In children aged >18 months with cerebral malaria, elevated erythropoietin levels were associated with increased mortality, prolonged coma duration, and a lack of neuroprotection. Caution is warranted in the use of systemic erythropoietin as adjunctive therapy in cerebral malaria.
Keywords: erythropoietin, cerebral malaria, mortality, neurologic deficits
Abstract
Background. Elevated endogenous plasma erythropoietin (EPO) levels have been associated with protection from acute neurologic deficits in Kenyan children with cerebral malaria (CM). Based on these findings and animal studies, clinical trials of recombinant human EPO (rHuEPO) have been started in children with CM. Recent clinical trials in adults with acute ischemic stroke have demonstrated increased mortality with rHuEPO treatment. We conducted a study in children with CM to assess the relationship of endogenous plasma and cerebrospinal fluid (CSF) EPO levels with mortality and acute and long-term neurologic outcomes.
Methods. A total of 210 children between 18 months and 12 years of age with a diagnosis of CM, were enrolled at Mulago Hospital, Kampala, Uganda. Plasma (n = 204) and CSF (n = 147) EPO levels at admission were measured by radioimmunoassay and compared with mortality and neurologic outcomes.
Results. After adjustment for age and hemoglobin level, a 1-natural-log increase in plasma EPO level was associated with a 1.74-fold increase in mortality (95% confidence interval, 1.09–2.77, P = .02). Plasma and CSF EPO levels also correlated positively with coma duration (P = .05 and P = .02, respectively). Plasma and CSF EPO levels did not differ in children with vs those without acute or long-term neurologic deficits. Plasma EPO levels correlated positively with markers of endothelial and platelet activation and histidine-rich protein-2 levels, but remained associated with mortality after adjustment for these factors.
Conclusions. High endogenous plasma EPO levels are associated with prolonged coma duration and increased mortality in children >18 months of age with CM.
Cerebral malaria (CM) is a leading cause of morbidity and mortality from Plasmodium falciparum infection, affecting >500 000 children yearly, with a mortality rate of approximately 20% [1]. CM is also an important cause of short-term [2] and long-term [3] neurocognitive impairment in African children. In CM, sequestration of infected red blood cells as well as leukocytes and platelets to the blood-brain barrier endothelium, combined with an imbalance of systemic and local pro- and anti-inflammatory cytokines, is thought to lead to blood-brain barrier dysfunction and adverse clinical outcomes [4]. To date, no adjunctive treatment for CM in humans has decreased mortality or neurologic complications [5].
Erythropoietin (EPO) is a hematopoietic factor that promotes survival and proliferation of bone marrow progenitor cells during erythropoiesis [6]. Expression of EPO under the control of hypoxia-inducible factor 1-alpha (HIF1-α) occurs primarily in the kidney, but EPO and EPO receptor expression have been identified in other organs, including the brain of rodents, monkeys, and humans [7]. In vitro studies demonstrating that EPO reduced glutamate-induced neuronal apoptosis [8] initiated intensive investigations on the use of EPO as a neuroprotective agent.
Exogenous recombinant human EPO (rHuEPO) has been shown to be neuroprotective in animal models of cerebral ischemic neuronal damage [9], autoimmune encephalomyelitis [10], and cerebral malaria [11, 12]. Consequently, rHuEPO was rapidly introduced into human clinical trials. A large phase I trial in patients with acute ischemic stroke showed improved clinical outcome at the 1-month primary endpoint [13]. However, longitudinal follow-up of these patients revealed increased mortality in the rHuEPO arm and provided evidence, along with other studies, that in critically ill patients, EPO can increase the risk of thrombosis, endothelial cell activation, and platelet aggregation [14–16]. The use of rHuEPO has been shown to correlate with better cognitive outcomes in preterm infants [17]; however, the use of high doses of rHuEPO in children undergoing dialysis has been associated with an increased risk of hypertension [18], raising questions regarding the safety of rHuEPO as a neurotropic agent in children, particularly children with a prothrombotic disease process.
Severe malaria induces upregulation of endogenous EPO levels [19, 20], which can be greater than the increase induced by similar anemia without malaria [21]. High plasma levels of endogenous EPO were associated with protection from acute neurologic deficits in Kenyan children with CM [22]. This study and murine cerebral malaria studies [11, 12] prompted a small (n = 35) phase I clinical trial in which EPO-β adjunctive treatment for children with CM did not cause obvious adverse events [23]. In this study, clinical assessment was limited and children were followed for only 7 days. In light of recent studies showing increased mortality with rHuEPO in other neurologic conditions [14] and the lack of studies confirming the Kenyan study findings, we conducted a study to assess the relationship of plasma and CSF EPO levels with mortality and acute and long-term neurologic deficits in children with CM. As EPO can increase endothelial activation [16], which can further promote sequestration [4, 24], the relationships between plasma and CSF EPO levels and markers of endothelial activation and levels of P. falciparum histidine-rich protein-2 (PfHRP-2), a measure of sequestered and circulating parasite biomass [25], were also assessed.
METHODS
Study Design
This study was conducted at Mulago National Referral and Teaching Hospital in Kampala, Uganda. Children between 18 months and 12 years of age, with a diagnosis of CM, were recruited between 2008 and 2012. Cerebral malaria was defined as (1) coma (Blantyre coma score ≤2 or Glasgow coma score ≤8); (2) P. falciparum on blood smear; and (3) no other known cause of coma. Exclusion criteria included (1) known chronic illness requiring medical care; (2) known developmental delay; or (3) prior history of coma, head trauma, hospitalization for malnutrition, or cerebral palsy.
