increase in the number of Koreans who chronically consume ethanol has been associated with such problems. One health problem with ethanol intake is dysregulated hepatic lipid metabolism. For example, patients abusing alcohol are observed to have triglyceride accumulation in their hepatocytes, a critical process in disease progression leading to late stage hepatitis and cirrhosis [1,2,3]. Abnormal lipid metabolism includes fat accumulation in the liver caused by an imbalance between the degradation and synthesis of fatty acids. Development of hepatic steatosis is therefore initially seen in deleterious effects of excessive alcohol intake [4,5,6]. In addition, hypertriglyceridemia (increased blood triglyceride levels) caused by elevated very low-density lipoprotein and chylomicron levels in the blood [7], and hypercholesterolemia caused by enhanced cholesterol biosynthesis and decreased bile acid excretion [8] were observed in chronic alcohol consumption patients.
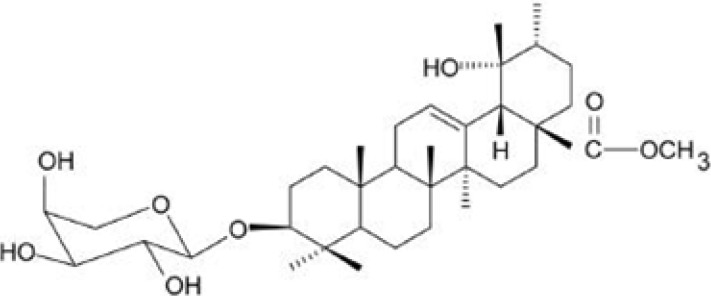
DWP208, a sodium succinate form of ZYM-201 (Fig. 1), is a saponin derivative obtained from a ziyu-glycoside of Sanguisorba officinalis L. (Rosaceae). This compound has been reported to be an orally available drug. Orally administered DWP208 dose-dependently inhibited in vivo tissue factor (TF) activity with an ED50 of 1.7 mg/kg [9]. Furthermore, ZYM-201 diminished both in vitro and in vivo tumor necrosis factor (TNF)-production with an IC50 and ED50 of 69.4 M and 87.4 mg/kg, respectively [9], suggesting that DWP208 may have therapeutic activity against tissue TF- and TNF-mediated diseases such as septic shock and against hyperlipidemic conditions. Indeed, this compound strongly attenuated the hyperlipidemia induced by diabetic and high fat diet conditions as did other saponin components from Sapindus rarak DC, floratheasaponins, and Eugenia jambolana [10]. However, because a major cause of hyperlipidemia is chronic alcohol intake, we investigated the curative activity of DWP208 in a rat model of chronic alcohol consumption.
Fig. 1.
Chemical structure of DWP208.
METHODS
Materials
DWP208, a sodium succinate form of ZYM-201 (ziyuglycoside II methylester) [9], was synthesized by adding succinic anhydride and sodium-2-ethylhexanoic acid. The yield of DWP208 was 83% and its chromatographic purity was greater than 95.3% according to HPLC analysis. Fenofibrate, cholesterol, (3-4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a tetrazole (MTT), succinic anhydride, and sodium-2-ethylhexanoic acid were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Absolute alcohol was purchased from Fluka (Buchs, Switzerland). All other chemicals and reagents used were of pharmaceutical grade. Fetal bovine serum and RPMI1640 were obtained from GIBCO (Grand Island, NY, USA). RAW264.7 cells were purchased from ATCC (Rockville, MD, USA).
Animals
Male Sprague-Dawley (SD) rats weighing 150-160 g were purchased from Daehan Biolink (Daejeon, Korea) and were given ad libitum access to a commercial diet (Samyang, Korea) and water. They were housed in a temperature-controlled room at 20℃ with lighting on for 12 h. Humidity was maintained between 50 and 70%. Our study was approved by the Animal Ethics Committee of Kangwon National University (Chuncheon, Korea) and was performed in accordance with the "Guide for the Care and Use of Laboratory Animals" published by the Korea National Institute of Health.
Induction of hyperlipidemic conditions
To induce hyperlipidemic conditions, 10% (w/w) aqueous ethanol was chronically treated for 8 weeks as reported previously [11]. At the end of the treatment, using these rats, DWP208 (0 to 10 mg/kg) or fenofibrate (5 mg/kg) were orally administered once a day for 1 week. According to previous methods [12], animals were anesthetized and blood was drawn by cardiac puncture 2 h after the final drug administration. Serum was obtained by centrifugation of blood at 3,000 rpm for 15 min.
Biochemical analysis of serum
Serum was separated by centrifugation at 1,000 g for 15 min. The levels of total cholesterol, total triglyceride, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and very low-density lipoprotein (VLDL) were determined using an automatic analyzer (Hitachi 7020, Japan) [13]. Determination of hydroxyl radicals was done by a method previously reported [14]. Lipase activity in serum was also determined by a method previously published [15]. Lipid peroxidation levels in liver and serum were measured using an assay for thiobarbituric acid reactive substances (TBARS) [16]. SOD activity was assayed in the serum and liver using a technique that involves inhibition of pyrogallol auto oxidation at pH 8.0 [17].
