Abstract
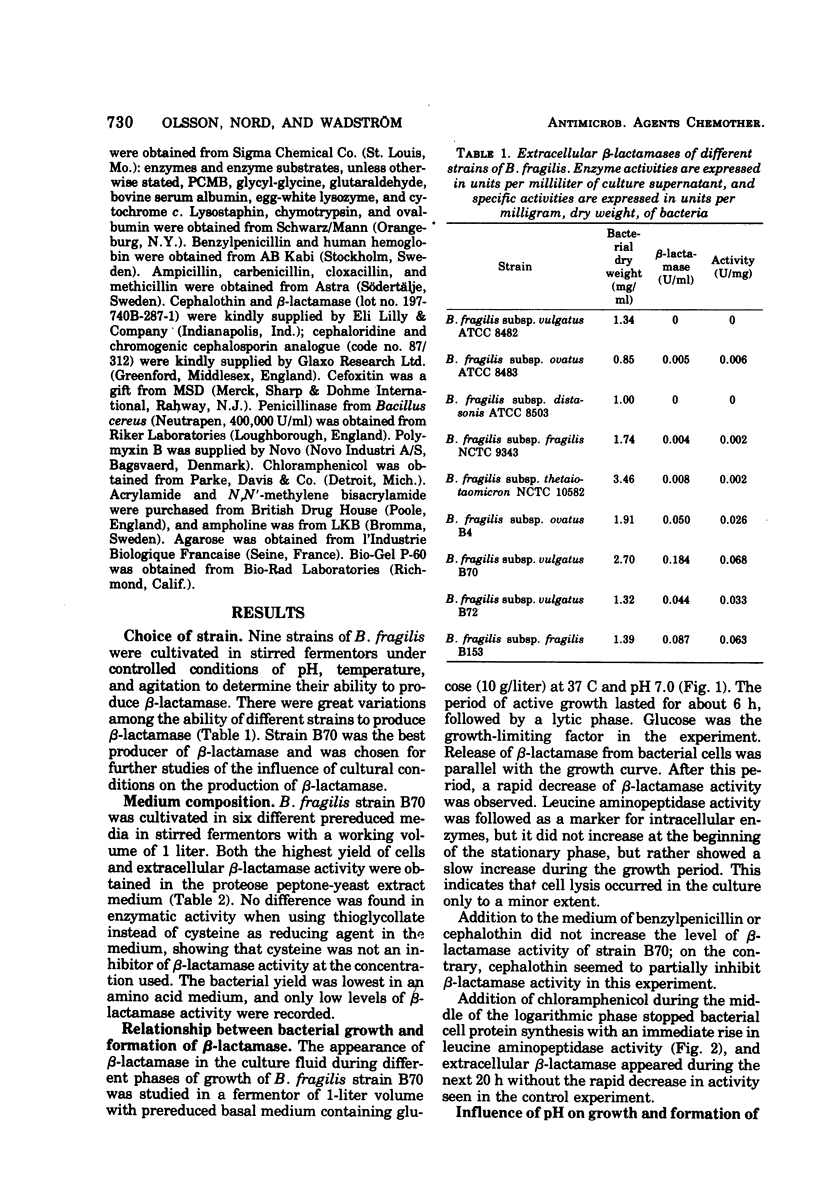
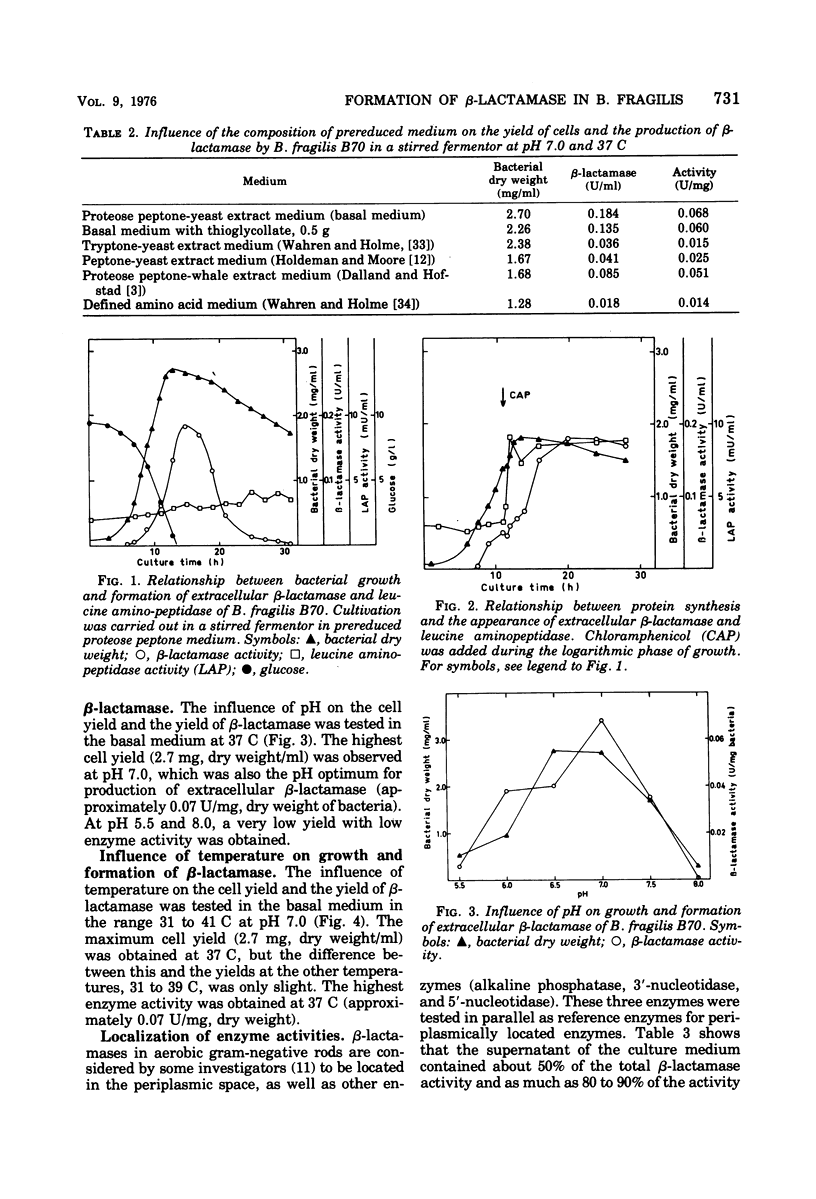
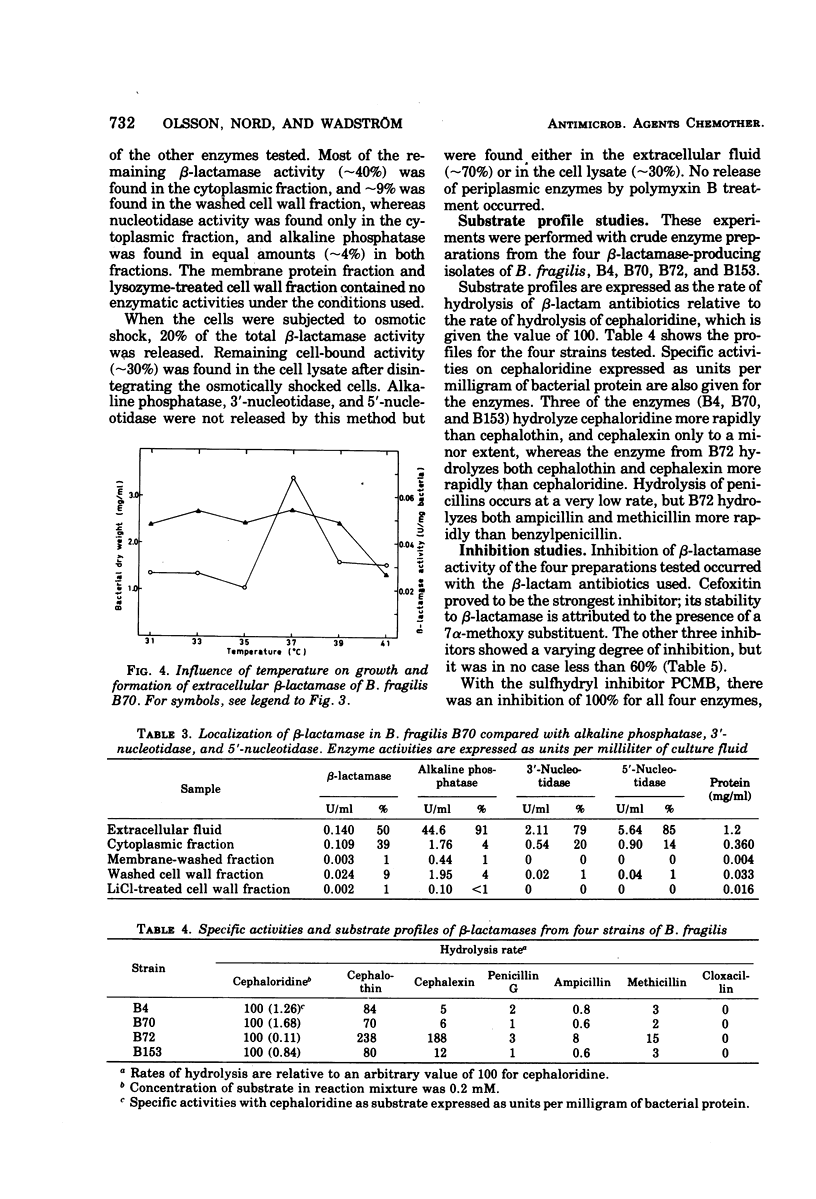
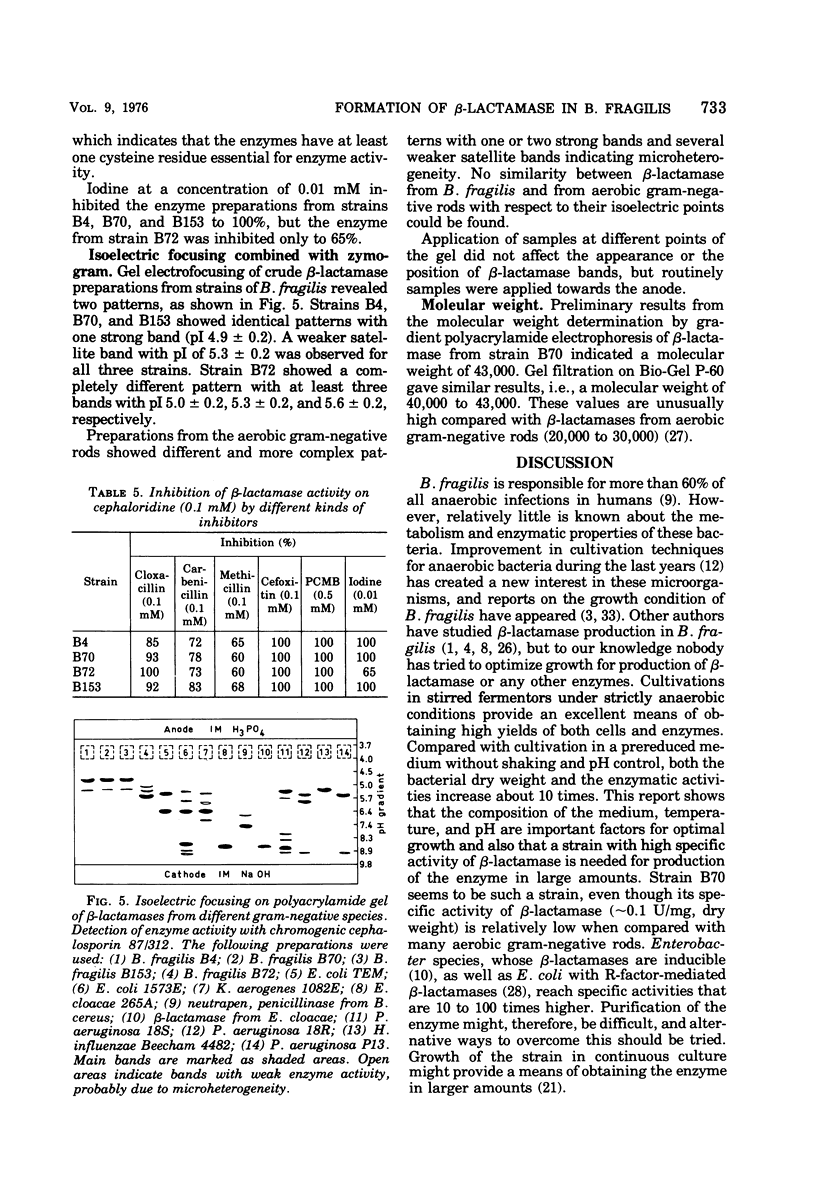
Nine strains of Bacteroides fragilis were cultivated in stirred fermentors and tested for their ability to produce β-lactamase. There was a correlation between formation of β-lactamase and high values of the minimal inhibitory concentration against β-lactam antibiotics. B. fragilis strain B70 was used for optimizing the production of β-lactamase. The highest bacterial yield was obtained in a proteose peptone-yeast extract medium. Optimal conditions for growth and β-lactamase production were obtained at 37 C and pH 7.0. The β-lactamase was released into the surrounding medium during the growth period to about 50%. Osmotic shock released about 20% of the total activity, and remaining activity was found in the cytoplasmic fraction. Substrate profile studies on four β-lactamase-producing strains showed that the enzymes were mainly cephalosporinases. They are inhibited by cloxacillin, p-chloromercuribenzoate, and iodine. Analytical isoelectric focusing in polyacrylamide gel gave an isoelectric point of 4.9 ± 0.2 for three of the strains and 5.6 ± 0.2 for one. Comparison with β-lactamases from aerobic gram-negative species with regard to isoelectric points showed no similarities. Also the molecular weight of the β-lactamase