Children with CM were managed according to Ugandan Ministry of Health guidelines (inpatient quinine, follow-up outpatient artemisinin combination therapy). A detailed neurologic examination was performed at discharge and 6 months later. A neurologic deficit was defined as the presence of motor deficits, ataxia, movement disorder, behavior, or speech or visual disorders, in a child with no known prior deficits.
Ethical Review and Approval
The study was reviewed and approved by the Ugandan National Council for Science and Technology, the Makerere University School of Medicine Research and Ethics Committee, and the University of Minnesota Institutional Review Board.
Laboratory Testing
Plasma and CSF EPO levels were tested via a high-sensitivity radioimmunoassay, as previously described [26]. Peripheral blood smears were assessed for the presence of Plasmodium species by microscopy with Giemsa staining. Two independent readings were conducted and, if not consistent, were resolved by a third reading. PfHRP-2 readings were performed using the Malaria Antigen Cellabs Enzyme-Linked Immunosorbent Assay (Cellabs, Brookvale, Australia).
Plasma Analyte Testing
Plasma levels of soluble intercellular adhesion molecule -1 (sICAM-1), soluble vascular cellular adhesion molecule -1 (sVCAM-1), and soluble P-selectin and E-selectin (sP-selectin and sE-selectin, respectively) were measured by cytometric bead assay according to the manufacturer's instructions in plasma diluted 1:300 (R&D Systems, Minneapolis, Minnesota) with a Bioplex-200 system (Bio-Rad, Hercules, California).
Statistical Analysis
Clinical and laboratory factors in children with vs those without neurologic deficits and in survivors vs nonsurvivors were compared by χ2 testing if categorical and by Student t test or by the Wilcoxon rank-sum test for measures with skewed distributions if continuous. Variables with a P value <.2 in these analyses were adjusted for in the respective regression models. Plasma and CSF EPO levels, coma duration, and number of seizures had skewed distributions, so for these variables, Spearman rank correlation (ρ) was used for unadjusted analyses, and log-transformed (natural log) values were used for regression analyses. Associations between log-transformed EPO levels and neurologic outcomes and mortality were tested by logistic regression for categorical variables and linear regression for continuous variables, with adjustment for potential confounding factors after initial testing for interactions of pairs of potential confounding factors and of log EPO with each potential confounder.
RESULTS
Study Cohort Follow-up, Sample Collection, and Baseline Characteristics
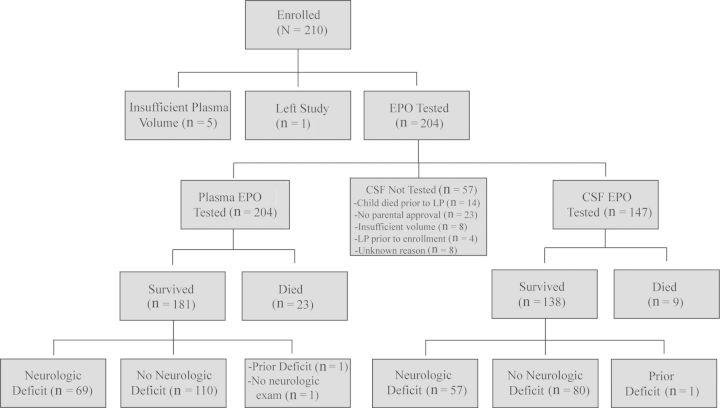
A total of 210 children with CM were enrolled in the study. Figure 1 shows the number of children tested for plasma and CSF EPO levels, the number of survivors and those who died, and the number of surviving children with neurologic deficits at discharge. Of the 204 children who had plasma tested, 147 had CSF tested. CSF was not tested on 57 of the 204 children for reasons specified in Figure 1. Children with human immunodeficiency virus (n = 5) and sickle cell disease (n = 1) were not excluded from the study.
Figure 1.
Study profile. Abbreviations: CSF, cerebrospinal fluid; EPO, erythropoietin; LP, lumbar puncture.
Clinical and Laboratory Predictors of Neurologic Deficits and Mortality
At discharge, 69 of 179 children who survived CM and had a neurologic exam (38.5%) had neurologic deficits, including motor deficits (n = 37), ataxia (n = 35), disorders of movement (n = 5), behavior (n = 10), hearing (n = 5), or speech or vision (n = 34). At 6 months’ follow-up, 10 of 173 children who survived and completed follow-up (5.8%) had neurologic deficits, including motor deficits (n = 4), ataxia (n = 4), movement disorder (n = 2), behavior (n = 1), and speech or visual disorders (n = 6).
Clinical and laboratory variables that were biologically plausible as predictors of neurologic deficits or mortality in children with CM were compared in children with vs those without neurologic deficits at discharge and 6 months, and in children who survived vs children who died (Tables 1 and 2). Among these factors, children with neurologic deficits at discharge had a lower age and higher sP-selectin levels than children without deficits, and children with neurologic deficits at 6 months of follow-up had a lower weight-for-age z score and lower Blantyre coma score and received a blood transfusion more frequently than children without deficits (Table 1). Lower sP-selectin levels and a trend toward lower hemoglobin levels were seen in children who survived vs children who died (Table 2).
Table 1.