Biochemical analysis of liver samples
Liver samples for enzymatic analyses were prepared according to the methods of a previous report [18]. Each liver sample (1 g) was homogenized (10%, w/v) in four volumes of 0.1 M potassium phosphate buffer (pH 7.5), then centrifuged at 600 g for 10 min, 10,000 g for 20 min, and 105,000 g for 60 min to prepare the microsomal fraction. The microsomal fraction was used for the assay of 3-hydroxy-3-methyl-glutaryl (HMG)-CoA reductase. Total lipids, total cholesterol, and triglyceride in the liver homogenate prepared with 0.9% NaCl were enzymatically determined using assay kits (Asan Pharmacy, Seoul, Korea). Protein concentrations were determined by the Bradford method [19] using BSA as the standard.
Statistical analysis
Student's t-test and a one-way ANOVA were used to determine the statistical significance of differences between values for the various experimental and control groups. Data are expressed as mean±standard error (SEM) and the results are taken from at least four independent experiments performed in 10 rats. p<0.05 was considered to be statistically different unless otherwise indicated.
RESULTS AND DISCUSSION
Since we found that DWP208, a water-soluble salt form of ZYM-201, is able to normalize the up-regulated levels of hyperlipidemic parameters in an animal model of streptozotocin-induced diabetes that was simultaneously fed a high fat diet [20], we examined the effects of DWP208 on alcohol-induced hyperlipidemia. Chronic alcohol addiction is more prevalent in the Korean population and causes serious effects such as liver damage, leading to the generation of fatty liver, cirrhosis, and liver cancer. Although getting the patient to abstain from alcohol drinking would be ideal, alcohol-induced liver damage and subsequent malfunction of liver metabolism, causing the generation of various vascular diseases, such as atherosclerosis and stroke should be restored. To date, no drug has been reported to be a strong candidate for treating these symptoms of chronic alcohol intake. In the current study, we investigated the possibility that DWP208 can be used as a remedy to ameliorate alcohol-induced liver dysfunction in an animal model of alcohol-induced hyperlipidemia.
During chronic alcohol (10%) treatment, it has been shown that liver function and lipid metabolism are altered. Thus, alcohol treated individuals display striking hyperlipidemic symptoms such as (i) up-regulated levels of LDL, VLDL, atherosclerosis index (AI), serum triglyceride and total cholesterol, hepatic total lipid, cholesterol, and triglyceride levels, and serum lipoproteins and HMG-CoA reductase contents (Fig. 2, 3, 4, 5, Table 1), and (ii) down-regulated levels of body weight, HDL, and SOD activity (Table 2, Fig. 2A, and Fig. 5C). The alcohol-induced up-regulation and down-regulation of these parameters were previously reported [21,22], indicating that our experimental plan was feasible.
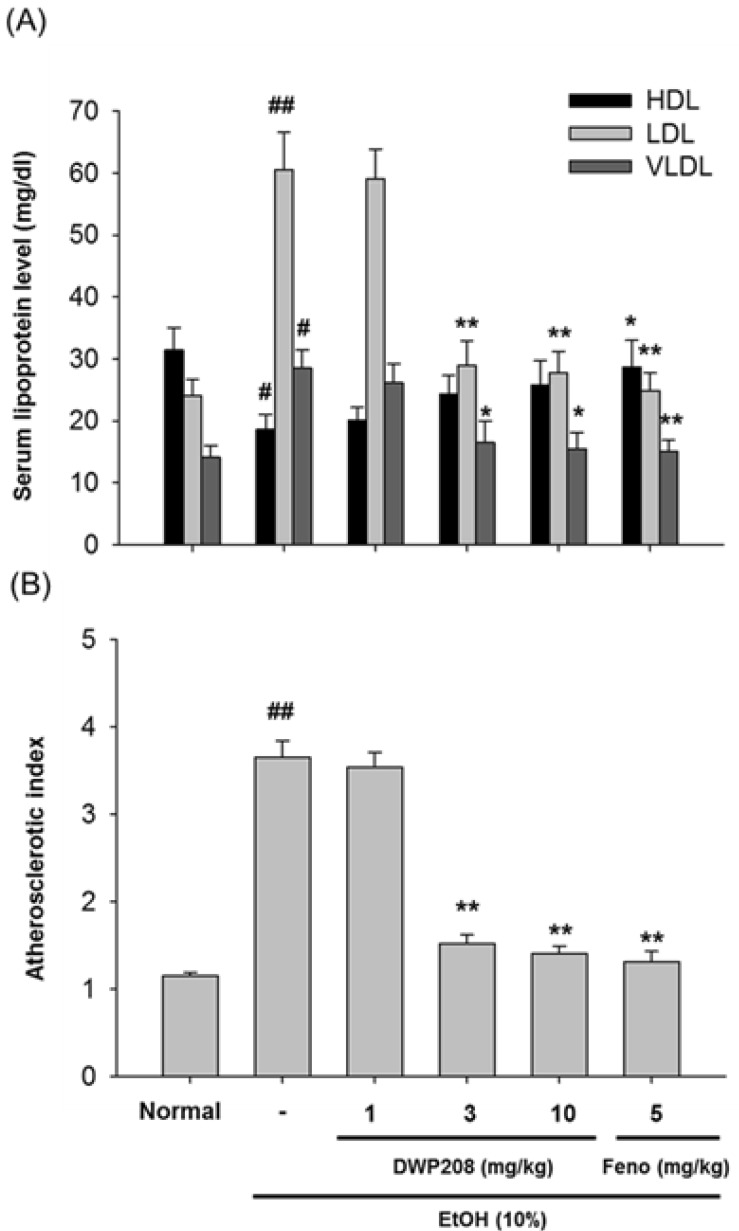
Fig. 2.
