The clinical validity of drug susceptibility testing (DST) for pyrazinamide, ethambutol, and second-line antituberculosis drugs is uncertain. In an individual patient data meta-analysis of 8955 patients with confirmed multidrug-resistant tuberculosis, DST results for these drugs were associated with treatment outcomes.
Keywords: tuberculosis, drug susceptibility test, treatment outcomes, multidrug resistant, meta-analysis
Abstract
Background. Individualized treatment for multidrug-resistant (MDR) tuberculosis and extensively drug-resistant (XDR) tuberculosis depends upon reliable and valid drug susceptibility testing (DST) for pyrazinamide, ethambutol, and second-line tuberculosis drugs. However, the reliability of these tests is uncertain, due to unresolved methodological issues. We estimated the association of DST results for pyrazinamide, ethambutol, and second-line drugs with treatment outcomes in patients with MDR tuberculosis and XDR tuberculosis.
Methods. We conducted an analysis of individual patient data assembled from 31 previously published cohort studies of patients with MDR and XDR tuberculosis. We used data on patients' clinical characteristics including DST results, treatment received, outcomes, and laboratory methods in each center.
Results. DST methods and treatment regimens used in different centers varied considerably. Among 8955 analyzed patients, in vitro susceptibility to individual drugs was consistently and significantly associated with higher odds of treatment success (compared with resistance to the drug), if that drug was used in the treatment regimen. Various adjusted and sensitivity analyses suggest that this was not explained by confounding. The adjusted odds of treatment success for ethambutol, pyrazinamide, and the group 4 drugs ranged from 1.7 to 2.3, whereas for second-line injectables and fluoroquinolones, odds ranged from 2.4 to 4.6.
Conclusions. DST for ethambutol, pyrazinamide, and second-line tuberculosis drugs appears to provide clinically useful information to guide selection of treatment regimens for MDR and XDR tuberculosis.
Multidrug-resistant (MDR) tuberculosis, defined as tuberculosis resistant to at least isoniazid and rifampin, and extensively drug-resistant (XDR) tuberculosis, defined as resistance to isoniazid and rifampin plus at least 1 fluoroquinolone and 1 second-line injectable drug, have become major public health concerns. The World Health Organization (WHO) estimates that 3.7% of new cases and 20% of previously tuberculosis treated cases, or >500 000 tuberculosis cases each year, are due to MDR strains [1]. Treatment of MDR tuberculosis requires the lengthy use of less effective and more toxic second-line drugs [2]. Recently, WHO recommended that MDR tuberculosis and XDR tuberculosis treatment should be individualized, that is, based on drug susceptibility testing (DST) results for first- and second-line drugs [3]. However, WHO estimates that DST is performed for <5% of all cases globally [1]. Moreover, testing methods for second-line drugs are not standardized, are considered unreliable [4–6], and have not been validated against clinical outcomes [7].
In view of the different available methods of DST for pyrazinamide (PZA), ethambutol (EMB), and second-line tuberculosis drugs [5], WHO published guidance on standardized methods of DST for second-line drugs in 2008 [4]. However, there is little published evidence regarding the relationship of these DST results to treatment outcomes. Additionally, the appropriate laboratory methods that will provide the most consistent and reliable results have not been well defined [4–6]. This has led to controversy about the clinical significance of DST for second-line tuberculosis drugs [7].
Using information from an international collaboration that assembled individual patient data of >9000 patients with MDR/XDR tuberculosis [8], this study assessed the relationship between treatment outcomes and results of culture-based DST for PZA, EMB, and the second-line drugs.
METHODS
MDR/XDR Tuberculosis Individual Patient Data
The collection and assembly of the individual patient dataset is described in detail elsewhere [8]. In brief, this work was conducted to address specific questions developed by an expert guideline development group convened by WHO to revise recommendations for treatment of drug-resistant tuberculosis [9]. The project was approved by the Research Ethics Board of the Montreal Chest Institute of the McGill University Health Center, Canada, and, for some of the original studies, by the local ethics boards. The study was determined to be non–human subjects research by the Office of the Associate Director for Science at the National Center for HIV/AIDS, Viral Hepatitis, STD and Tuberculosis Prevention, US Centers for Disease Control and Prevention.
