Abstract
Introduction
All living organisms have evolved sophisticated mechanisms to maintain appropriate iron levels in their cells and within their body. Recently our understanding of iron metabolism has dramatically increased. Overt labile plasma iron (LPI) represents a component of non-transferrin bound iron (NTBI) that is both redox active and chelatable, capable of permeating into organs and inducing tissue iron overload. The LPI measures the iron-specific capacity of a given sample to produce reactive oxygen species. We studied for the first time NTBI correlations with markers of iron status and inflammation in prevalent hemodialyzed patients.
Material and methods
Complete blood count, urea, serum lipids, fasting glucose, creatinine, ferritin, serum iron, total iron binding capacity (TIBC) were studied by standard laboratory method. The NTBI was assessed commercially available kits from Aferrix Ltd in Tel Aviv, Israel. A test result of 0.6 units of LPI or more indicates a potential for iron-mediated production of reactive oxygen species in the sample.
Results
Patients with LPI units ≥ 0.6 had higher serum iron, erythropoiesis stimulating agents (ESA) dose, ferritin, high-sensitivity C-reactive protein (hsCRP), hepcidin and lower hemojuvelin. In hemodialyzed patients NTBI correlated with hsCRP (r = 0.37, p < 0.01), ferritin (r = 0.41, p < 0.001), IL-6 (r = 0.43, p < 0.001). In multivariate analysis predictors of NTBI were hemoglobin and alkaline phosphatase, explaining 58% of the variability
Conclusions
Elevated NTBI in HD may be due to disturbed iron metabolism. Anemia and liver function might also contribute to the presence of NTBI in this population.
Keywords: iron metabolism, non-transferrin-bound iron, hemodialysis, anemia, hepcidin, ferritin, inflammation
Introduction
Before the era of erythropoietin stimulating agents, blood transfusion and iron administration were the major approaches to combat anemia in dialyzed patients; therefore we faced the problem of secondary iron overload. The overload appears as excess iron in the serum and is collectively known as non-transferrin-bound iron (NTBI) [1]. This is in excess of the iron binding capacity of transferrin, resulting in its binding to various proteins and other putative ligands in the circulation. A NTBI levels vary between 1 and 10 µM in overloaded patients [1]. Studies have also shown that in hemochromatosis patients, NTBI is present in spite of incomplete transferrin saturation [2]. Therefore, these classical parameters might not provide an accurate picture of the iron status of patients. For this reason, it is important to monitor and accurately quantify the NTBI fraction. Although iron status and iron deficiency have been extensively studied in dialyzed iron recipients, there have been a few reports on NTBI [3–6]. In hemodialysis (HD) patients, iron supplementation is frequent to ensure adequate iron stores for erythropoiesis and to maintain target hemoglobin. The source of NTBI in hemodialysis is not only intravenous iron therapy, but also the hemodialysis procedure per se. It may be due to microhemolysis during hemodialysis. The NTBI is, however, found to be catalytically inactive. However, the nature of NTBI was not clear in the dialysis population, but there was good evidence to suggest that it was neither heme nor ferritin bound iron [7, 8]. It is believed that free iron is potentially toxic, possibly leading to free radical generation with further consequences [9]. Therefore, the measurement of NTBI could serve as an early marker for tissue damage induced by reactive oxygen species. However, NTBI did not correlate with serum ferritin and oxidative markers [4] or with lipid peroxides [6]. We studied for the first time NTBI correlations with markers of iron status and inflammation in prevalent hemodialyzed patients. We also looked at NTBI in relation to comorbidities such as diabetes, and hepatitis B and C.
Material and methods
We conducted a cross-sectional, single center study. Fifty-three hemodialyzed patients (25 females) with overall mean age of 65 ±16 years were included in this study. Mean time on dialyses was 51 months. The causes of end-stage renal disease were diabetic nephropathy (n = 10), chronic glomerulonephritis (n = 18), autosomal dominant polycystic kidney disease (n = 6) and others or unknown (n = 19). There were 13 patients with HCV positive status and 5 with HBV positive status.
