Abstract
Introduction
The objective of this study was to investigate the effect of common single nucleotide genomic polymorphisms in the cyclooxygenase-1 (COX-1) gene on the thromboxane A2 (TxA2) metabolite concentrations in serum and urine, as well as on prostaglandin F2α (PGF2α) urinary excretion in the diabetic population on acetylsalicylic acid (ASA) therapy.
Material and methods
The study cohort consisted of 284 Caucasians with diabetes type 2 who had been taking ASA tablets at the dose of 75 mg/day for at least 3 months. Genotyping for the 4 selected SNPs within the COX-1 gene (two nonsynonymous-coding variants, rs3842787 [C50T, P17L] and rs5789 [C174A, L237M]; and two other synonymous SNPs, rs3842788 [G128A, Q41Q] and rs5788 [C644A]) was performed using the Sequenom iPLEX platform.
Results
No statistically significant results were observed for the investigated SNPs and measured metabolites in the investigated cohort of patients. Statistically significant differences in S-TxB2 could however be observed for rs5788 in the subgroup of patients with very high S-TxB2 concentrations. In particular, more patients who were carriers of the minor allele for this polymorphism were observed in the group with S-TxB2 levels > 95th percentile, when compared with similar carriers in the group with S-TxB2 < 95th percentile (20% vs. 1.1%, respectively, p < 0.001, Mann-Whitney test).
Conclusions
The results of our study suggest that the four investigated common SNPs in the COX1 gene are not associated with obviously altered TxA2 metabolism and PGF2α synthesis in the investigated diabetic cohort treated with ASA.
Keywords: acetylsalicylic acid, cyclooxygenase-1, diabetes mellitus, thromboxane, 8-iso-prostaglandin F2α
Introduction
Several mechanisms caused by metabolic and cellular abnormalities have been suggested to play a role in increased platelet reactivity. Endogenous and environmental factors – age, cholesterol and inflammatory markers levels, hypertension, diabetes mellitus, and cigarette smoking – explain only part of the variation in platelet function observed in persons with these conditions [1–9]. Although inherited and genetic factors have known links to cardiovascular disease, the evidence for genetic influences that enhance platelet function is much weaker [10–12].
Patients with type 2 diabetes mellitus (T2DM) are characterized by an elevated risk of recurrent atherothrombotic events. In addition, acetylsalicylic acid (ASA) therapy may be less effective in high-risk populations [11, 13, 14]. Patients with T2DM exhibit platelet hyperreactivity both in vitro and in vivo coupled with biochemical evidence of persistently increased thromboxane-dependent platelet activation [3–6]. Furthermore, polymorphisms in different genes have been associated with variability for increased platelet reactivity on ASA therapy in T2DM patients [14–16].
The problems with determining ASA response and the different methodologies available for determining the biochemical and functional effects of ASA have compounded the problems of attempting to define increased platelet reactivity despite ASA therapy, with some investigators suggesting that the term should be used only when production of thromboxane A2 (TxA2) (or its breakdown products) is blocked regardless of platelet function [11, 17].
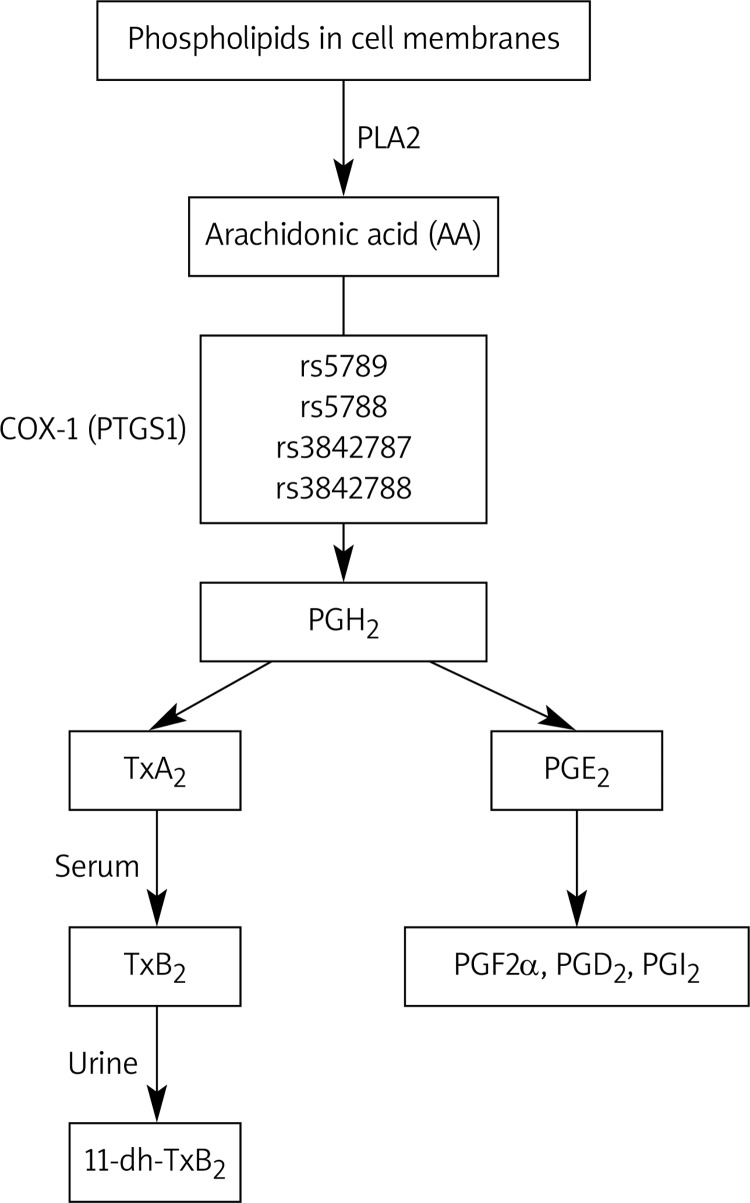
Acetylsalicylic acid irreversibly inhibits cyclooxygenase (COX)-1 in human platelets. COX-1 (prostaglandin endoperoxide G/H synthase) catalyzes the formation of prostaglandin H2 (PGH2) from arachidonic acid (AA). This action is the committed step in prostaglandin synthesis, which yields the bioactive eicosanoids, prostaglandin D2, prostaglandin F2α (PGF2α), prostaglandin I2, and TxA2 through branching enzymatic pathways (Figure 1) [18]. The effect of ASA repeatedly administered once daily is irreversible, cumulative and saturable, reaching a ceiling effect in the low dose range [19]. The TxA2 production can be determined by measuring stable metabolites of TxA2, such as thromboxane B2 (TxB2) in the serum and 11-dehydro-TxB2 (11-dh-TxB2) in the urine. Because serum TxB2 production is predominantly dependent on platelet COX-1, it has been used as a primary measure of the inhibitory effects of low-dose ASA on platelets [20]. In addition to TxA2, platelets release F2-isoprostanes, in particular 8-iso-prostaglandin F2α (8-iso-PGF2α), a chemically stable compound derived from enzymatic and nonenzymatic oxidation of AA [21]. Platelet 8-iso-PGF2α is synthesized in vivo as a minor product of the COX-1 enzyme in human platelets and the COX-2 isoform in human monocytes, as well as through the free radical-catalyzed peroxidation of AA in biological membranes [22, 23]. Because 8-iso-PGF2α formation might correlate with the rate of TxA2 biosynthesis, it was hypothesized that increased oxidant stress in T2DM could induce enhanced generation of 8-iso-PGF2α, which can contribute to platelet activation [24]. Even if it was demonstrated previously that enhanced 8-iso-PGF2α synthesis may be associated with advanced age, cigarette smoking, hypercholesterolemia, and unstable angina, as well as after coronary artery reperfusion, the role of 8-iso-PGF2α in ASA's effect on TxA2 synthesis remains uncertain [25–33].
Figure 1.
Arachidonic acid cascade and investigated single nucleotide polymorphisms (SNPs)
PLA2 – phospholipase A2, COX-1 – cy clooxygenase-1, PGH2 – prostaglandin H2, PGF2α – pro staglandin F2α, PGD2 – prostaglandin D2, PGJ2 – pro staglandin J2, TxA2 – thromboxane A2, TxB2 – thromboxane B2, 11-dh-TxB2 – 11-dehydro- TxB2
Genetic variation in COX-1 may affect enzyme expression, biochemical function or interaction with pharmacological agents [21]. The gene encoding human COX-1 (PTGS1), mapped to chromosome 9q32-q33.3, is approximately 22 kb in length, and contains 11 exons [22, 34]. COX-1 is constitutively expressed and is responsible for the biosynthesis of PGs involved in various functions, such as the regulation of renal, gastrointestinal, and platelet activity [18]. Several single nucleotide polymorphisms (SNPs) in both coding and noncoding regions of human COX-1 have been previously identified and were postulated to change its activity; however, all these studies were performed in healthy individuals or in patients with coronary artery disease (CAD) [11, 12, 35–37]. In addition, there is no systemic information published about the effects of these common SNPs on the function of COX-1, as measured directly by TxA2 metabolites and 8-iso-PGF2α concentrations in the diabetic population. To our knowledge, there is also no information about the effects of these common SNPs in the COX-1 gene on the related metabolic activity in the Polish population.
Thus, the objective of this study was to evaluate the impact of the four frequently investigated COX-1 gene polymorphisms in the entirely diabetic population on ASA therapy on TxA2 synthesis, measured as serum and urinary TxA2 metabolite concentrations, as well as on 8-iso-PGF2α synthesis measured as its urinary excretion. The genotyping was attempted for four COX-1 SNPs (two missense variants, rs3842787 [C50T, P17L] and rs5789 [C174A, L237M]; and two other synonymous SNPs, rs3842788 [G128A, Q41Q] and rs5788 [C644A]).
Material and methods
Patient population and study design
The ethics committee of the Medical University of Warsaw approved both the study protocol and the informed consent form. The study was conducted in accordance with the current version of the Declaration of Helsinki at the time when the study was designed, and informed written consent was obtained. The genotyping part of the study was reviewed and approved by the Institutional Review Board of Penn State Hershey Medical Center (Hershey, PA, USA). The study subjects were recruited consecutively from patients with T2DM participating in the multi-center, prospective, randomized, and open-label AVOCADO (Aspirin Vs/Or Clopidogrel in Aspirin-resistant Diabetics inflammation Outcomes) study presenting to the outpatient clinic of the Central Teaching Hospital of the Medical University of Warsaw. The full characteristics of the study population, including the inclusion and exclusion criteria, were published previously [14, 38]. Briefly, the study recruited 304 subjects with T2DM who, at the time of enrollment, had been taking ASA tablets at the dose of 75 mg per day for at least 3 months for primary or secondary prevention of myocardial infarction (MI). No clopidogrel or antiplatelet drugs other than ASA were used in any of the investigated patients. All patients had been taking oral antidiabetic agents and/or insulin for at least 6 months; diet-controlled diabetic patients were not included. Compliance with ASA therapy was determined at the study entry based upon the patient's own statement and serum thromboxane B2 (S-TxB2) levels below 7.2 ng/ml in all patients as described previously in a diabetic population by Mortensen et al. [6].