Effect of DWP208 on serum parameters in alcohol-induced hyperlipidemic rats. (A) Alcohol-treated rats were orally administered with DWP208 or fenofibrate (Feno) for 1 week. After preparing serum from rats, levels of HDL, LDL, and VLDL were examined. (B) AI values were calculated from an equation [AI=(total cholesterol-HDL-cholesterol)/HDL-cholesterol]. Data represent mean±SEM of four independent observations performed with 10 rats. #p<0.05 and ##p<0.01 compared to normal group, *p<0.05 and **p<0.01 compared to control group.
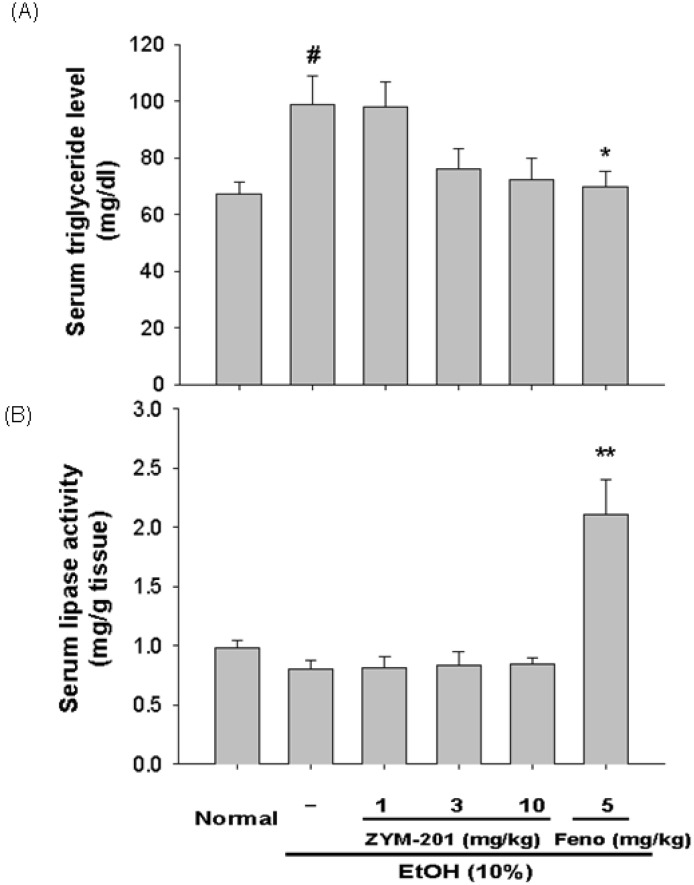
Fig. 3.
Effect of DWP208 on serum parameters in alcohol-induced hyperlipidemic rats. (A) Alcohol-treated rats were orally administered with DWP208 or fenofibrate (Feno) for 1 week. After preparing serum from rats, levels of triglyceride were examined. (B) Lipase activity in serum was determined. Data represent mean±SEM of four independent observations performed with 10 rats. #p<0.05 compared to normal group, *p<0.05 and **p<0.01 compared to control group.
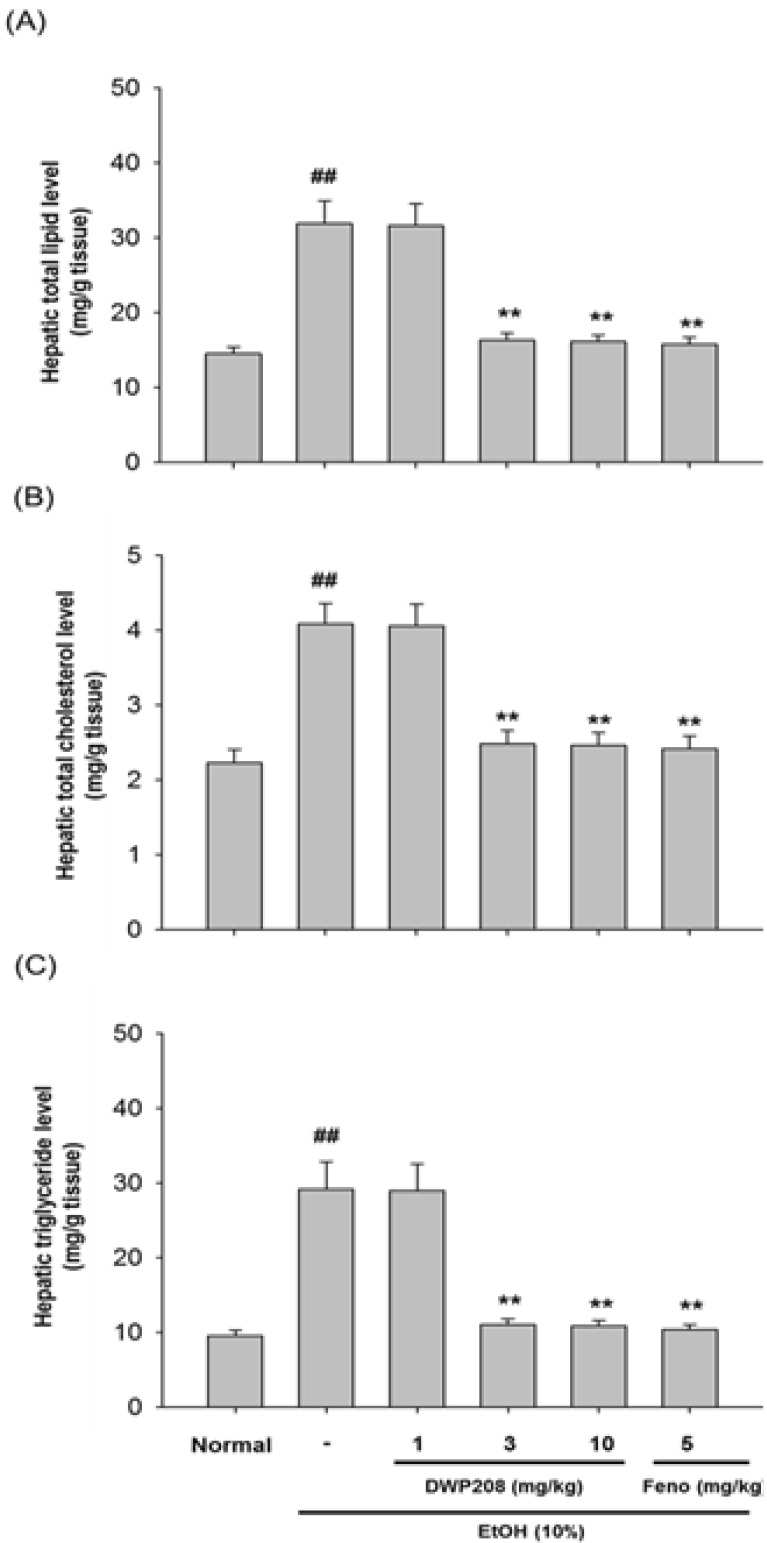
Fig. 4.