from strain B70 of 43,000 indicates that this is a new class of β-lactamase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. D., Sykes R. B. Characterisation of a -lactamase obtained from a strain of Bacteroides fragilis resistant to -lactam antibiotics. J Med Microbiol. 1973 May;6(2):201–206. doi: 10.1099/00222615-6-2-201. [DOI] [PubMed] [Google Scholar]
- Cerny G., Teuber M. Differential release of periplasmic versus cytoplasmic enzymes from Escherichia coli B by polymixin B. Arch Mikrobiol. 1971;78(2):166–179. doi: 10.1007/BF00424873. [DOI] [PubMed] [Google Scholar]
- Dalland E., Hofstad T. Growth of Bacteroides fragilis in continuous culture and in batch cultures at controlled pH. Appl Microbiol. 1974 Nov;28(5):856–860. doi: 10.1128/am.28.5.856-860.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bene V. E., Farrar W. E., Jr Cephalosporinase activity in Bacteroides fragilis. Antimicrob Agents Chemother. 1973 Mar;3(3):369–372. doi: 10.1128/aac.3.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornbusch K., Nord C. E., Olsson B. Antibiotic susceptibility testing of anaerobic bacteria by the standardized disc diffusion method with special reference to bacteroides fragilis. Scand J Infect Dis. 1975;7(1):59–66. doi: 10.3109/inf.1975.7.issue-1.11. [DOI] [PubMed] [Google Scholar]