All the dialyzed patients met the following criteria: a stable clinical state, C-reactive protein (CRP) below 6 mg/l (using the qualitative method for screening), no oral contraception in women of child-bearing age, stable and no more than twice the normal alanine and aspartate aminotranspherases (ALT, ASP) activities, no evidence of blood loss other than that related to dialysis during the last 6 months, and no cause of anemia other than renal. None of the patients investigated had received blood transfusions for at least 1.5 months and no drugs known to affect platelet function and coagulation were administered for at least 2 weeks prior to the study (except heparin during a hemodialysis session). The study was approved by the Ethic Committee. All the patients were on chronic maintenance hemodialyses (three times a week for 4–4.5 h per hemodialysis procedure). All the patients were given ESA (epoetin α or darbepoetin α) for at least 6 months and a maintenance dose of i.v. iron (iron sucrose, median dose 100 mg per week). All HD patients were receiving treatment with polysulfone membranes and bicarbonate-based dialysate with heparinization. Blood was drawn without stasis in the morning when patients appeared for routine office assessment after an overnight fast, to avoid circadian variations. The following biochemical parameters were assessed: complete blood count, serum iron, total iron binding capacity, ferritin, CRP, total protein, lipids, alanine and aspartate aminotransferases, alkaline phosphatase, uric acid, and glucose by means of standard laboratory methods in the central laboratory.
Non-transferrin-bound iron was assessed using a FeROS eLPI kit (a fluorescence-based assay intended for the in vitro semi-quantitative detection of both overt and cryptic redox-active forms of NTBI) by Aferrix Ltd in Tel Aviv, Israel. LPI levels less than 0.4 were considered as negative. A test result of more than 0.6 units of eLPI indicates a potential for iron-mediated production of reactive oxygen species in the sample. Results of 0.4–0.6 eLPI units are considered as low positive. High-sensitivity CRP was studied using kits from American Diagnostica, Greenwich, CT, USA. Soluble transferrin receptor (sTfR), interleukin-6 (high-sensitivity), and tumor necrisis factor-α (TNF-α) were studied using kits from R&D (Abington, UK). Hepcidin was measured using an assay from Bachem, UK, hemojuvelin from Uscn Life Sci, Wuhan, China.
Statistical analysis
Data were expressed as means ± SD or median and interquartile ranges. The data given were analyzed using Statistica 10.0 computer software (Tulsa, OK, USA). The examination of the distribution normality of variables was done using the Shapiro-Wilk W test. The Mann-Whitney rank sum U test or Student t test was used to compare differences between groups, with p < 0.05 considered statistically significant. Multiple regression analysis was used to determine independent factors affecting the dependent variable. Factors showing linear correlations with NTBI (p < 0.1) were included in the analysis.