Effect of DWP208 on hepatic parameters in alcohol-induced hyperlipidemic rats. (A) Alcohol-treated rats were orally administered with DWP208 or fenofibrate (Feno) for 1 week. After preparing liver homogenates from rats, level of total lipid was examined. (B) Level of total cholesterol in liver was determined. (C) Level of triglyceride in liver was determined. Data represent the mean±SEM of four independent observations performed with 10 rats. ##p<0.01 compared to normal group, **p<0.01 compared to control group.
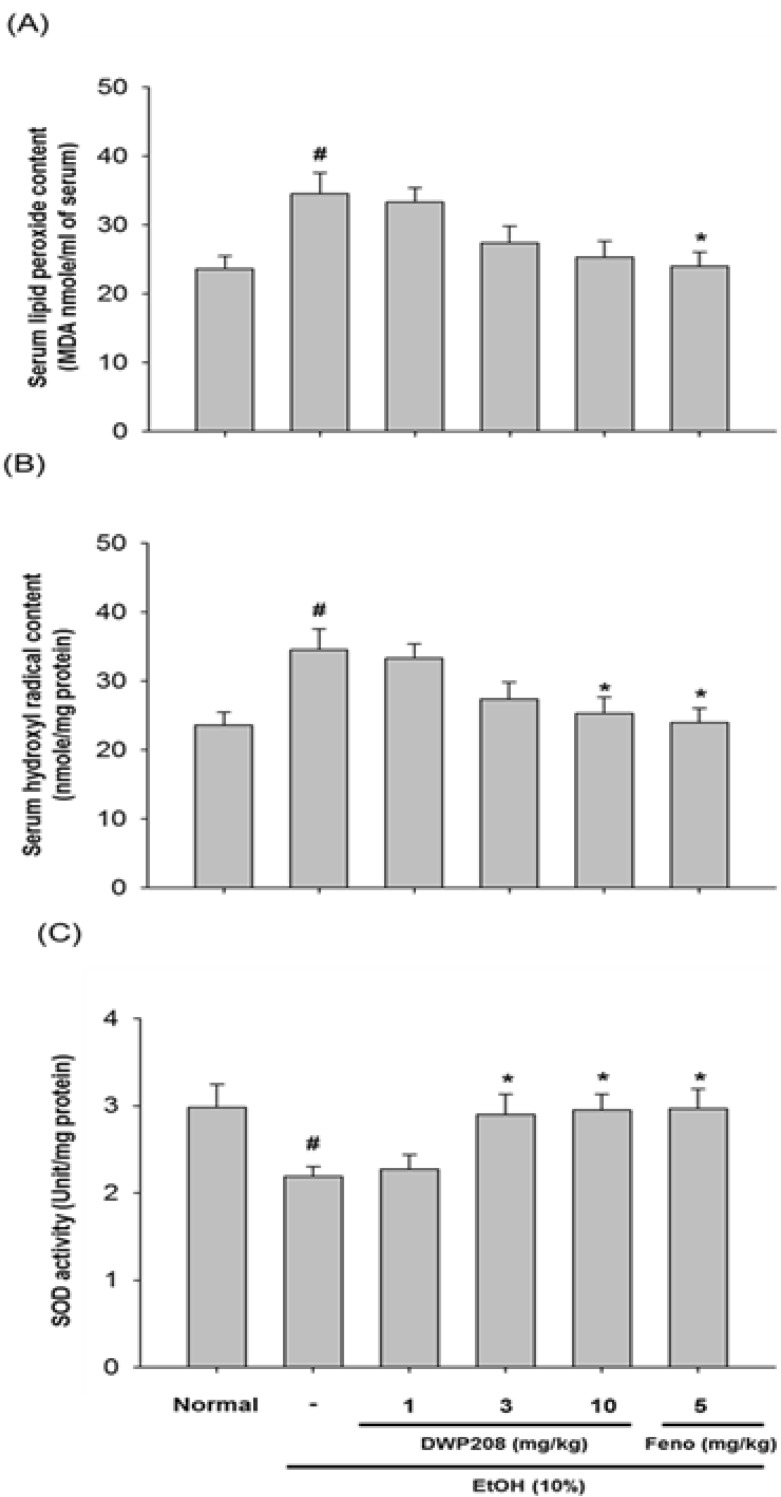
Fig. 5.