Clinical and Laboratory Findings in Children With Cerebral Malaria (With Versus Those Without) Neurologic Deficits, at Discharge and 6-Months of Follow-up
| Characteristic | Discharge |
6-mo Follow-up |
||||
|---|---|---|---|---|---|---|
| Deficits (n = 69) | No Deficits (n = 110) | P Valuea | Deficits (n = 10) | No Deficits (n = 163) | P Valuea | |
| Demographic and clinical findings | ||||||
| Age, mo, median (IQR) | 38.14 (26.71–47.84) | 43.66 (32.46–61.63) | .02 | 37.08 (24.80–42.25) | 41.49 (31.11–52.86) | .09 |
| Sex, male, No. (%) | 45 (65.22) | 60 (54.55) | .16 | 3 (30.00) | 97 (59.51) | .07 |
| Weight-for-age z score, mean (SD) | −1.46 (1.29) | −1.11 (1.10) | .06 | −1.96 (1.11) | −1.20 (1.15) | .04 |
| Seizures before admission, No. (%) | 65 (94.20) | 105 (95.45) | .71 | 10 (100.00) | 157 (96.32) | .54 |
| Deep acidotic breathing, No. (%) | 4 (5.80) | 9 (8.18) | .55 | 0 (0) | 12 (7.36) | .37 |
| Abnormal posturing, No. (%) | 4 (5.80) | 8 (7.27) | .70 | 1 (10.00) | 11 (6.75) | .69 |
| Blantyre coma scoreb, median (IQR) | 2 (1–2) | 2 (2–2) | .18 | 1 (1–1) | 2 (2–2) | <.0001 |
| Coma duration, h, median (IQR) | 16.00 (6.50–24.00) | 12.00 (5.00–23.00) | .28 | 11.00 (5.00–26.00) | 12.00 (5.00–24.00) | .65 |
| Seizures after admission, No. (%) | 33 (47.83) | 66 (60.00) | .11 | 6 (60.00) | 90 (55.21) | .77 |
| No. of seizures after admission, median (IQR) | 0 (0–1) | 1 (0–2) | .29 | 3 (0–8) | 1 (0–2) | .11 |
| Transfused, No. (%) | 47 (68.12) | 65 (59.09) | .23 | 10 (100) | 97 (59.51) | .01 |
| Clinical laboratory tests | ||||||
| Hemoglobin, g/dL, mean (SD) | 6.84 (2.33) | 6.60 (2.21) | .49 | 6.39 (1.47) | 6.68 (2.23) | .68 |
| White blood cell count, median (IQR) | 10.20 (6.70–16.05) | 8.90 (6.50–12.30) | .11 | 8.30 (5.30–15.70) | 9.10 (6.70–12.90) | .74 |
| Platelet count, median (IQR) | 63.00 (33.50–130) | 61.00 (37.00–105) | .96 | 58.00 (31.00–75.00) | 61.00 (37.00–115) | .62 |
| Hypoglycemiac, No. (%) | 6 (8.70) | 6 (5.45) | .40 | 1 (10.00) | 11 (6.75) | .69 |
| Plasmodium falciparum peripheral blood density, median (IQR) | 37 580 (8160–249 560) | 59 180 (14 180–407 940) | .20 | 72 370 (6420–801 060) | 43 990 (12 680–309 060) | .62 |
| PfHRP-2 level, ng/mL, median (IQR) | 2678 (1042–5369) | 2274 (943–4949) | .54 | 1001 (470–3036) | 2561 (996–5148) | .14 |
| Endothelial and platelet activation markers | ||||||
| Soluble VCAM-1, ng/mL, median (IQR) | 4034 (2551–6815) | 3944 (2869–6601) | .78 | 5836 (3781–11 977) | 3845 (2715–6364) | .09 |
| Soluble ICAM-1, ng/mL, median (IQR) | 729 (225–1433) | 505 (238–1340) | .60 | 657 (220–1170) | 521 (225–1433) | .86 |
| Soluble E-selectin, ng/mL, median (IQR) | 172 (121–238) | 187 (142–254) | .22 | 211 (158–246) | 179 (134–250) | .78 |
| Soluble P-selectin, ng/mL, median (IQR) | 60.98 (40.60–83.14) | 48.62 (35.82–72.78) | .05 | 58.82 (51.68–83.19) | 50.03 (36.82–76.13) | .40 |
| EPO levels | ||||||
| Plasma EPO, mU/mL, median (IQR) | 741 (330–3234) | 933 (288–2560) | .76 | 680 (549–1763) | 783 (259–2835) | .76 |
| CSF EPO, mU/mLd, median (IQR) | 8.34 (4.21–14.84) | 8.50 (4.17–18.35) | .99 | 8.34 (4.21–11.96) | 8.76 (4.20–17.89) | .71 |
Abbreviations: CSF, cerebrospinal fluid; EPO, erythropoietin; ICAM-1, intercellular adhesion molecule-1; IQR, interquartile range; PfHRP-2, Plasmodium falciparum histidine-rich protein-2; SD, standard deviation; VCAM-1, vascular adhesion molecule-1.
a Variables with medians reported compared by Wilcoxon rank-sum score; means compared by Student t test; proportions compared by χ2 test.
b Blantyre coma score assessed in children <5 years of age. At discharge: deficits, n = 56, no deficits = 82; at 6 months: deficits = 9, no deficits = 125.
c Hypoglycemia defined as blood glucose <2 mmol/L.
d CSF EPO tested on admission in 57 and 80 children with and without deficits, respectively, and at 6 months, in 9 and 122 children with and without deficits, respectively.
Table 2.