Results
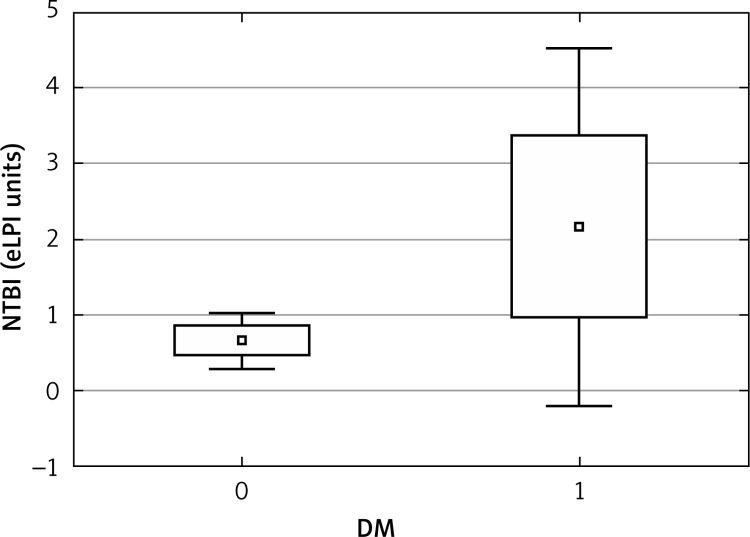
Labile plasma iron ≥ 0.6 units was found in 19 out of 53 (36%) hemodialyzed patients. Median number of LPI units was 0.3 (mean 0.9), with a range of 0.0–11.0 units. Clinical and biochemical characteristics of hemodialyzed patients are given in Table I, while iron status and inflammatory markers are presented in Table II. Patients with LPI units ≥ 0.6 had higher serum iron, ESA dose, ferritin, hsCRP and hepcidin, and lower hemojuvelin (Table II). In hemodialyzed patients, NTBI correlated with presence of diabetes (r = 0.30, p < 0.05), hemoglobin (r = –0.31, p < 0.05), hsCRP (r = 0.36, p < 0.01), ferritin (r = 0.31, p < 0.05), alkaline phosphatase (r = 0.51, p < 0.01), alanine aminotransferase (r = 0.51, p < 0.01), aspartate aminotransferase (r = 0.43, p < 0.01), serum glucose (r = 0.27, p < 0.05), HDL (r = –0.29, p < 0.05), and IL-6 (r = 0.42, p < 0.001). In multivariate analysis predictors of NTBI were hemoglobin (β value –0.27, p = 0.0027) and alkaline phosphatase (β value 0.42, p = 0.004), explaining 58% of the variability. F = 98, p < 0.00046 and SE was 1.44. The 10 diabetic patients in our population had higher NTBI than nondiabetic patients (Figure 1). Moreover, 13 anemic patients (with anemia defined as hemoglobin below 10 g/dl) had higher NTBI relative to nonanemic patients (0.6; 0.4–1.2 eLPI units, vs. 0.2; 0.0–0.8 eLPI units, p < 0.05) as well as 13 HCV positive patients versus HCV negative ones (0.5; 0.3–1.0 eLPI units vs, 0.25; 0.0–0.7 eLPI units, p < 0.05).
Table I.
Clinical and biochemical characteristics of hemodialyzed patients with NTBI values
| Parameter | NTBI ≥ 0.6 eLPI units | NTBI < 0.6 eLPI units |
|---|---|---|
| Age [years] | 65.63 ±12.03 | 63.76 ±18.39 |
| Time on HD [months] | 37 (20–84) | 35 (8–65) |
| Hemoglobin [g/dl] | 10.76 ±1.47 | 11.16 ±1.26 |
| Erythrocyte count [× 1012/l] | 3.55 ±0.55 | 3.66 ±0.54 |
| MCHC [pg] | 30.18 ±1.80 | 30.89 ±0.85* |
| Leukocyte count [× 103/l] | 5.69 ±1.65 | 6.21 ±1.96 |
| Platelet count [× 106/l] | 193.22 ±62.29 | 184.39 ±62.80 |
| Glucose [mg/dl] | 136.95 ±96.03 | 120.41 ±51.70 |
| Uric acid [mg/dl] | 4.47 ±1.61 | 4.50 ±0.84 |
| Total protein [g/dl] | 6.42 ±0.56 | 6.76 ±0.53* |
| Albumin [g/dl] | 3.71 ±0.36 | 3.95 ±0.37* |
| Total cholesterol [mg/dl] | 237.26 ±39.92 | 238.44 ±39.38 |
| HDL [mg/dl] | 42.89 ±16.51 | 46.25 ±14.91 |
| Triglycerides [mg/dl] | 139.26 ±57.35 | 139.69 ±90.88 |
| Alkaline phosphatase [U/l] | 110.5 (78.5–149) | 92 (73–140) |
| Aanine aminotransferase [U/l] | 16 (11–18) | 14.5 (10–19.5) |
| Aspartate aminotransferase [U/l] | 17 (14–22) | 17 (13.5–19) |
Data given are means ± SD, or medians and interquartile ranges
p < 0.05.
Table II.