Effect of DWP208 on the contents of lipid peroxide and hydroxyl radicals, and the activity of SOD in serum from alcohol-treated rats. (A) Alcohol-treated rats were orally treated with DWP208 or fenofibrate (Feno) for 1 week. After preparing serum, serum lipid peroxide contents were examined. (B) Hydroxyl radicals were examined from serum. (C) SOD activity was examined in serum. Data represent the mean±SEM of four independent observations performed with 10 rats. #p<0.05 compared to normal group, *p<0.05 compared to control group.
Table 1.
Effect of DWP208 on serum total cholesterol levels in alcoholic hyperlipidemic rats
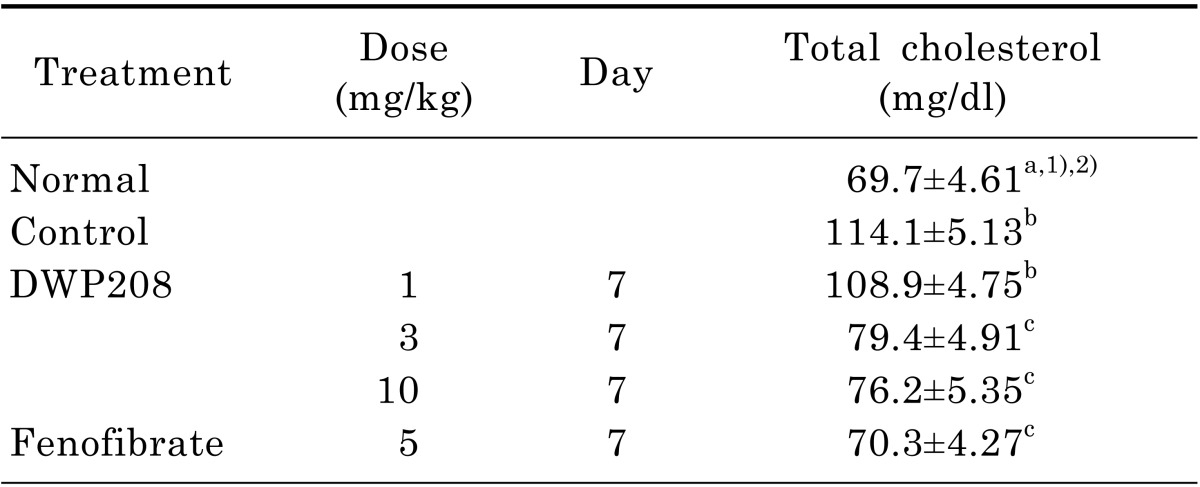
Rats were orally administered DWP208 (1, 3, or 10 mg/kg) daily for seven consecutive days after alcohol-induced hyperlipidemia. Rats were sacrificed seven days later. The assay procedure is described in the experimental methods.
1)Values are expressed mean±SEM (n=4).
2)Values sharing the same superscript letter are not significantly different from each other (p<0.05) by Duncan's multiple range test.
Table 2.
Effect of DWP208 on body and liver/body weights in alcoholic hyperlipidemic rats
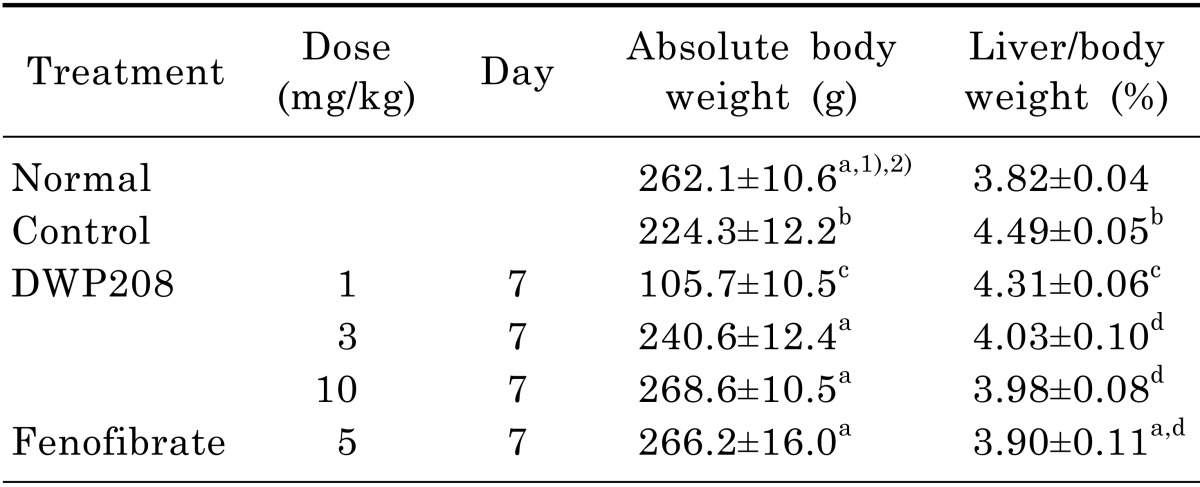
Rats were orally administered DWP208 (1, 3, or 10 mg/kg) daily for seven consecutive days after alcohol-induced hyperlipidemia. The assay procedure is described in the experimental methods.
1)Values expressed are mean±SEM (n=4).
2)Values sharing the same alphabetical superscript letter were not significantly different from each other (p<0.05) by Duncan's multiple range test.