Blood sample and assay procedures
Blood samples were taken in the morning 2-3 h after the last ASA dose. Blood was taken from the antecubital vein, and the initial 2 ml of blood were discarded to avoid spontaneous platelet activation. Whole blood for S-TxB2 was allowed to clot at 37°C for 1 h before separating serum by centrifugation. Serum was obtained from venous blood by centrifugation at 1000 g for 15 min at 4°C, and aliquots were stored at –80°C for further analysis. Regular laboratory testing was performed at the laboratory of the Central Teaching Hospital, Medical University of Warsaw, using standard techniques, and included complete blood cell and platelet counts, fasting glycemia, glycosylated hemoglobin (HbA1c), lipid profile, C-reactive protein and serum creatinine concentrations.
Urinary 11-dehydro-thromboxane B2 (11-dh-TxB2)
11-dh-TxB2 was measured using an enzyme immunoassay kit according to the manufacturer's instructions (EIA kit, Cayman Chemical) after extraction and purification on SPE (C18) columns (Waters Associates, Milford, MA, USA). Data were normalized for urinary creatinine concentration.
Serum thromboxane B2 (S-TxB2)
S-TxB2 was measured also with an enzyme immunoassay kit according to the manufacturer's instructions (Cayman Chemicals, Ann Arbor, MI, USA). Each lot of TxB2 EIA kit was tested for the impact of interferences. The correlation of results in three dilutions of five random samples was assessed, as was proposed in the kit protocol. The decision to use the assay without purification was taken after analysis of results, as differences of results did not exceed 20%. Samples with results outside the standard curve were re-assayed with appropriate dilutions.
Urinary isoprostane measurement
Quantification of 8-iso-PGF2α was performed on a morning urine sample with an enzyme immunoassay kit (8-isoprostane EIA Kit, Cayman Chemical, Ann Arbor, MI, USA) and normalized to urinary creatinine concentration.
DNA extraction, quality control, and quantification
DNA was obtained from whole blood samples by the membrane ultrafiltration method using a Fuji MiniGene 80 extractor (FujiFilm Life Sciences distributed by Autogene, Holliston, MA), as described previously [15, 16].
Individual SNP genotyping
Genotyping was performed at Boston Children's Hospital using a custom Sequenom iPLEX assay in conjunction with the Mass ARRAY platform (Sequenom Inc., La Jolla, CA, USA). One panel of SNP markers was designed using Sequenom Assay Design 3.2 software.
Statistical analysis
Power analysis
We planned a prospective, observational study of diabetic patients treated with ASA. The required sample size (n = 265) of the study was designed to have a power of > 80% to detect a change in S-TxB2 level corresponding to a significant 2-fold increase in S-TxB2 concentration between alleles of the 4 investigated SNPs at a 0.05/4 = 0.0125 level of significance, with Bonferroni correction applied.
Statistical calculations
The statistical analyses were performed using IBM-SPSS ver. 19 and Stata (Stata Corporation, College Station, TX) software. Deviations from Hardy-Weinberg equilibrium (HWE) were calculated using the χ2 test. The recorded clinical data, when normally distributed in the analyzed group of patients, are presented as mean and SD, and non-normally distributed data are presented as medians and interquartile range (IQR). In the first stage of the analysis we compared the distribution of median S-TxB2, 11-dh-TxB2 and 8-iso-PGF2α levels among all genotypes of successfully genotyped SNPs using the Kruskal-Wallis test. The SNPs with nominal statistically significant (i.e. p < 0.05 before applying correction for multiple comparisons) differences in the measurements (S-TxB2, 11-dh-TxB2 and 8-iso-PGF2α levels) between medians for each of three genotypes (i.e., homozygotes for minor allele, heterozygotes and homozygotes for major allele) were subjected to further testing based on the dominant, recessive or additive genetic model by Mann-Whitney test.
Results
From the initially enrolled 304 patients, complete clinical data and blood samples finally became available for 298 patients. Subsequently, 8 patients were eliminated from the analysis based on the suspected ASA non-compliance (patients’ own statement and S-TxB2 concentrations > 7.2 ng/ml) and a further 6 patients were eliminated because of the lack of corresponding biochemical and genotype data. Demographic characteristics and clinical data for the remaining 284 patients are summarized in Table I.
Table I.