Iron status and inflammatory markers of hemodialyzed patients with NTBI values
| Parameter | NTBI ≥ 0.6 eLPI units | NTBI < 0.6 eLPI units |
|---|---|---|
| hsCRP [mg/l] | 8.86 (1.81–12.48) | 5.82 (1.81–9.80)** |
| TNF-α [pg/ml] | 6.15 (0.1–14.01) | 5.94 (0.1–8.92) |
| IL-6 [pg/ml] | 2.82 (0.1–6.16) | 0.57 (0.1–2.96)*** |
| Fibrinogen [mg/dl] | 364.16 ±73.13 | 323.65 ±80.94* |
| Erythropoietin dose [U/week] | 7235 ±2772 | 4939 ±3630* |
| sTfR [nmol/l] | 42.63 ±16.43 | 34.77 ±14.71* |
| Iron [µg/dl] | 76.14 ±29.14 | 57.06 ±25.40* |
| TIBC [µg/dl] | 282.00 ±58.29 | 247.69 ±59.86* |
| Ferritin [ng/ml] | 401.7 (114.1–704.6) | 303 (11.4–492.2)** |
| Hepcidin [ng/ml] | 26.65 ±12.98 | 20.18 ±14.16 |
| Hemojuvelin [ng/ml] | 1.37 ±0.93 | 2.72 ±1.84* |
Data given are means ± SD, or medians and interquartile ranges
p < 0.05
p < 0.01
p < 0.001.
Figure 1.
NTBI in HD population according to diabetic status
Discussion
There are few studies of LPI, or other related iron species classified as NTBI, in end-stage renal disease (ESRD) patients [3–6, 10]. In our cohort we found that LPI ≥ 0.6 units was found in 19 out of 53 patients (36%), whereas LPI ≥ 0.4 units was found in 22 out of 53 hemodialyzed patients (42%). In a cohort of dialysis patients at a single center, up to 20% of the patients tested were positive for LPI (with the cut-off point of 0.5 LPI units) [5]. In the study of Prakash et al. [4], NTBI as well as ferritin were significantly higher in 22 hemodialyzed patients not on intravenous iron therapy than in 24 patients with chronic kidney disease and 20 normal controls. Driss et al. [3] studied 65 hemodialyzed patients; 20 patients received weekly infusions of iron polymaltose (Maltofer), whereas 45 patients had been off iron therapy for more than 2 months. Then they investigated 12 patients during two consecutive HD sessions, one session without and one session with infusion of 100 mg of Maltofer over 4 h [3]. They found that NTBI was detected in 41% of the patients and the proportion of NTBI-positive patients was the same whether or not they received iron therapy. Thus, the prevalence of NTBI positivity was very similar to our studied population. In fact, it has been shown previously in a Dutch cohort of hemodialysis patients that NTBI was detectable and that treatment with iron saccharate leads to a fivefold increase [9]. This strongly suggests that intravenous iron is important in the generation of NTBI [10]. In our previous study on kidney transplant patients, NTBI was related to ferritin [11]. In all heart transplant recipients, NTBI was negative [12]. Dresow et al. [13] described for the first time the occurrence of NTBI after oral iron medication in subjects with iron deficiency anemia. Values for NTBI found in plasma were similar to those reported in iron overload diseases [14]; however, in all subjects transferrin saturation was far below 100% [14]. The term LPI is an operational definition for non-transferrin-bound iron (NTBI), and denotes all iron in the plasma that is loosely bound and hence may have redox-active potential. The NTBI is associated with reactive oxygen species formation. The LPI is usually not observed in healthy individuals. Esposito et al. [5] did not detect LPI in healthy controls in their study, as we also did not in our previous study [12]. They suggested that increased frequency of LPI-positive ESRD patients is probably indicative of impaired iron metabolism. Whether LPI is a sign of ‘cryptic’ or overt iron overload in these patients was not clear. They also stressed that the diagnostic value of serum ferritin was limited and, accordingly, they found that it failed to show any correlation with LPI [5].