Also, DWP208 was able to ameliorate the hyperlipidemia induced by a high fat diet or streptozotocin [20]. Furthermore, DWP208 protected against the hyperlipidemia induced by chronic alcohol intake. The most striking activity of DWP208 was its effects on LDL levels. Alcohol-induced up-regulation of LDL was completely restored by DWP208 treatment at 3 to 10 mg/kg (Fig. 2A). Reduced HDL levels were also restored and enhanced VLDL level were normalized. Consequently, AI value increased in alcohol treatment was strongly recovered in DWP208 treatment groups (3 and 10 mg/kg), as standard compound fenofibrate did (Fig. 2B). DWP208 also attenuated the up-regulated triglyceride and total cholesterol levels in serum (Fig. 3A, Table 1).
To see if the regulation of serum metabolism of lipid molecules by DWP208 was due to modulation of serum lipase levels, the activity of this enzyme was tested. However, chronic alcohol treatment failed to significantly increase the serum activity of lipase (Fig. 3B); indeed, there was a slight decrease. DWP208 did not alter serum lipase activity, while fenofibrate increased it up to 2 fold, suggesting that DWP208-mediated regulation of serum parameters is not serum lipase dependent. In fact, hepatic levels of total lipid (Fig. 4A), total cholesterol (Fig. 4B), and triglycerides (Fig. 4C) upregulated by 10% alcohol treatment were apparently restored by DWP208 (Fig. 5), suggesting that altered hepatic lipid metabolism can be targeted by DWP208. However, HMG-CoA reductase, a critical enzyme for cholesterol biosynthesis [23], did not appear to be a target enzyme involved in DWP208-mediated amelioration of hyperlipidemic conditions. Thus, DWP208 at doses up to 10 mg/kg did not suppress the activity of this enzyme, although the enhancement of HMG-CoA reductase activity was very marginal in alcohol treatment group (Table 3), suggesting that other metabolic enzymes or pathological events could be the target of DWP208.
Table 3.
Effect of DWP208 on hepatic HMG-CoA reductase activity in alcoholic hyperlipidemic rats
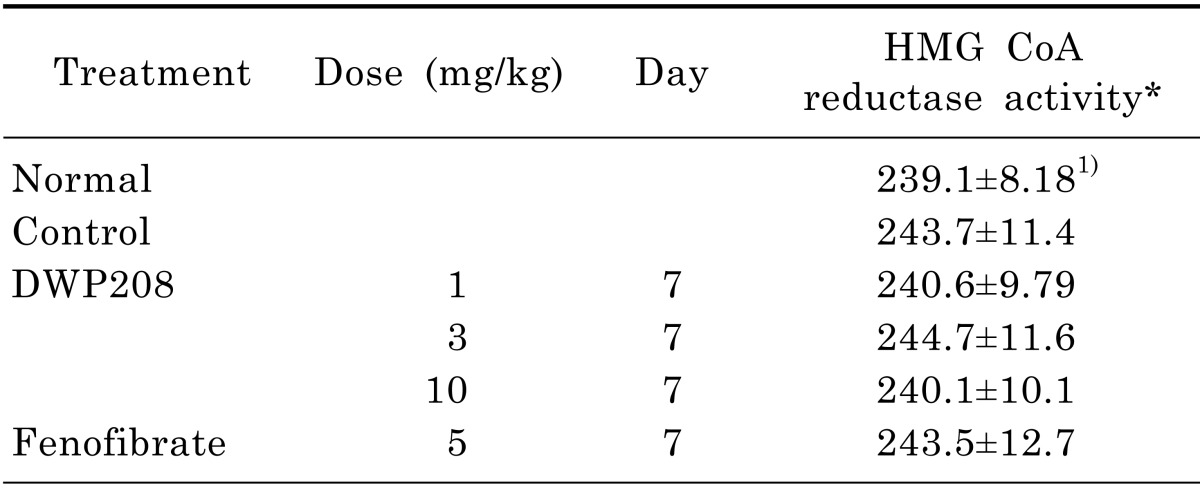
The assay procedure is described in the experimental methods.
*HMG-CoA reductase activity: oxidized NADPH pmole/mg protein/min
1)Values are expressed mean±SEM (n=4).
Since finding a direct target of DWP208 is not easy, we focused on exploring the effect of this compound on the generation of major pathological molecules. Numerous literature reports have commonly stressed the critical role of radicals generated during alcohol intake in the malfunction of hepatic lipid metabolism [5,24]. We found that DWP208 suppresses radical generation under streptozotocin treatment during high fat diet conditions [20]. Indeed, Fig. 5 strongly supported an anti-oxidative role of DWP208 in alcohol-treated conditions. Thus, this compound dose-dependently suppressed lipid peroxide (Fig. 5A) content and serum hydroxyl radical levels (Fig. 5B). Furthermore, the activity of SOD, an anti-oxidative enzyme against superoxide and hydroperoxide [25], was restored to normal levels after alcohol treatment had clearly reduced it (Fig. 5C). This suggested that the anti-oxidative property of DWP208 may be a critical factor in its anti-hyperlipidemic effects.