Demographic and clinical characteristics of the study patients (n = 284)
| Demographics | |
| Age [years] | 67.6 ±8.7 |
| Female, n (%) | 135 (47.5) |
| BMI [kg/m2] | 31.19 ±12.0 |
| SBP [mm Hg] | 142.3 ±18.9 |
| DBP [mm Hg] | 80.5 ±11.3 |
| Dyslipidemia | 234 (82.4%) |
| Hypertension | 262 (92.3%) |
| CAD | 162 (57.0%) |
| Prior MI | 87 (30.6%) |
| Prior stroke | 23 (8.1%) |
| Heart failure | 107 (37.7%) |
| History of smoking | 160 (56.3%) |
| Current smoking | 28 (9.8%) |
| Concurrent medications | |
| Oral hypoglycemic | 243 (85.6%) |
| Insulin | 93 (32.7%) |
| β-Blockers | 205 (72.2%) |
| ACE inhibitors | 185 (65.1%) |
| Statins | 206 (72.6%) |
| Biochemical and hematological parameters | |
| HGB [g/dl] | 13.8 ±1.3 |
| HCT [%] | 41.3 ±4.5 |
| WBC [103/mm3] | 7.1 ±2.2 |
| PLT [103/mm3] | 227.8 ±58.3 |
| MPV [fl] | 9.9 ±1.2 |
| eGFR [ml/min/1.73] | 70.8 ±20.9 |
| HbA1c [%] | 7.0 ±1.3 |
| hsCRP | 4.1 ±5.6 |
Data are presented as mean ± SD unless otherwise indicated. BMI – body mass index, SBP – systolic blood pressure, DBP – diastolic blood pressure, CAD – coronary artery disease, MI – myocardial infarction, ACE – angiotensin-converting enzyme, HGB – hemoglobin, HCT – hematocrit, WBC – white blood cells, PLT – platelet count, MPV – mean platelet volume, eGFR – estimated glomerular filtration rate, HbA1c – glycosylated hemoglobin, hsCRP – high-sensitivity C-reactive protein.
The summary results of the allele and genotype frequencies for all genotyped SNPs are summarized in Table II. All 4 SNPs genotyped well (> 86% success rate) and were in Hardy-Weinberg equilibrium. For each of the successfully genotyped SNPs, we initially compared the corresponding S-TxB2, and urine 11-dh-TxB2 and 8-iso-PGF2α excretion data between 3 allelic groups (e.g. homozygotes for minor and major alleles, as well as heterozygotes) using the non-parametric Kruskal-Wallis test. The obtained results did not reveal any statistically significant differences in S-TxB2, 11-dh-TxB2 and 8-iso-PGF2α concentrations for carriers and non-carriers of the investigated SNP variants.
Table II.
The effects of different analyzed genotypes of SNPs in COX-1 on plasma levels of S-TxB2 and urine excretion of 11-dh-TxB2 and 8-iso-PGF2α in diabetic patients on ASA
| Analyzed SNPs in COX-1 | S-TxB2 [ng/ml] | 11-dh-TxB2 [ng/mmol Cr] | 8-iso-PGF2α [ng/mg Cr] |
|---|---|---|---|
| Rs5788 C > A (MAF = 0.180) | |||
| Homozygotes for major allele (n = 193) | 0.155 (0.6) | 40.485 (40.82) | 0.411 (0.3) |
| Heterozygotes (n = 81) | 0.159 (0.6) | 38.990 (41.55) | 0.410 (0.4) |
| Homozygotes for minor (variant) allele (n = 10) | 0.107 (1.7) | 61.690 (108.70) | 0.405 (0.2) |
| P* (KW test) | 0.945 | 0.288 | 0.885 |
| Rs5789 C > A (MAF = 0.017) | |||
| Homozygotes for major allele (n = 275) | 0.153 (0.6) | 40.490 (41.56) | 0.410 (0.3) |
| Heterozygotes (n = 8) | 0.223 (0.5) | 26.200 (38.89) | 0.352 (0.5) |
| Homozygotes for minor (variant) allele (n = 1) | n/a | n/a | n/a |
| P (MW test) | 0.509 | 0.247 | 0.597 |
| Rs3842787 C > T (MAF = 0.061) | |||
| Homozygotes for major allele (n = 250) | 0.154 (0.6) | 40.430 (41.68) | 0.406 (0.3) |
| Heterozygotes (n = 28) | 0.157 (0.6) | 42.800 (42.07) | 0.494 (0.4) |
| Homozygotes for minor (variant) allele (n = 3) | 0.033 (–) | 36.980 (–) | 0.301 (–) |
| P (MW test) | 0.602 | 0.978 | 0.284 |
| Rs3842788 G > A (MAF = 0.015) | |||
| Homozygotes for major allele (n = 275) | 0.154 (0.5) | 40.450 (40.45) | 0.410 (0.3) |
| Heterozygotes (n = 9) | 0.071 (1.1) | 39.505 (57.38) | 0.342 (0.4) |
| Homozygotes for minor (variant) allele (n = 0) | n/a | n/a | n/a |
| P (MW test) | 0.815 | 0.897 | 0.945 |
Data are shown as median and interquartile range (IQR). *P using Kruskal-Wallis (KW) test for differences between 3 analyzed genotypes for each SNP or using Mann-Whitney test (MW) for differences between 2 genotypes (carriers of wild-type allele and pooled carriers of variant allele); n – number of carriers for each genotype, MAF – minor allele frequency for each analyzed SNP in investigated cohort, SNPs – single nucleotide polymorphisms, COX-1 – cyclooxygenase-1, S-TxB2 – serum thromboxane B2, 11-dh-TxB2 – urinary 11-dehydro-thromboxane B2, 8-iso-PGF2α – 8-iso-prostaglandin F2α.