Clinical and Laboratory Findings in Children With Cerebral Malaria Who Survived Compared With Those Who Died
| Characteristic | Survived (n = 181) | Died (n = 23) | P Valuea |
|---|---|---|---|
| Demographic and clinical findings | |||
| Age, mo, median (IQR) | 41.46 (31.05–52.17) | 35.63 (25.79–46.98) | .13 |
| Sex, male, No. (%) | 106 (58.56) | 14 (60.87) | .83 |
| Weight-for-age z score, mean (SD) | −1.25 (1.18) | −1.66 (1.29) | .13 |
| Seizures before admission, No. (%) | 171 (94.48) | 22 (95.65) | .81 |
| Deep acidotic breathing, No. (%) | 13 (7.18) | 3 (13.04) | .33 |
| Abnormal posturing, No. (%) | 12 (6.63) | 0 (0) | .20 |
| Blantyre coma scoreb, median (IQR) | 2 (1–2) | 2 (1–2) | .41 |
| Coma duration, h, median (IQR) | 13.00 (5.00–24.00) | 18.00 (8.00–24.00) | .17 |
| Seizures after admission, No. (%) | 100 (55.25) | 13 (56.52) | .91 |
| No. of seizures after admission, median (IQR) | 1 (0–2) | 1 (0–2) | .73 |
| Transfused, No. (%) | 112 (61.88) | 12 (52.17) | .37 |
| Clinical laboratory tests | |||
| Hemoglobin, g/dL, mean (SD) | 6.69 (2.24) | 7.63 (2.35) | .06 |
| White blood cell count, median (IQR) | 9.30 (6.70–13.90) | 10.70 (7.40–13.20) | .38 |
| Platelet count, median (IQR) | 61.00 (35.00–113) | 55.00 (35.00–83.00) | .34 |
| Hypoglycemiac, No. (%) | 12 (6.63) | 3 (13.04) | .27 |
| Plasmodium falciparum peripheral blood density, median (IQR) | 45 600 (11 780–302 060) | 49 040 (6480–121 100) | .53 |
| PfHRP-2 level, ng/mL, median (IQR) | 2486 (996–5112) | 3532 (1598–5822) | .15 |
| Endothelial and platelet activation markers | |||
| Soluble VCAM-1, ng/mL, median (IQR) | 3945 (2770–6601) | 3225 (2532–6018) | .23 |
| Soluble ICAM-1, ng/mL, median (IQR) | 621 (238–1400) | 976 (321–1864) | .16 |
| Soluble E-selectin, ng/mL, median (IQR) | 180 (137–246) | 189 (158–283) | .25 |
| Soluble P-selectin, ng/mL, median (IQR) | 53.07 (37.45–76.78) | 67.09 (48.53–82.99) | .05 |
| EPO levels | |||
| Plasma EPO, mU/mL, median (IQR) | 783 (288–2759) | 1566 (473–2852) | .39 |
| CSF EPO, mU/mLd, median (IQR) | 8.42 (4.20–17.64) | 9.70 (6.66–89.00) | .21 |
Abbreviations: CSF, cerebrospinal fluid; EPO, erythropoietin; ICAM-1, intercellular adhesion molecule-1; IQR, interquartile range; PfHRP-2, Plasmodium falciparum histidine-rich protein-2; SD, standard deviation; VCAM-1, vascular cellular adhesion molecule-1.
a Variables with medians reported compared by Wilcoxon rank-sum score; means compared by Student t test; proportions compared by χ2 test.
b Blantyre coma score assessed in children <5 years of age; survived n = 139, died = 17.
c Hypoglycemia defined as blood glucose <2 mmol/L.
d CSF EPO testing performed in 138 children who survived and 9 children who died.
Plasma and CSF EPO Levels and Neurologic Outcomes, Adjusted for Hemoglobin Level and Age
Plasma and CSF EPO levels were strongly correlated (ρ= 0.68, P < .0001), and both plasma and CSF EPO correlated inversely with age (ρ = −0.31, P < .0001 and ρ = −0.29, P = .0002, respectively) and hemoglobin level (ρ = −0.78, P < .0001 and ρ = −0.54, P < .0001, respectively). Age and hemoglobin level were therefore included as adjusters when assessing the relationship of plasma and CSF EPO levels to primary outcomes. After adjusting for age and hemoglobin level, endogenous plasma and CSF EPO levels were not associated with neurologic deficits (at discharge or 6 months of follow-up) or number of seizures postadmission, but plasma and CSF EPO levels positively correlated with increased coma duration during hospitalization (Table 3). Plasma EPO levels in a cohort of asymptomatic and otherwise healthy children from this area were significantly lower than in children with CM (n = 136, median, 20.02 mU/mL [25th percentile–75th percentile, 15.62–35.25], P < .0001 vs children with CM).
Table 3.
Association of Plasma and Cerebrospinal Fluid Erythropoietin Levels With Neurologic Deficits, Number of Seizures, and Coma Duration
| EPO | Neurologic Deficit (Discharge) |
Neurologic Deficit (6 mo) |
No. of Seizures After Admission |
Coma Duration, h |
||||
|---|---|---|---|---|---|---|---|---|
| ORa (95% CI) | P Value | ORa (95% CI) | P Value | βa Coefficient (95% CI) | P Value | βa Coefficient (95% CI) | P Value | |
| Plasma EPO, mU/mL | 1.16 (.83–1.62) | .39 | 0.98 (.49–1.98) | .96 | −.01 (−.14 to .12) | .85 | .15 (.001–.29) | .05 |
| CSF EPO, mU/mL | 1.15 (.75–1.75) | .53 | 0.74 (.31–1.76) | .49 | .09 (−.08 to .27) | .30 | .22 (.03–.42) | .02 |
Odds ratios denote the increase in odds of the clinical outcome (neurologic deficit or log of number of seizures or coma duration) for each log increase in EPO level. β-coefficients denote the increase in clinical outcome (neurologic outcome) or log of clinical outcome (number of seizures, coma duration) for each log increase in EPO level.