- Evans D. J., Evans D. G., Gorbach S. L. Polymyxin B-Induced Release of Low-Molecular-Weight, Heat-Labile Enterotoxin from Escherichia coli. Infect Immun. 1974 Nov;10(5):1010–1017. doi: 10.1128/iai.10.5.1010-1017.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARROD L. P. Sensitivity of four species of bacteroides to antibiotics. Br Med J. 1955 Dec 24;2(4955):1529–1531. doi: 10.1136/bmj.2.4955.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbach S. L., Bartlett J. G. Anaerobic infections. 1. N Engl J Med. 1974 May 23;290(21):1177–1184. doi: 10.1056/NEJM197405232902106. [DOI] [PubMed] [Google Scholar]
- Hennessey T. D. Inducible beta-lactamase in Enterobacter. J Gen Microbiol. 1967 Nov;49(2):277–285. doi: 10.1099/00221287-49-2-277. [DOI] [PubMed] [Google Scholar]
- Kasper D. L., Seiler M. W. Immunochemical characterization of the outer membrane complex of Bacteroides fragilis subspecies fragilis. J Infect Dis. 1975 Oct;132(4):440–450. doi: 10.1093/infdis/132.4.440. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linder L., Lindquist L., Söder P. O., Holme T. Estimation of cell lysis. Determination of aminopeptidase in extracts of Streptococcus mitis, ATCC 903. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Oct;82B(5):602–607. doi: 10.1111/j.1699-0463.1974.tb00226.x. [DOI] [PubMed] [Google Scholar]