In order to more closely analyze the relationship between S-TxB2 and COX-1 polymorphisms (in particular in the outlier sub-cohort with S-TxB2 > 75th percentile), we also established several empirical cut-off criteria (e.g., at 75th percentile 0.612 ng/ml, 1.5 × 75th percentile 0.918 ng/ml, and at 95th percentile 4.05 ng/ml), which divided the investigated patients into 2 categories, based on S-TxB2 concentration. No effects of any investigated polymorphisms in COX-1 were observed for the analyzed patient cohort with the S-TxB2 cut-off at the 75th percentile or 1.5 × 75th percentile (not shown). When the investigated cohort was subsequently divided into two parts, based on the 95th percentile, a statistically significant difference could be established for the rs5788 polymorphism, based on the recessive model, i.e., in carriers of a variant minor allele. In particular, more patients who were carriers of the minor allele for this polymorphism were observed in the group with S-TxB2 levels > 95th percentile, when compared with similar carriers in the group with S-TxB2 < 95th percentile (20% vs. 1.1%, respectively, p < 0.001, Mann-Whitney test). No changes were observed for the analyzed outliers in S-TxB2, 11-dh-TxB2 or 8-iso-PGF2α or for the other three investigated SNPs in COX-1.
The presented statistical analysis was performed on 284 patients with observed S-TxB2 levels above the non-compliance threshold (i.e. S-TxB2 > 7.2 ng/ ml). Very similar results were obtained when this analysis was repeated in all 292 patients (i.e. without removal of any of them for suspected non-compliance), including the effect of rs5788 polymorphism on outlier S-TxB2 levels (data not shown).
Discussion
The current genetic study of platelet reactivity during chronic ASA therapy represents, to our knowledge, the first comprehensive analysis of SNPs in the COX-1 gene during chronic ASA therapy in diabetic patients and aims to directly correlate genetic variants within the COX-1 gene with AA metabolites (i.e. TxB2, 11-dehydro TxB2, 8-iso-PGF2α) in an ethnically homogeneous and entirely diabetic population from central Poland. In our study COX-1 inhibition was based on TxA2-related measurements, such as serum TxB2 levels (index of the maximum biosynthetic capacity of platelet COX-1 ex vivo) and urinary 11-dehydro TxB2 excretion (index of in vivo TxA2 biosynthesis) [39, 40].
We found that four common COX-1 gene polymorphisms (two missense variants, rs3842787 [C50T, P17L] and rs5789 [C174A, L237M]; and two other synonymous SNPs, rs3842788 [G128A, Q41Q] and rs5788 [C644A]) (Table II) had no statistically significant effects on TxA2-related indexes and 8-iso-PGF2α excretion in the investigated diabetic patients treated with ASA. A possible exception was observed for SNP rs5788, for which the carriers of the minor allele for this polymorphism were more frequently observed in the group with S-TxB2 levels > 95th percentile, when compared with similar carriers in the group with S-TxB2 < 95th percentile (20% vs. 1.1%, respectively, p < 0.001, Mann-Whitney test). The clinical significance of this last finding remains unclear, but may indicate that this SNP could be responsible for the inter-individual differences in the S-TxB2 concentrations in at least a small portion of the patients with high COX-1 activity or may be due to known or unknown genetic variants in linkage disequilibrium.
Several genetic variants in human COX-1 were found previously to alter COX-1-mediated AA metabolism in the non-diabetic population; however, only the rs3842787 (or so-called C50T) polymorphism in COX-1 was repeatedly shown to be of functional significance in relation to the antiplatelet effect of ASA [11, 12, 41, 42]. Some studies demonstrated an association between this SNP and reduced ASA effect on platelet aggregation and TxB2 formation [21, 43, 44]. Gonzalez-Conejero et al. demonstrated that ASA-treated healthy subjects with the COX-1 rs3842787 polymorphism had an almost two-fold increase in-vivo TxB2 production [43]. Similar findings were observed in a trial including 144 patients with stable CAD, which showed higher AA-induced platelet aggregation and serum TxB2 synthesis in carriers with a haplotype containing the variant of rs3842787 [21]. Lepantalo et al. examined the polymorphisms of COX-1 on antiplatelet effect of ASA in coronary artery disease patients and observed a correlation with AA-induced aggregometry and PFA-100, but not with serum TxB2 levels [44]. However, a recent comprehensive systematic review revealed no significant association of rs3842787 with so-called “ASA resistance”, as the number of studies and of subjects used was small, making it difficult to exclude definitively any contribution of these polymorphisms to the effect of ASA (OR for aspirin resistance 1.07; 95% CI 0.41, 2.77; p = 0.89) [11].