The univariate correlation between NTBI and markers of inflammation, including ferritin, supports the role of iron in the pathogenesis of infection/inflammation, as elegantly reviewed recently [15]. The relation between iron and inflammation might also explain the fact that anemic patients had higher NTBI relative to nonanemic patients. In our study, we found that predictors of NTBI were hemoglobin and alkaline phosphatase, and at the same time we observed that NTBI was higher in hepatitis C positive patients. This suggests that the liver plays an important role in iron metabolism, in the presence of hepatitis C. In the previous studies, Detivaud et al. [16] found that parameters reflecting hepatic function were correlated with hepcidin levels, while Aoki et al. [17] observed that in chronic hepatitis C, the hepcidin mRNA level was associated with iron stores. In has been reported that NTBI is efficiently taken up by the liver [18]. In rats using single-pass perfused liver, iron uptake as NTBI ranged from 58% to 75% when compared to less than 1% of transferrin-bound iron [19]. The NTBI then mainly targets the hepatocytes; however, NTBI uptake by the liver is not down-regulated by hepatocytic iron excess [20, 21]. In liver diseases, NTBI is likely to be observed through hepcidin deficiency related to either etiology or the severity of the liver dysfunction [16, 22]. In active chronic alcoholics, NTBI was found in more than 83% of patients, and subjects with cirrhosis had higher NTBI that the control group [23]. To date, there are no data available on hepatitis C and NTBI.
Kalantar-Zadeh et al. [24] found that in the national database of DaVita of 38 328 long-term hemodialyzed patients the ESA-hyporesponsive group (n = 9581) had the lowest serum albumin, total iron binding capacity (TIBC), iron, transferrin saturation (TSAT) and ferritin and highest intact parathyroid hormone (PTH) and alkaline phosphatase levels. They concluded that these findings may have important clinical implications in the management of anemia in chronic kidney disease (CKD) patients, especially those undergoing HD treatment, and serum alkaline phosphatase levels, presumably a biomarker of bone turnover activity, may be related to non-bone related conditions such as liver disease, particularly since they assessed only total alkaline phosphatase. Therefore we might speculate that alkaline phosphatase as an indicator of renal osteodystrophy could be associated with ESA-hyporesponsiveness by diminishing endogenous erythropoietin synthesis, reducing bone marrow erythroid progenitors, and shortening erythrocyte survival [25]. Indirect effects include the association of renal osteodystrophy with bone marrow fibrosis [26]. In addition, it is possible to have higher labile plasma iron because of inadequate protein intake, since the binding plasma proteins, including transferrin, could be decreased; therefore lower albumin in subjects with high NTBI would be a result of inflammation/malnutrition.
The 10 diabetic patients in our population had higher NTBI than nondiabetic patients. The NTBI (measured by HPLC) was also detected in patients with type 2 diabetes in 59% of patients with newly diagnosed diabetes NTBI was present and in 92% of patients with long-term diabetes [27]. Mean NTBI values varied significantly among the three groups, with the highest values being observed in patients with known diabetes and the lowest in the control subjects [27]. Analysis of modified albumins has shown that both glycation and oxidation increase the albumin iron binding capacity [28]. Non-enzymatic modifications of albumin, that are prevalent in diabetes and increased oxidative stress, may have a fundamental role in NTBI speciation. This result would indicate that the low NTBI levels so far measured in diabetic sera may be understated and that different analytical techniques are required to determine NTBI levels in diabetic patients [28]. However, in the modern assay of eLPI we used in our study, the difference in NTBI between diabetic and non-diabetic patients should not be ascribed to the assay performance [29]. The NTBI was found to be an independent predictor of mortality after myocardial infarction in individuals with diabetes [30], probably due to the link between diabetes, cardiovascular complications [31–35], and iron as a pro-oxidant cofactor for cardiovascular risk [36].
The NTBI was first described in conditions of iron overload, when TSAT exceeded 100% [1]. Using this concept, intravenous iron therapy would add large amounts of NTBI to the plasma. It was demonstrated that parenteral iron preparations increased the intracellular labile iron pool of the human hepatoma HepG2 cells [37]. Previously, we studied iron parameters including hepcidin in patients treated with intravenous iron saccharate [38]. In these patients later we also assessed NTBI and found a non-significant rise in NTBI after intravenous administration of 1 γ of iron sucrose (data not published).