Abbreviations: CI, confidence interval; CSF, cerebrospinal fluid; EPO,erythropoietin; OR, odds ratio.
a Adjusted for age and hemoglobin level; EPO levels, seizures after admission, and coma duration were log-transformed (natural log).
Plasma and CSF EPO Levels and Mortality
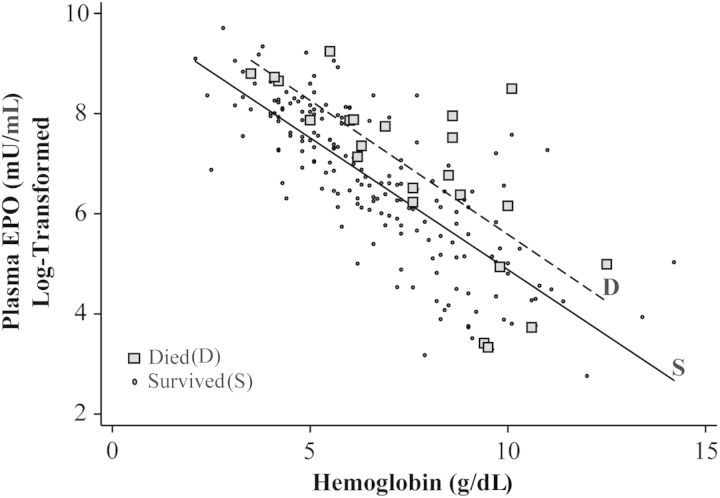
Unadjusted plasma and CSF EPO levels did not differ in CM survivors compared to those who died (Table 2), but after adjustment for age and hemoglobin level, log-transformed plasma EPO levels were associated with mortality (odds ratio [OR], 1.74 [95% confidence interval {CI}, 1.09–2.77], P = .02; Table 4). In this analysis, hemoglobin level (OR, 1.70 [95% CI, 1.27–2.26], P < .001) but not age (OR, 0.78 [95% CI, .58–1.06], P = .11) was also independently associated with mortality. For any given hemoglobin level, children who died typically had a higher EPO level than children who survived (Figure 2). Using an 8-g/dL cutoff for moderate anemia, plasma EPO was associated with mortality and prolonged coma duration in children with hemoglobin levels <8 g/dL (adjusted OR, 3.11 [95% CI, 1.30–7.41], P = .01 and β = .23 [95% CI, .02–.44], P = .03, respectively) but not in children with hemoglobin levels ≥8 g/dL (adjusted OR, 1.37 [95% CI, .76–2.47], P = .29 and β = 0.11 [95% CI, −.12 to .34], P = .34, respectively). Plasma EPO levels remained associated with mortality and prolonged coma after adjustment for receipt of a blood transfusion or number of transfusions (data not shown).
Table 4.
Association of Plasma and Cerebrospinal Fluid Erythropoietin Levels With Mortality
| EPO | ORa (95% CI) | P Value | ORb (95% CI) | P Value |
|---|---|---|---|---|
| Plasma EPO, mU/mL | 1.74 (1.09–2.77) | .02 | 1.69 (1.03–2.77) | .04 |
| CSF EPO, mU/mL | 1.70 (.81–3.56) | .16 | 1.73 (.80–3.74) | .17 |
Abbreviations: CI, confidence interval; CSF, cerebrospinal fluid; EPO,erythropoietin; OR, odds ratio.
a Adjusted for age and hemoglobin level; EPO levels were log-transformed (natural log).
b Adjusted for age and levels of hemoglobin, soluble P-selectin, soluble intercellular adhesion molecule-1, and Plasmodium falciparum histidine-rich protein-2; EPO levels were log-transformed (natural log).
Figure 2.
Hemoglobin and erythropoietin (EPO) levels in children with cerebral malaria who survived vs those who died. EPO levels (log-transformed) and hemoglobin level are depicted for children who died and survived. Solid and dashed lines show average EPO level for each hemoglobin level for children who survived (S) vs children who died (D), respectively. In 19 of the 23 children who died, EPO values were higher than the mean EPO value of survivors for the same hemoglobin level.
CSF EPO levels showed a very similar trend toward association with mortality as plasma EPO levels (1-natural-log increase in CSF EPO level; OR, 1.70 [95% CI, .81–3.56], P = .16; Table 4), but the trend did not achieve significance, likely because of the smaller number of children with CSF samples for testing (n = 147).
Plasma EPO Levels, Markers of Endothelial Activation, and PfHRP-2
Log-transformed plasma EPO levels, adjusted for age and hemoglobin level, were positively associated with levels of sP-selectin, sE-selectin, sICAM-1, and sVCAM-1 (Table 5). Plasma and CSF EPO levels also correlated strongly with plasma PfHRP-2 levels after adjustment for age and hemoglobin level (β = .44 [95% CI, .27–.61], P < .001 and β = .50 [95% CI, .27–.73], P < .001, respectively), but not with peripheral parasite density (P > .71 for both). Levels of PfHRP-2, sP-selectin, and sICAM-1 met the predetermined adjusted cutoff for differences between children who survived vs died (Table 2), so they were adjusted for in a final model assessing plasma EPO levels and mortality. Log-transformed plasma EPO levels remained independently associated with mortality after this further adjustment (OR, 1.69 [95% CI, 1.03–2.77], P = .04; Table 4).