In general, it has been known that most saponin components are not strong anti-oxidants due to lack of chemical features [26]. Instead, some saponins such as ginsenoside Rb1 and gypenosides indirectly protect against cell damage induced by radicals by suppressing NF-B [27] and increasing glutathione levels [28]. We also failed to find direct neutralizing activity of DWP208 against SNP-induced radical generation [20]. Therefore, our results and previous reports suggest that DWP208 is protective by indirectly increasing cellular anti-oxidant systems. Since various biological activities of saponins - such as acute hepatic failure induced by lipopolysaccharide and D-galactosamine in mice [29], gastric ulcer induced by HCl/EtOH [30], and oxidative injury of dopaminergic neurons induced by 1-methyl-4-phenylpyridinium ion (MPP(+) [28] - are derived from their neutralizing activity against oxidative stress, DWP208 seems to have similar protective activities against these pathological symptoms. Further tests of the pharmacological actions of DWP208 in such models are now warranted.
In summary, we found that orally administered DWP208 can reverse the hyperlipidemia generated by chronic intake of alcohol. This compound normalized both up-regulated hyperlipidemic parameters such as LDL, VLDL, AI, triglyceride, total cholesterol, and down-regulated hyperlipidemia parameters such as absolute body weight, SOD activity, and HDL in serum and liver. According to our data, the ameliorative activity of DWP208 seems to be due to its indirect anti-oxidative activity by which lipid peroxide and hydroxyl radical levels are reduced and the activity of SOD is increased. Considering that chronic alcohol abuse is a serious social problem and is increasing particularly fast in Far East Asian countries, the complications of alcohol consumption, including hyperlipidemia and atherosclerosis, will be greatly increased. Our data strongly suggest that DWP208 can be used as a remedy against alcohol-induced hyperlipidemia.
ACKNOWLEDGEMENTS
This research was supported by a grant from Sungkyunkwan University (2014).
ABBREVIATIONS
- VLDL
very low-density lipoprotein
- AI
atherosclerotic index
- SOD
superoxide dismutase
- TF
tissue factor
- TNF
tumor necrosis factor
- TBARS
thiobarbituric acid reactive substances
- HMG
3-hydroxy-3-methyl-glutaryl
References
- 1.Maxwell JC. Update: comparison of drug use in Australia and the United States as seen in the 2001 National Household Surveys. Drug Alcohol Rev. 2003;22:347–357. doi: 10.1080/0959523031000154490. [DOI] [PubMed] [Google Scholar]
- 2.Thomson SJ, Westlake S, Rahman TM, Cowan ML, Majeed A, Maxwell JD, Kang JY. Chronic liver disease--an increasing problem: a study of hospital admission and mortality rates in England, 1979-2005, with particular reference to alcoholic liver disease. Alcohol Alcohol. 2008;43:416–422. doi: 10.1093/alcalc/agn020. [DOI] [PubMed] [Google Scholar]
- 3.You M, Crabb DW. Recent advances in alcoholic liver disease II. Minireview: molecular mechanisms of alcoholic fatty liver. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1–G6. doi: 10.1152/ajpgi.00056.2004. [DOI] [PubMed] [Google Scholar]
- 4.Arteel GE, Iimuro Y, Yin M, Raleigh JA, Thurman RG. Chronic enteral ethanol treatment causes hypoxia in rat liver tissue in vivo. Hepatology. 1997;25:920–926. doi: 10.1002/hep.510250422. [DOI] [PubMed] [Google Scholar]
- 5.Albano E. Oxidative mechanisms in the pathogenesis of alcoholic liver disease. Mol Aspects Med. 2008;29:9–16. doi: 10.1016/j.mam.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Deaciuc IV, Doherty DE, Burikhanov R, Lee EY, Stromberg AJ, Peng X, de Villiers WJ. Large-scale gene profiling of the liver in a mouse model of chronic, intragastric ethanol infusion. J Hepatol. 2004;40:219–227. doi: 10.1016/j.jhep.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Chait A, Mancini M, February AW, Lewis B. Clinical and metabolic study of alcoholic hyperlipidaemia. Lancet. 1972;2:62–64. doi: 10.1016/s0140-6736(72)91552-8. [DOI] [PubMed] [Google Scholar]
- 8.Lefevre AF, DeCarli LM, Lieber CS. Effect of ethanol on cholesterol and bile acid metabolism. J Lipid Res. 1972;13:48–55. [PubMed] [Google Scholar]
- 9.Cho JY, Yoo ES, Cha BC, Park HJ, Rhee MH, Han YN. The inhibitory effect of triterpenoid glycosides originating from Sanguisorba officinalis on tissue factor activity and the production of TNF-alpha. Planta Med. 2006;72:1279–1284. doi: 10.1055/s-2006-947257. [DOI] [PubMed] [Google Scholar]
- 10.Asao Y, Morikawa T, Xie Y, Okamoto M, Hamao M, Matsuda H, Muraoka O, Yuan D, Yoshikawa M. Structures of acetylated oleanane-type triterpene saponins, rarasaponins IV, V, and VI, and anti-hyperlipidemic constituents from the pericarps of Sapindus rarak. Chem Pharm Bull (Tokyo) 2009;57:198–203. doi: 10.1248/cpb.57.198. [DOI] [PubMed] [Google Scholar]