However, in the recent review mounting evidence suggesting that abnormalities in the normal pathophysiology of bone/mineral axis, iron, and erythropoietin play a role in accelerating CKD and CVD was underlined [39]. Nowadays, multiple lines of evidence suggest that in addition to the direct effects of uremia on cardiovascular pathophysiology [40], secondary changes in bone and mineral metabolism, as well as iron deficiency and anemia, have the potential to be important contributors to the pathogenesis of chronic cardiorenal syndrome.
We are fully aware of the limitation of this study due to its cross-sectional design, single center and moderate sample size.
To conclude, elevated NTBI in HD may be due to disturbed iron metabolism. Anemia and liver function might also contribute to the presence of NTBI in this population.
References
- 1.Breuer W, Hershko C, Cabantchik ZI. The importance of non-transferrin iron in disorders of iron metabolism. Transfusion Sci. 2000;23:185–92. doi: 10.1016/s0955-3886(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 2.Cabantchik ZI, Breuer W, Zanninelli G, Cianciulli P. LPI-labile plasma iron in iron overload. Best Pract Res Clin Haematol. 2005;18:277–87. doi: 10.1016/j.beha.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Driss F, Vrtovsnik F, Katsahian S, et al. Effects of intravenous polymaltose iron on oxidant stress and non-transferrin-bound iron in hemodialysis patients. Nephron Clin Pract. 2005;99:c63–7. doi: 10.1159/000083208. [DOI] [PubMed] [Google Scholar]
- 4.Prakash M, Upadhya S, Prabhu R. Serum non-transferrin bound iron in hemodialysis patients not receiving intravenous iron. Clin Chim Acta. 2005;360:194–8. doi: 10.1016/j.cccn.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 5.Espósito BP, Breuer W, Slotki I, Cabantchik ZI. Labile iron in parenteral iron formulations and its potential for generating plasma nontransferrin-bound iron in dialysis patients. Eur J Clin Invest. 2002;32(Suppl 1):42–9. doi: 10.1046/j.1365-2362.2002.0320s1042.x. [DOI] [PubMed] [Google Scholar]
- 6.Scheiber-Mojdehkar B, Lutzky B, Schaufler R, Sturm B, Goldenberg H. Non-transferrin-bound iron in the serum of hemodialysis patients who receive ferric sacharate: no correlation to peroxide generation. J Am Soc Nephrol. 2004;6:1648–55. doi: 10.1097/01.asn.0000130149.18412.56. [DOI] [PubMed] [Google Scholar]
- 7.Hershko C, Peto TEA. Non-transferrin plasma iron. Br J Haematol. 1987;66:149–51. doi: 10.1111/j.1365-2141.1987.tb01291.x. [DOI] [PubMed] [Google Scholar]
- 8.Baker E, Morgan EH, Brock JH, Halliday JW, Pippard MJ, Poowell LW, editors. Iron metabolism in health and disease. London: Saunders; 1994. Iron transport; pp. 63–96. [Google Scholar]
- 9.Gutteridge JMC, Rowely DA, Griffiths E, Halliwell B. Low molecular-weight iron complexes and oxygen radical reactions in idiopathic hemochromatosis. Clin Sci. 1995;68:463–7. doi: 10.1042/cs0680463. [DOI] [PubMed] [Google Scholar]
- 10.Kooistra MP, Kersting S, Gosriwatana I, Lu S, Hider RC, Marx JJM. Non transferrin-bound iron in plasma of haemodialysis patients after intravenous iron saccharate infusion. Eur J Clin Invest. 2002;32(Suppl. 1):36. doi: 10.1046/j.1365-2362.2002.0320s1036.x. [DOI] [PubMed] [Google Scholar]
- 11.Malyszko J, Głowińska I, Malyszko JS, Levin-Iaina N, Koc-Zorawska E, Mysliwiec M. Iron metabolism in kidney allograft recipients: still a mystery? Transplant Proc. 2011;43:2973–5. doi: 10.1016/j.transproceed.2011.08.055. [DOI] [PubMed] [Google Scholar]