Table 5.
Association of Plasma Erythropoietin With Markers of Platelet and Endothelium Activation
| Marker | β Coefficienta (95% CI) | P Value |
|---|---|---|
| Soluble P-selectin | .11 (.04–.18) | .002 |
| Soluble E-selectin | .11 (.06–.17) | <.001 |
| sICAM-1 | .19 (.007–.38) | .04 |
| sVCAM-1 | .11 (.02–.19) | .01 |
Abbreviations: CI, confidence interval; EPO,erythropoietin; sICAM-1, soluble intercellular adhesion molecule-1; sVCAM-1, soluble vascular cellular adhesion molecule-1.
a Adjusted for age and hemoglobin level; EPO levels were log-transformed (natural log).
DISCUSSION
The present study showed that in children with CM, both high plasma and CSF levels of endogenous EPO are associated with prolonged coma duration, whereas only plasma EPO levels are associated with increased mortality. In contrast to an earlier study in Kenyan children with CM [22], we found no association between plasma and CSF EPO levels and protection from acute neurologic deficits; we also found no association with protection from long-term neurologic deficits. Further studies are needed to confirm these findings, but in conjunction with studies showing no neuroprotective benefit of endogenous EPO in adult CM patients [27] and increased risks of exogenous rHuEPO in other diseases [14, 18], the present study's results argue for caution in using systemic rHuEPO as adjunctive therapy for children with CM.
Association of endogenous and exogenous EPO with adverse outcomes has precedent in several severe diseases such as chronic kidney disease, dialysis, and stroke [14, 15, 18]. These studies, along with others that found an increase of exogenous EPO-mediated platelet and endothelial activation [16, 28], suggest that a balance between neuroprotective and erythropoietic events is needed for erythropoietin to be both useful and safe. We observed a positive correlation of endogenous plasma EPO levels with markers of platelet and endothelial activation, factors important in CM pathogenesis [24]. EPO could lead to increased disease severity in CM by endothelial and platelet activation, as this increased activation could lead to sequestration [4, 24]. In support of an association with sequestration, plasma EPO was not associated with parasite density at enrollment but was associated with plasma PfHRP-2 levels, a marker of total parasite biomass, including sequestered parasite biomass [25].
In vitro and in vivo studies have shown that EPO as a neurotropic agent can be neuroprotective or harmful [29, 30]. Continued presence of high levels of EPO, when combined with moderate hypoxia, leads to increased neuronal apoptosis in cultured rat neurons [29]. Impaired local perfusion due to sequestration, rosette formation, and reduced nitric oxide bioavailability leads to hypoxia in CM, which could tip the balance of EPO from neuroprotective to damaging. In addition, EPO levels at 10 nM or higher increased apoptosis in cultures of rat hippocampal neurons [30]. The levels of endogenous plasma EPO seen in this study are lower than the levels of EPO reached in adults after rHuEPO treatment [13], and because the affinity of EPO for its receptor in the brain is lower than for its homodimeric receptor on erythroid progenitors, it is possible that EPO levels above those produced endogenously are neuroprotective [31]. Moreover, the timing of plasma EPO elevation could have detrimental effects. Untimely onset of EPO-induced reticulocytosis augmented parasitemia and was fatal in a mouse malaria model [32]. In our study, endogenous EPO was not associated with parasitemia at enrollment, but we do not have consecutive EPO and parasitemia measurements to compare our findings with those of the mouse model.
Our study findings contrast with those of a previous study done in Kenyan children with CM, in which high EPO levels were associated with protection from acute neurologic deficits and mortality [22]. Children in the present study had a lower mortality rate and slightly more frequent neurologic deficits at discharge than children in the prior study, but these differences should not strongly alter associations between EPO and neurologic deficits. Differences in age might partially explain the different study findings. The median age was higher in the present study, and increased age has been associated with greater upregulation of endogenous EPO levels in response to a similar decrease in hemoglobin levels [33], and with decreased clearance of both endogenous and exogenous EPO [33, 34]. Slower clearance of high EPO levels could increase the risk of thrombotic events. Our regression models controlled for age, but we did not enroll children as young as the youngest children in the Kenyan study. The other primary differences seen between the current study population and the Kenyan study population were that children in the current study had lower mean hemoglobin levels and platelet counts and had higher median EPO levels. Among children in the present study, the elevated EPO levels were likely due to the lower hemoglobin levels, and the lower platelet counts could reflect increased platelet sequestration. Elevated EPO levels via their prothrombotic effect could have aggravated already increased platelet and infected erythrocyte sequestration in the children in our study. The association of EPO with mortality in children with a hemoglobin level <8 g/dL, but not in children with a hemoglobin level ≥8 g/dL, is consistent with low hemoglobin levels being a driver of mortality in our study cohort, although transfusion did not alter outcomes. In the Kenyan study, high levels of EPO were associated with decreased mortality, after adjustment for deep breathing, number of seizures, coma duration, hyperparasitemia, and papilledema. In our cohort, the association of EPO with mortality was unaltered in a model that included these predictors (adjusted OR for log-transformed plasma EPO, 1.68 [95% CI, 1.03–2.76], P = .04). In summary, age, severity of anemia, and degree of sequestration could explain some of the differences between the Kenyan study and the present study, but studies in additional cohorts are needed to resolve the study differences.