- Mathew A., Harris A. M., Marshall M. J., Ross G. W. The use of analytical isoelectric focusing for detection and identification of beta-lactamases. J Gen Microbiol. 1975 May;88(1):169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- Neu H. C. The 5'-nucleotidase of Escherichia coli. I. Purification and properties. J Biol Chem. 1967 Sep 10;242(17):3896–3904. [PubMed] [Google Scholar]
- Neu H. C. The cyclic phosphodiesterases (3'-nucleotidases) of the enterobacteriaceae. Biochemistry. 1968 Oct;7(10):3774–3780. doi: 10.1021/bi00850a060. [DOI] [PubMed] [Google Scholar]
- Neu H. C. The surface localization of penicillinases in Escherichia coli and Salmonella typhimurium. Biochem Biophys Res Commun. 1968 Jul 26;32(2):258–263. doi: 10.1016/0006-291x(68)90378-1. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Winshell E. B. Relation of beta-lactamase activity and cellular location to resistance of Enterobacter to penicillins and cephalosporins. Antimicrob Agents Chemother. 1972 Feb;1(2):107–111. doi: 10.1128/aac.1.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nord C. E., Möllby R., Smyth C., Wadström T. Formation of phospholipase C and theta-haemolysin in pre-reduced media in batch anc continuous culture of Clostridium perfringens type A. J Gen Microbiol. 1974 Sep;84(1):117–127. doi: 10.1099/00221287-84-1-117. [DOI] [PubMed] [Google Scholar]
- Nord C. E., Wadström T. Formation of -L- and -D-fucosidase in cultures of Streptococcus mitis. Med Microbiol Immunol. 1972;158(2):95–103. doi: 10.1007/BF02120474. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C., Morris A. Inhibition of beta-lactamases by beta-lactam antibiotics. Antimicrob Agents Chemother. 1972 Dec;2(6):442–448. doi: 10.1128/aac.2.6.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkus G., Veo G., Braude A. I. Bacteroides penicillinase. J Bacteriol. 1968 Oct;96(4):1437–1438. doi: 10.1128/jb.96.4.1437-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]
- Rubin F. A., Smith D. H. Characterization of R factor beta-lactamases by the acidimetric method. Antimicrob Agents Chemother. 1973 Jan;3(1):68–73. doi: 10.1128/aac.3.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. T., Wyatt J. M. Relation of R factor and chromosomal beta-lactamase with the periplasmic space. J Bacteriol. 1974 Mar;117(3):931–939. doi: 10.1128/jb.117.3.931-939.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderholm J., Allestam P., Wadström T. A rapid method for isoelectric focusing in polyacrylamide gel. FEBS Lett. 1972 Jul 15;24(1):89–92. doi: 10.1016/0014-5793(72)80833-0. [DOI] [PubMed] [Google Scholar]
- Wahren A., Holme T. Amino acid and peptide requirement of Fusiformis necrophorus. J Bacteriol. 1973 Oct;116(1):279–284. doi: 10.1128/jb.116.1.279-284.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren A., Holme T. Growth of Bacteroidaceae in stirred fermentors. Appl Microbiol. 1969 Aug;18(2):235–239. doi: 10.1128/am.18.2.235-239.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