- 12.Przybylowski P, Malyszko J, Levin-Iaina N, Malyszko JS. Lack of non-transferrin-bound iron in heart transplant recipients. Transplant Proc. 2011;43:3068–70. doi: 10.1016/j.transproceed.2011.08.033. [DOI] [PubMed] [Google Scholar]
- 13.Dresow B, Petersen D, Fischer R, Nielsen P. Non-transferrin-bound iron in plasma following administration of oral iron drugs. Biometals. 2008;21:273–6. doi: 10.1007/s10534-007-9116-5. [DOI] [PubMed] [Google Scholar]
- 14.Wang WC, Ahmed N, Hanna M. Non-transferrin-bound iron in long-term transfusion in children with congenital anemias. J Pediatr. 1986;108:552–7. doi: 10.1016/s0022-3476(86)80832-0. [DOI] [PubMed] [Google Scholar]
- 15.Małyszko J, Levin-Iaina N, Myśliwiec M, Przybyłowski P, Durlik M. Iron metabolism in solid-organ transplantation: how far are we from solving the mystery? Pol Arch Med Wewn. 2012;122:504–11. [PubMed] [Google Scholar]
- 16.Detivaud L, Nemeth E, Boudjema K, et al. Hepcidin levels in humans are correlated with hepatic iron stores, hemoglobin levels and hepatic function. Blood. 2005;106:746–8. doi: 10.1182/blood-2004-12-4855. [DOI] [PubMed] [Google Scholar]
- 17.Aoki CA, Rossaro L, Ramsamooj RR, Brandhagen D, Burritt MF, Bowlus CL. Liver hepcidin mRNA corrrelates with iron stores, but not inflammation, in patients with chronic hepatitis. C. J Clin Gastroenterol. 2005;39:71–4. [PubMed] [Google Scholar]
- 18.Brissot P, Wright TL, Ma WL, Weisiger RA. Efficient clearance of nontransferrin-bound iron by rat liver. Implications for hepatic iron loading in iron overload states. J Clin Invest. 1985;76:1463–70. doi: 10.1172/JCI112125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craven CM, Alexander J, Eldridge M, Kushner JP, Bernstein S, Kaplan J. Tissue distribution and clearance kinetics of non-transferrin-bound iron in the hypotransferrinemic mouse: a rodent model for hemochromatosis. Proc Natl Acad Sci USA. 1987;84:3457–61. doi: 10.1073/pnas.84.10.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker E, Baker SM, Morgan EH. Characterisation of non-transferrin-bound iron (ferric citrate) uptake by rat hepatocytes in culture. Biochim Biophys Acta. 1998;1380:21–30. doi: 10.1016/s0304-4165(97)00120-7. [DOI] [PubMed] [Google Scholar]
- 21.Wright TL, Brissot P, Ma WL, Weisiger RA. Characterization of nontransferrin-bound iron clearance by rat liver. J Biol Chem. 1986;261:10909–14. [PubMed] [Google Scholar]
- 22.Harrison-Findik DD, Klein E, Crist C, Evans J, Timchenko N, Gollan J. Ironmediated regulation of liver hepcidin expression in rats and mice is abolished by alcohol. Hepatology. 2007;46:1979–85. doi: 10.1002/hep.21895. [DOI] [PubMed] [Google Scholar]
- 23.De Feo TM, Fargion S, Duca L, et al. Non-transferrin-bound iron in alcohol abusers. Alcohol Clin Exp Res. 2001;25:1494–9. doi: 10.1097/00000374-200110000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Kalantar-Zadeh K, Lee GH, Miller JE, et al. Predictors of hyporesponsiveness to erythropoiesis-stimulating agents in hemodialysis patients. Am J Kidney Dis. 2009;53:823–34. doi: 10.1053/j.ajkd.2008.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brancaccio D, Cozzolino M, Gallieni M. Hyperparathyroidism and anemia in uremic subjects: a combined therapeutic approach. J Am Soc Nephrol. 2004;15(Suppl. 1):S21–4. doi: 10.1097/01.asn.0000093369.09194.12. [DOI] [PubMed] [Google Scholar]