As a longitudinal, observational study, the present study cannot determine causality. Given the multiple factors that can cause EPO levels to increase, such as hypoxia, inflammation, and suppression of erythropoiesis, it will be important to determine whether the association of high endogenous EPO levels with coma duration and mortality is causative. Several factors lead us to believe that endogenous EPO levels are most likely causally related to prolonged coma and mortality. First, in randomized clinical trials of rHuEPO in stroke, which has some similarities in pathogenesis to CM, individuals in the rHuEPO arm had increased mortality [14]. Second, in our study, the association between EPO and mortality remained after adjustment for important confounding factors, including age, hemoglobin level, and PfHRP-2 level [25]. Third, although exogenous EPO can be neuroprotective, evidence of EPO-related adverse events has been demonstrated in animal models and human studies of other diseases [14, 18, 29, 30]. However, hypoxia, a major driver of EPO levels, can be caused by multiple factors, and it remains possible that elevated endogenous EPO is a marker for another as-yet undefined process that leads to mortality in CM. New analogues of EPO have been formulated that lack erythropoietic effects but retain the neuroprotective characteristics of EPO, such as carbamylated EPO [28, 35]. These derivatives have been tested as neuroprotective agents in animal models of stroke and autoimmune encephalomyelitis [35], and may hold promise in CM treatment.
In summary, the present study showed that high plasma levels of endogenous EPO are associated with prolonged coma duration and increased mortality in CM children aged >18 months, and not with protection from neurologic deficits. In conjunction with other studies showing adverse effects from systemic rHuEPO therapy in adults and children with prothrombotic disease states, the present study findings suggest caution in considering recombinant systemic rHuEPO as adjunctive therapy for children aged >18 months with cerebral malaria.
Notes
Acknowledgments. We thank the study teams at Makerere University, Mulago Hospital, and the University of Minnesota for their essential work on this study. We are grateful to the children and parents who took part in the study.
Author contributions. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH). The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Financial support. This work was supported by the National Institute of Neurologic Disorders and Stroke (R01 NS05534) and NIH Fogarty International Center (D43 NS078280).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Murphy SC, Breman JG. Gaps in the childhood malaria burden in Africa: cerebral malaria, neurological sequelae, anemia, respiratory distress, hypoglycemia, and complications of pregnancy. Am J Trop Med Hyg. 2001;64(1–2 suppl):57–67. doi: 10.4269/ajtmh.2001.64.57. [DOI] [PubMed] [Google Scholar]
- 2.Boivin MJ, Bangirana P, Byarugaba J, et al. Cognitive impairment after cerebral malaria in children: a prospective study. Pediatrics. 2007;119:e360–6. doi: 10.1542/peds.2006-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.John CC, Bangirana P, Byarugaba J, et al. Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics. 2008;122:e92–9. doi: 10.1542/peds.2007-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Idro R, Marsh K, John CC, Newton CR. Cerebral malaria: mechanisms of brain injury and strategies for improved neurocognitive outcome. Pediatr Res. 2010;68:267–74. doi: 10.1203/PDR.0b013e3181eee738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.John CC, Kutamba E, Mugarura K, Opoka RO. Adjunctive therapy for cerebral malaria and other severe forms of Plasmodium falciparum malaria. Expert Rev Anti Infect Ther. 2010;8:997–1008. doi: 10.1586/eri.10.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobson LO, Plazak L, Fried W, Goldwasser E. Plasma factor(s) influencing red cell production. Nature. 1956;177:1240. doi: 10.1038/1771240a0. [DOI] [PubMed] [Google Scholar]
- 7.Marti HH, Wenger RH, Rivas LA, et al. Erythropoietin gene expression in human, monkey and murine brain. Eur J Neurosci. 1996;8:666–76. doi: 10.1111/j.1460-9568.1996.tb01252.x. [DOI] [PubMed] [Google Scholar]
- 8.Morishita E, Masuda S, Nagao M, Yasuda Y, Sasaki R. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience. 1997;76:105–16. doi: 10.1016/s0306-4522(96)00306-5. [DOI] [PubMed] [Google Scholar]
- 9.Siren AL, Fratelli M, Brines M, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A. 2001;98:4044–9. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agnello D, Bigini P, Villa P, et al. Erythropoietin exerts an anti-inflammatory effect on the CNS in a model of experimental autoimmune encephalomyelitis. Brain Res. 2002;952:128–34. doi: 10.1016/s0006-8993(02)03239-0. [DOI] [PubMed] [Google Scholar]
- 11.Kaiser K, Texier A, Ferrandiz J, et al. Recombinant human erythropoietin prevents the death of mice during cerebral malaria. J Infect Dis. 2006;193:987–95. doi: 10.1086/500844. [DOI] [PubMed] [Google Scholar]