Platelet 8-iso-PGF2α is synthesized in vivo as a minor product of the COX-1 enzyme in human platelets and the COX-2 isoform in human monocytes and mostly independently of the activity of COX-1, through the free radical- catalyzed peroxidation of AA in biological membranes [22, 23]. Contrary to TxA2, 8-iso-PGF2α does not directly induce platelet aggregation, but it elicits shape change, amplifies the platelet response to common agonists, and enhances platelet recruitment, and thus can modulate platelet aggregation through the thromboxane receptor (TP) or a closely related receptor, regardless of COX-1 inhibition by ASA [23, 24, 32]. As thromboxane synthase inhibitors do not reliably inhibit AA-induced platelet aggregation, it was postulated that the measurement of 8-iso-PGF2α can serve as a better marker than TxB2 for analysis of the correlation between functional outcomes and genetic variants at the COX-1 locus. Because platelet COX activity can also produce small (when compared with TxB2) amounts of 8-iso-PGF2α, we tried to establish an association between enhanced urinary excretion of 8-iso-PGF2α and COX-1 polymorphisms that could influence its enzymatic activity. Halushka et al. reported that ASA has an enhanced inhibitory effect on in vitro PGH2 formation by platelets (measured as 8-iso-PGF2α) from healthy carriers of variant allele rs3842787 [35]. In contrast to those results, we did not find any association between rs3842787 (C50T) SNP and 8-iso-PGF2α levels in the diabetic population. These differences could be, in part, explained by the previous report that compared 8-iso-PGF2α excretion in the presence and absence of platelet COX blockade by low-dose aspirin. In this study COX-1 inhibition, as reflected by suppression of TxB2 biosynthesis, was not associated with any detectable difference in F2-isoprostane formation [32]. In another study it was also shown that urinary 8-iso-PGF2α excretion did not contribute to impaired platelet responsiveness to ASA in a COX-1 dependent mechanism [33].
According to Lee et al., the COX-1 rs5789 (L237M) variant can presumably influence catalytic activity of COX-1 through its predicted impact on dimerization [42]. Moreover, they found that COX-1 metabolic activity in vitro was significantly lower with the rs5789 (L237M) variant relative to wild type after inhibition with indomethacin as the carriers of the rs5789 (L237M) variant also had significantly lower basal activity [42]. However, this observation may not extend to all NSAIDs, and thus TxB2 formation on ASA therapy in vivo.
The inherent limitations of observational design apply to this study. The candidate SNPs within the COX-1 gene were chosen because of their established or suspected influence on platelet function, as assessed by numerous studies in several laboratories. However, it cannot be ruled out that some other non-analyzed SNPs (or other genetic variants) can alter COX-1 activity. As such, our current findings should be interpreted with caution and await further confirmation from more detailed studies. Moreover, pretreatment measurements (i.e. before beginning ASA treatment) could not be conducted because all patients included in this study had diagnosed CAD or multiple risk factors for CAD and therefore were on ASA therapy at the time of enrollment. Other weaknesses of our study include the lack of external replication in an independent cohort of diabetic patients. Lastly, our association might be either random or associated with the particular patient population (e.g. diabetes), and this question could not be answered in this study.
In conclusion, we were unable to confirm an association between investigated common COX-1 gene SNPs and COX-1 activity in the diabetic population treated with a low dose of ASA. Additional investigation is warranted to establish whether COX-1 activity in the T2DM population may be influenced by other, less frequent, genetic variants of COX-1, which were not investigated in this study. Further studies in larger and different ethnic diabetic populations are needed to validate and possibly extend the conclusions from this relatively small, but ethnically homogeneous, cohort.
Acknowledgments
Marek Postula and Piotr K. Janicki contributed equally to this work.
Dr Postula was supported by a Fulbright Fellowship. The authors thank Thutrang Nguyen, B.A. (Laboratory Manager), from the SNP Genotyping Core Facility at Boston Children's Hospital, Massachusetts, for performing the custom Sequenom iPLEX assay. The authors alone are responsible for the content and writing of this paper.
The AVOCADO study was supported financially as part of a research grant from the Polish Pharmaceutical Company ADAMED for a Young Scientist 2007 Award grant number: 1WR DAR1/2007.
References
- 1.Patrono C, Rocca B. Aspirin: promise and resistance in the new millennium. Arterioscler Thromb Vasc Biol. 2008;28:25–32. doi: 10.1161/ATVBAHA.107.160481. [DOI] [PubMed] [Google Scholar]