- 26.Rao DS, Shih MS, Mohini R. Effect of serum parathyroid hormone and bone marrow fibrosis on the response to erythropoietin in uremia. N Engl J Med. 1993;328:171–5. doi: 10.1056/NEJM199301213280304. [DOI] [PubMed] [Google Scholar]
- 27.Lee DH, Liu DY, Jakobs DR, et al. Common presence of nontransferrin-bound iron among patients with type 2 diabetes. Diabetes Care. 2006;29:1090–5. doi: 10.2337/diacare.2951090. [DOI] [PubMed] [Google Scholar]
- 28.Silva AM, Hider RC. Influence of non-enzymatic post-translation modifications on the ability of human serum albumin to bind iron. Implications for non-transferrin-bound iron speciation. Biochim Biophys Acta. 2009;1794:1449–58. doi: 10.1016/j.bbapap.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Breuer W, Ghoti H, Shattat A, et al. Non-transferrin bound iron in Thalassemia: differential detection of redox active forms in children and older patients. Am J Hematol. 2012;87:55–61. doi: 10.1002/ajh.22203. [DOI] [PubMed] [Google Scholar]
- 30.Sulieman M, Asleh R, Cabantchik ZI, et al. Serum chelatable redox-active iron is an independent predictor of mortality after myocardial infarction in individuals with diabetes. Diabetes Care. 2004;27:2730–2. doi: 10.2337/diacare.27.11.2730. [DOI] [PubMed] [Google Scholar]
- 31.Athyros VG, Hatzitolios AI, Karagiannis A, et al. IMproving the imPlemEntation of cuRrent guidelines for the mAnagement of major coronary hearT disease rIsk factors by multifactorial interVEntion. The IMPERATIVE renal analysis. Arch Med Sci. 2011;7:984–92. doi: 10.5114/aoms.2011.26610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aronow WS. Editorial on management of diabetes mellitus with coronary artery disease. Arch Med Sci. 2011;7:928–30. doi: 10.5114/aoms.2011.26601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyers DG. The iron hypothesis – does iron cause atherosclerosis? Clin Cardiol. 1996;19:925–9. doi: 10.1002/clc.4960191205. [DOI] [PubMed] [Google Scholar]
- 34.Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci. 2013;9:191–200. doi: 10.5114/aoms.2013.33181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franczyk-Skóra B, Gluba A, Banach M, Rysz J. Treatment of non-ST-elevation myocardial infarction and ST-elevation myocardial infarction in patients with chronic kidney disease. Arch Med Sci. 2013;9:1019–27. doi: 10.5114/aoms.2013.39792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salonen JT, Nyyssonen K, Korpela H, Tuomilehto J, Seppanen R, Salonen R. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation. 1992;86:803–8. doi: 10.1161/01.cir.86.3.803. [DOI] [PubMed] [Google Scholar]
- 37.Sturm B, Goldenberg H, Scheiber-Mojdehkar B. Transient increase of the labile iron pool in HepG2 cells by intravenous iron preparations. Eur J Biochem. 2003;270:3731–8. doi: 10.1046/j.1432-1033.2003.03759.x. [DOI] [PubMed] [Google Scholar]
- 38.Malyszko J, Malyszko JS, Mysliwiec M. Serum prohepcidin and hepcidin in hemodialyzed patients undergoing iron therapy. Kidney Blood Press Res. 2009;32:235–8. doi: 10.1159/000235747. [DOI] [PubMed] [Google Scholar]
- 39.Charytan D, Fishbane S, Malyszko J, McCullough PA, Goldsmith D, editors. Cardiorenal syndrome and the role of the bone-mineral axis and anemia; Am J Kidney Dis in press; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin CJ, Chuang CK, Jayakumar T, et al. Serum p-cresyl sulfate predicts cardiovascular disease and mortality in elderly hemodialysis patients. Arch Med Sci. 2013;9:662–8. doi: 10.5114/aoms.2013.36901. [DOI] [PMC free article] [PubMed] [Google Scholar]