- 11.Liu SJ, Ramsey RK, Fallon HJ. Effects of ethanol on hepatic microsomal drug-metabolizing enzymes in the rat. Biochem Pharmacol. 1975;24:369–378. doi: 10.1016/0006-2952(75)90220-8. [DOI] [PubMed] [Google Scholar]
- 12.Yuan Q, Shi H. Treatment of hyperlipemia by acupoint catgutembedding in 34 cases. J Tradit Chin Med. 2003;23:208. [PubMed] [Google Scholar]
- 13.Takahashi T, Hirano T, Okada K, Adachi M. Apolipoprotein CIII deficiency prevents the development of hypertriglyceridemia in streptozotocin-induced diabetic mice. Metabolism. 2003;52:1354–1359. doi: 10.1016/s0026-0495(03)00202-6. [DOI] [PubMed] [Google Scholar]
- 14.Sagone AL, Jr, Decker MA, Wells RM, Democko C. A new method for the detection of hydroxyl radical production by phagocytic cells. Biochim Biophys Acta. 1980;628:90–97. doi: 10.1016/0304-4165(80)90354-2. [DOI] [PubMed] [Google Scholar]
- 15.Giada F, Baldo-Enzi G, Balocchi MR, Zuliani G, Baroni L, Fellin R. Heparin-released plasma lipase activities, lipoprotein and apoprotein levels in young adult cyclists and sedentary men. Int J Sports Med. 1988;9:270–274. doi: 10.1055/s-2007-1025020. [DOI] [PubMed] [Google Scholar]
- 16.Fraga CG, Leibovitz BE, Tappel AL. Lipid peroxidation measured as thiobarbituric acid-reactive substances in tissue slices: characterization and comparison with homogenates and microsomes. Free Radic Biol Med. 1988;4:155–161. doi: 10.1016/0891-5849(88)90023-8. [DOI] [PubMed] [Google Scholar]
- 17.Wahlund G, Marklund SL, Sjöquist PO. Extracellular-superoxide dismutase type C (EC-SOD C) reduces myocardial damage in rats subjected to coronary occlusion and 24 hours of reperfusion. Free Radic Res Commun. 1992;17:41–47. doi: 10.3109/10715769209061087. [DOI] [PubMed] [Google Scholar]
- 18.Ametaj BN, Bobe G, Lu Y, Young JW, Beitz DC. Effect of sample preparation, length of time, and sample size on quantification of total lipids from bovine liver. J Agric Food Chem. 2003;51:2105–2110. doi: 10.1021/jf0259011. [DOI] [PubMed] [Google Scholar]
- 19.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 20.Choi J, Kim MY, Cha BC, Yoo ES, Yoon K, Lee J, Rho HS, Kim SY, Cho JY. ZYM-201 sodium succinate ameliorates streptozotocin-induced hyperlipidemic conditions. Planta Med. 2012;78:12–17. doi: 10.1055/s-0031-1280219. [DOI] [PubMed] [Google Scholar]
- 21.Grauvogel J, Daemmrich TD, Ryschich E, Gebhard MM, Werner J. Chronic alcohol intake increases the severity of pancreatitis induced by acute alcohol administration, hyperlipidemia and pancreatic duct obstruction in rats. Pancreatology. 2010;10:603–612. doi: 10.1159/000288707. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Yao T, Song Z. Chronic alcohol consumption disrupted cholesterol homeostasis in rats: down-regulation of low-density lipoprotein receptor and enhancement of cholesterol biosynthesis pathway in the liver. Alcohol Clin Exp Res. 2010;34:471–478. doi: 10.1111/j.1530-0277.2009.01111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vinik AI. The metabolic basis of atherogenic dyslipidemia. Clin Cornerstone. 2005;7:27–35. doi: 10.1016/s1098-3597(05)80065-1. [DOI] [PubMed] [Google Scholar]
- 24.Albano E. Alcohol, oxidative stress and free radical damage. Proc Nutr Soc. 2006;65:278–290. doi: 10.1079/pns2006496. [DOI] [PubMed] [Google Scholar]
- 25.Hiramatsu M, Edamatsu R, Mori A. Free radicals, lipid peroxidation, SOD activity, neurotransmitters and choline acetyltransferase activity in the aged rat brain. EXS. 1992;62:213–218. doi: 10.1007/978-3-0348-7460-1_21. [DOI] [PubMed] [Google Scholar]
- 26.Liu ZQ, Luo XY, Liu GZ, Chen YP, Wang ZC, Sun YX. In vitro study of the relationship between the structure of ginsenoside and its antioxidative or prooxidative activity in free radical induced hemolysis of human erythrocytes. J Agric Food Chem. 2003;51:2555–2558. doi: 10.1021/jf026228i. [DOI] [PubMed] [Google Scholar]
- 27.Liu TZ, Lee KT, Chern CL, Cheng JT, Stern A, Tsai LY. Free radical-triggered hepatic injury of experimental obstructive jaundice of rats involves overproduction of proinflammatory cytokines and enhanced activation of nuclear factor kappaB. Ann Clin Lab Sci. 2001;31:383–390. [PubMed] [Google Scholar]
- 28.Wang P, Niu L, Guo XD, Gao L, Li WX, Jia D, Wang XL, Ma LT, Gao GD. Gypenosides protects dopaminergic neurons in primary culture against MPP(+)-induced oxidative injury. Brain Res Bull. 2010;83:266–271. doi: 10.1016/j.brainresbull.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Ying-Wan, Wu YL, Feng XC, Lian LH, Jiang YZ, Nan JX. The protective effects of total saponins from Ornithogalum saundersiae (Liliaceae) on acute hepatic failure induced by lipopolysaccharide and D-galactosamine in mice. J Ethnopharmacol. 2010;132:450–455. doi: 10.1016/j.jep.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 30.Rujjanawate C, Kanjanapothi D, Amornlerdpison D. The antigastric ulcer effect of Gynostemma pentaphyllum Makino. Phytomedicine. 2004;11:431–435. doi: 10.1016/j.phymed.2003.07.001. [DOI] [PubMed] [Google Scholar]