- 2.Boncler M, Gresner P, Nocun M, et al. Elevated cholesterol reduces acetylsalicylic acid-mediated platelet acetylation. Biochim Biophys Acta. 2007;1770:1651–9. doi: 10.1016/j.bbagen.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Ferroni P, Basili S, Falco A, Davi G. Platelet activation in type 2 diabetes mellitus. J Thromb Haemost. 2004;2:1282–91. doi: 10.1111/j.1538-7836.2004.00836.x. [DOI] [PubMed] [Google Scholar]
- 4.DiChiara J, Bliden KP, Tantry US, et al. The effect of aspirin dosing on platelet function in diabetic and nondiabetic patients: an analysis from the aspirin-induced platelet effect (ASPECT) study. Diabetes. 2007;56:3014–9. doi: 10.2337/db07-0707. [DOI] [PubMed] [Google Scholar]
- 5.Evangelista V, de Berardis G, Totani L, et al. Persistent platelet activation in patients with type 2 diabetes treated with low doses of aspirin. J Thromb Haemost. 2007;5:2197–203. doi: 10.1111/j.1538-7836.2007.02728.x. [DOI] [PubMed] [Google Scholar]
- 6.Mortensen SB, Larsen SB, Grove EL, Kristensen SD, Hvas AM. Reduced platelet response to aspirin in patients with coronary artery disease and type 2 diabetes mellitus. Thromb Res. 2010;126:e318–22. doi: 10.1016/j.thromres.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Suwalski G, Suwalski P, Filipiak KJ, et al. The effect of off-pump coronary artery bypass grafting on platelet activation in patients on aspirin therapy until surgery day. Eur J Cardiothorac Surg. 2008;34:365–9. doi: 10.1016/j.ejcts.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Postula M, Tarchalska-Kryńska B, Filipiak KJ, et al. Factors responsible for “aspirin resistance“ – can we identify them? Kardiol Pol. 2010;68:403–11. [PubMed] [Google Scholar]
- 9.Rosiak M, Postula M, Kaplon-Cieslicka A, et al. Effect of ASA dose doubling versus switching to clopidogrel on plasma inflammatory markers concentration in patients with type 2 diabetes and high platelet reactivity: the AVOCADO study. Cardiol J. 2013 doi: 10.5603/CJ.2013.0045.. in press. [DOI] [PubMed] [Google Scholar]
- 10.Gluba A, Banach M, Mikhailidis DP, Rysz J. Genetic determinants of cardiovascular disease: the renin-angiotensin-aldosterone system, paraoxonases, endothelin-1, nitric oxide synthase and adrenergic receptors. In Vivo. 2009;23:797–812. [PubMed] [Google Scholar]
- 11.Goodman T, Ferro A, Sharma P. Pharmacogenetics of aspirin resistance: a comprehensive systematic review. Br J Clin Pharmacol. 2008;66:222–32. doi: 10.1111/j.1365-2125.2008.03183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Würtz M, Kristensen SD, Hvas AM, Grove EL. Pharmacogenetics of the antiplatelet effect of aspirin. Curr Pharm Des. 2012;18:5294–308. doi: 10.2174/138161212803251907. [DOI] [PubMed] [Google Scholar]
- 13.Catakoglu AB, Aytekin S, Celebi H, et al. The influence of aspirin resistance on non-fatal coronary events following percutaneous coronary interventions. Arch Med Sci. 2009;5:531–8. [Google Scholar]
- 14.Baigent C, Sudlow C, Collins R, et al. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Postula M, Kaplon-Cieslicka A, Rosiak M, et al. Genetic determinants of platelet reactivity during acetylsalicylic acid therapy in diabetic patients: evaluation of 27 polymorphisms within candidate genes. J Thromb Haemost. 2011;9:2291–301. doi: 10.1111/j.1538-7836.2011.04482.x. [DOI] [PubMed] [Google Scholar]
- 16.Postula M, Janicki PK, Rosiak M, et al. New single nucleotide polymorphisms associated with differences in platelets reactivity in patients with type 2 diabetes treated with acetylsalicylic acid: genome-wide association approach and pooled DNA strategy. J Thromb Thrombolysis. 2012 doi: 10.1007/s11239-012-0823-6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 17.Gasparyan AY. Aspirin resistance: more emphasis on definition and appropriate use of platelet function tests is needed. Arch Med Sci. 2009;5:539–41. [Google Scholar]
- 18.Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:33157–60. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 19.Patrono C, Ciabattoni G, Patrignani P, et al. Clinical pharmacology of platelet cyclooxygenase inhibition. Circulation. 1985;72:1177–84. doi: 10.1161/01.cir.72.6.1177. [DOI] [PubMed] [Google Scholar]
- 20.Krasopoulos G, Brister SJ, Beattie WS, Buchanan MR. Aspirin “resistance“ and risk of cardiovascular morbidity: systematic review and meta-analysis. BMJ. 2008;336:195–8. doi: 10.1136/bmj.39430.529549.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maree AO, Curtin RJ, Chubb A, et al. Cyclooxygenase-1 haplotype modulates platelet response to aspirin. J Thromb Haemost. 2005;3:2340–5. doi: 10.1111/j.1538-7836.2005.01555.x. [DOI] [PubMed] [Google Scholar]
- 22.Yokoyama C, Tanabe T. Cloning of human gene encoding prostaglandin endoperoxide synthase and primary structure of the enzyme. Biochem Biophys Res Commun. 1989;165:888–94. doi: 10.1016/s0006-291x(89)80049-x. [DOI] [PubMed] [Google Scholar]
- 23.Pratico‘ D, Lawson JA, FitzGerald GA. Cyclooxygenase-dependent formation of the isoprostane 8-epi-prostaglandin F2alpha. J Biol Chem. 1995;270:9800–8. doi: 10.1074/jbc.270.17.9800. [DOI] [PubMed] [Google Scholar]