- 12.Wiese L, Hempel C, Penkowa M, Kirkby N, Kurtzhals JA. Recombinant human erythropoietin increases survival and reduces neuronal apoptosis in a murine model of cerebral malaria. Malar J. 2008;7:3. doi: 10.1186/1475-2875-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehrenreich H, Hasselblatt M, Dembowski C, et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- 14.Ehrenreich H, Weissenborn K, Prange H, et al. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009;40:e647–56. doi: 10.1161/STROKEAHA.109.564872. [DOI] [PubMed] [Google Scholar]
- 15.Wagner M, Alam A, Zimmermann J, et al. Endogenous erythropoietin and the association with inflammation and mortality in diabetic chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:1573–9. doi: 10.2215/CJN.00380111. [DOI] [PubMed] [Google Scholar]
- 16.Stohlawetz PJ, Dzirlo L, Hergovich N, et al. Effects of erythropoietin on platelet reactivity and thrombopoiesis in humans. Blood. 2000;95:2983–9. [PubMed] [Google Scholar]
- 17.Ohls RK, Kamath-Rayne BD, Christensen RD, et al. Cognitive outcomes of preterm infants randomized to darbepoetin, erythropoietin, or placebo. Pediatrics. 2014;133:1023–30. doi: 10.1542/peds.2013-4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yalcinkaya F, Tumer N, Cakar N, Ozkaya N. Low-dose erythropoietin is effective and safe in children on continuous ambulatory peritoneal dialysis. Pediatr Nephrol. 1997;11:350–2. doi: 10.1007/s004670050294. [DOI] [PubMed] [Google Scholar]
- 19.Burchard GD, Radloff P, Philipps J, Nkeyi M, Knobloch J, Kremsner PG. Increased erythropoietin production in children with severe malarial anemia. Am J Trop Med Hyg. 1995;53:547–51. doi: 10.4269/ajtmh.1995.53.547. [DOI] [PubMed] [Google Scholar]
- 20.Diez-Padrisa N, Aguilar R, Machevo S, et al. Erythropoietin levels are not independently associated with malaria-attributable severe disease in Mozambican children. PLoS One. 2011;6:e24090. doi: 10.1371/journal.pone.0024090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casals-Pascual C, Kai O, Cheung JO, et al. Suppression of erythropoiesis in malarial anemia is associated with hemozoin in vitro and in vivo. Blood. 2006;108:2569–77. doi: 10.1182/blood-2006-05-018697. [DOI] [PubMed] [Google Scholar]
- 22.Casals-Pascual C, Idro R, Gicheru N, et al. High levels of erythropoietin are associated with protection against neurological sequelae in African children with cerebral malaria. Proc Natl Acad Sci U S A. 2008;105:2634–9. doi: 10.1073/pnas.0709715105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Picot S, Bienvenu AL, Konate S, et al. Safety of epoietin beta-quinine drug combination in children with cerebral malaria in Mali. Malar J. 2009;8:169. doi: 10.1186/1475-2875-8-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faille D, El-Assaad F, Alessi MC, Fusai T, Combes V, Grau GE. Platelet-endothelial cell interactions in cerebral malaria: the end of a cordial understanding. Thromb Haemost. 2009;102:1093–102. doi: 10.1160/TH09-05-0337. [DOI] [PubMed] [Google Scholar]
- 25.Dondorp AM, Desakorn V, Pongtavornpinyo W, et al. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med. 2005;2:e204. doi: 10.1371/journal.pmed.0020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Georgieff MK, Landon MB, Mills MM, et al. Abnormal iron distribution in infants of diabetic mothers: spectrum and maternal antecedents. J Pediatr. 1990;117:455–61. doi: 10.1016/s0022-3476(05)81097-2. [DOI] [PubMed] [Google Scholar]
- 27.Medana IM, Day NP, Hien TT, White NJ, Turner GD. Erythropoietin and its receptors in the brainstem of adults with fatal falciparum malaria. Malar J. 2009;8:261. doi: 10.1186/1475-2875-8-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirkeby A, Torup L, Bochsen L, et al. High-dose erythropoietin alters platelet reactivity and bleeding time in rodents in contrast to the neuroprotective variant carbamyl-erythropoietin (CEPO) Thromb Haemost. 2008;99:720–8. doi: 10.1160/TH07-03-0208. [DOI] [PubMed] [Google Scholar]
- 29.Weber A, Dzietko M, Berns M, et al. Neuronal damage after moderate hypoxia and erythropoietin. Neurobiol Dis. 2005;20:594–600. doi: 10.1016/j.nbd.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 30.Ehrenreich H, Hasselblatt M, Knerlich F, et al. A hematopoietic growth factor, thrombopoietin, has a proapoptotic role in the brain. Proc Natl Acad Sci U S A. 2005;102:862–7. doi: 10.1073/pnas.0406008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masuda S, Nagao M, Takahata K, et al. Functional erythropoietin receptor of the cells with neural characteristics. Comparison with receptor properties of erythroid cells. J Biol Chem. 1993;268:11208–16. [PubMed] [Google Scholar]
- 32.Chang KH, Tam M, Stevenson MM. Modulation of the course and outcome of blood-stage malaria by erythropoietin-induced reticulocytosis. J Infect Dis. 2004;189:735–43. doi: 10.1086/381458. [DOI] [PubMed] [Google Scholar]
- 33.Kling PJ, Schmidt RL, Roberts RA, Widness JA. Serum erythropoietin levels during infancy: associations with erythropoiesis. J Pediatr. 1996;128:791–6. doi: 10.1016/s0022-3476(96)70331-1. [DOI] [PubMed] [Google Scholar]
- 34.Widness JA, Veng-Pedersen P, Peters C, Pereira LM, Schmidt RL, Lowe LS. Erythropoietin pharmacokinetics in premature infants: developmental, nonlinearity, and treatment effects. J Appl Physiol. 1996;80:140–8. doi: 10.1152/jappl.1996.80.1.140. [DOI] [PubMed] [Google Scholar]
- 35.Leist M, Ghezzi P, Grasso G, et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–42. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]