- 24.Davì G, Ciabattoni G, Consoli A, et al. In vivo formation of 8-iso-prostaglandin F2alpha and platelet activation in diabetes mellitus: effects of improved metabolic control and vitamin E supplementation. Circulation. 1999;99:224–9. doi: 10.1161/01.cir.99.2.224. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Ciabattoni G, Cre‘minon C, et al. Immunological characterization of urinary 8-epi-PGF2alpha excretion in man. J Pharmacol Exp Ther. 1995;275:94–100. [PubMed] [Google Scholar]
- 26.Morrow JD, Frei B, Longmire AW, et al. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers: smoking as a cause of oxidative damage. N Engl J Med. 1995;332:1198–203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 27.Reilly M, Delanty N, Lawson JA, et al. Modulation of oxidant stress in vivo in chronic cigarette smokers. Circulation. 1996;94:19–25. doi: 10.1161/01.cir.94.1.19. [DOI] [PubMed] [Google Scholar]
- 28.Davì G, Alessandrini P, Mezzetti A, et al. In vivo formation of 8-epi-PGF2alpha is increased in hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1997;17:3230–5. doi: 10.1161/01.atv.17.11.3230. [DOI] [PubMed] [Google Scholar]
- 29.Reilly MP, Delanty N, Tremoli E, et al. Increased formation of distinct F2 isoprostanes in hypercholesterolemia. Circulation. 1998;98:2822–8. doi: 10.1161/01.cir.98.25.2822. [DOI] [PubMed] [Google Scholar]
- 30.Delanty N, Reilly M, Praticò D, et al. 8-Epi-PGF2alpha generation during coronary reperfusion: a potential quantitative marker of oxidant stress in vivo. Circulation. 1997;96:2492–9. doi: 10.1161/01.cir.95.11.2492. [DOI] [PubMed] [Google Scholar]
- 31.Reilly MP, Delanty N, O'Callaghan P, et al. Increased generation of the F2 isoprostanes, IPF2alpha-1 and 8-iso-PGF2alpha, in acute coronary angioplasty: evidence for oxidant stress during coronary reperfusion in humans. Circulation. 1997;96:3314–20. doi: 10.1161/01.cir.96.10.3314. [DOI] [PubMed] [Google Scholar]
- 32.Cipollone F, Ciabattoni G, Patrignani P, et al. Oxidant stress and aspirin-insensitive thromboxane biosynthesis in severe unstable angina. Circulation. 2000;102:1007–13. doi: 10.1161/01.cir.102.9.1007. [DOI] [PubMed] [Google Scholar]
- 33.Lordkipanidzé M, Diodati JG, Turgeon J, Schampaert E, Palisaitis DA, Pharand C. Platelet count, not oxidative stress, may contribute to inadequate platelet inhibition by aspirin. Int J Cardiol. 2010;143:43–50. doi: 10.1016/j.ijcard.2009.01.037. [DOI] [PubMed] [Google Scholar]
- 34.Wang LH, Hajibeigi A, Xu XM, Loose-Mitchell D, Wu KK. Characterization of the promoter of human prostaglandin H synthase-1 gene. Biochem Biophys Res Commun. 1993;190:406–11. doi: 10.1006/bbrc.1993.1062. [DOI] [PubMed] [Google Scholar]
- 35.Halushka MK, Walker LP, Halushka PV. Genetic variation in cyclooxygenase 1: effects on response to aspirin. Clin Pharmacol Ther. 2003;73:122–30. doi: 10.1067/mcp.2003.1. [DOI] [PubMed] [Google Scholar]
- 36.Hillarp A, Palmqvist B, Lethagen S, Villoutreix BO, Mattiasson I. Mutations within the cyclooxygenase-1 gene in aspirin non-responders with recurrence of stroke. Thromb Res. 2003;112:275–83. doi: 10.1016/j.thromres.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Ulrich CM, Bigler J, Sibert J, et al. Cyclooxygenase 1 (COX1) polymorphisms in African-American and Caucasian populations. Hum Mutat. 2002;20:409–10. doi: 10.1002/humu.9080. [DOI] [PubMed] [Google Scholar]
- 38.Rosiak M, Postula M, Kaplon-Cieslicka A, et al. The effect of doubling the dose of ASA versus switching to clopidogrel on platelet reactivity in patients with type 2 diabetes with high-on-treatment platelet reactivity: the AVOCADO study. Kardiol Pol. 2013 doi: 10.5603/KP.2013.0056.. in press. [DOI] [PubMed] [Google Scholar]
- 39.Patrono C, Ciabattoni G, Pinca E, et al. Low dose aspirin and inhibition of thromboxane B2 production in healthy subjects. Thromb Res. 1980;17:317–27. doi: 10.1016/0049-3848(80)90066-3. [DOI] [PubMed] [Google Scholar]
- 40.Ciabattoni G, Pugliese F, Davi G, Pierucci A, Simonetti BM, Patrono C. Fractional conversion of thromboxane B2 to urinary 11-dehydrothromboxane B2 in man. Biochim Biophys Acta. 1989;992:66–70. doi: 10.1016/0304-4165(89)90051-2. [DOI] [PubMed] [Google Scholar]
- 41.Helmersson J, Arnlov J, Axelsson T, Basu S. A polymorphism in the cyclooxygenase 1 gene is associated with decreased inflammatory prostaglandin F2alpha formation and lower risk of cardiovascular disease. Prostaglandins Leukot Essent Fatty Acids. 2009;80:51–6. doi: 10.1016/j.plefa.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Lee CR, Bottone FG, Jr, Krahn JM, et al. Identification and functional characterization of polymorphisms in human cyclooxygenase-1 (PTGS1) Pharmacogenet Genomics. 2007;17:145–60. doi: 10.1097/01.fpc.0000236340.87540.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonzalez-Conejero R, Rivera J, Corral J, Acuna C, Guerrero JA, Vicente V. Biological assessment of aspirin efficacy on healthy individuals: heterogeneous response or aspirin failure? Stroke. 2005;36:276–80. doi: 10.1161/01.STR.0000151362.65339.f9. [DOI] [PubMed] [Google Scholar]
- 44.Lepantalo A, Mikkelsson J, Resendiz JC, et al. Polymorphisms of COX-1 and GPVI associate with the antiplatelet effect of aspirin in coronary artery disease patients. Thromb Haemost. 2006;95:253–9. doi: 10.1160/TH05-07-0516. [DOI] [PubMed] [Google Scholar]