Studies included in this analysis were identified from original studies published in 3 recent systematic reviews of MDR treatment outcomes [10–12]. These reviews searched Embase and Medline databases, the Cochrane Library, and the Institute for Scientific Information Web of Science, and included original studies published after 1970 that reported at least 1 treatment outcome that conformed with agreed definitions [13] for patients with bacteriologically confirmed MDR tuberculosis. All studies identified consisted of observational studies of patient groups; none were randomized trials. Most patients were treated with individualized regimens in specialized referral centers.
Methods for the individual patient data were based on criteria established by the Cochrane collaboration [14]. The additional inclusion criteria were that the study authors could be contacted; that they were willing to share their data, and that the cohort included at least 25 patients with MDR/XDR tuberculosis. Participating centers provided anonymized information including patient demographics (age and sex), clinical features (site of disease, sputum direct smear results for acid-fast bacilli, culture results for mycobacteria, chest radiography, human immunodeficiency virus (HIV) infection, use of antiretroviral therapy, initial DST results to first- and second-line drugs used, treatment factors (drugs and duration of initial and continuous phases of treatment, surgical resection), and treatment outcomes. Individual patients were excluded from the datasets if they had only extrapulmonary tuberculosis or were missing information on prescribed drug regimens or treatment outcomes. Standardized definitions for treatment outcomes of cure, completion, failure, death, and relapse were used [13].
Information on DST Methods
Methods for performance of DST and critical concentrations used for streptomycin, PZA, EMB, and tested second-line drugs were provided by members of the individual patient data collaborative group from each participating center. The information was reviewed by experts at WHO to assess the completeness of the description of the laboratory methods. DST for second-line drugs was routinely requested for patients with MDR tuberculosis. Laboratory technicians performing the DST were not blinded to the patients' clinical status.
The following groups of drugs were analyzed: PZA, EMB, injectable drugs (streptomycin, kanamycin, amikacin, or capreomycin), fluoroquinolones (ofloxacin, levofloxacin, and other later-generation quinolones) and drugs from group 4 (ethionamide/prothionamide, cycloserine, or para-aminosalicylic acid [PAS]). Ciprofloxacin was not assessed, as this is no longer recommended for MDR tuberculosis treatment. Kanamycin and amikacin were analyzed together given the high levels of cross-resistance between these drugs. Prothionamide and ethionamide were also considered equivalent and analyzed together. Levofloxacin, moxifloxacin, gatifloxacin, and sparfloxacin were defined as later-generation quinolones and were analyzed together. Drugs from group 5 (clofazimine, amoxicillin/clavulanate, clarithromycin, azithromycin, linezolid, thioacetazone) were not analyzed because very few centers performed DST for these drugs. Patients who received >1 quinolone or injectable drug were excluded from this analysis.
Data Analysis
We defined treatment outcomes as successful if cure was achieved or treatment was completed, whereas an unsuccessful outcome was defined in 2 ways: (1) as failure or relapse, or (2) as failure or relapse or death [13].
The primary analyses estimated odds of treatment success (vs fail/relapse or fail/relapse/death) associated with use of each drug when their Mycobacterium tuberculosis isolate was susceptible vs resistant to that drug. In secondary analysis; treatment outcomes were assessed in 2 strata: when critical concentrations used to define drug resistance were as recommended, or higher than recommended by WHO in 2008 [4]. Data from centers that used critical concentrations values below those recommended or could not provide data on critical concentrations were excluded. Analysis was also stratified by whether cultures for DST were performed on liquid or solid media.
For all adjusted analyses, we used a random-effects multivariable logistic regression (random intercept and random slope) with penalized quasi-likelihood [15], using PROC GLIMMIX in SAS software (version 9.2, SAS Institute, Cary, North Carolina) [16–19]. Patients were considered to be clustered within studies, and intercepts and slopes of the main exposure variables were allowed to vary across studies; this is to account for otherwise unmeasured interstudy differences in patient populations, as well as center-specific differences in data ascertainment, measurement, and other factors. Estimates were adjusted for 5 covariates: age, sex, HIV infection, extent of disease (a composite covariate scored by merging sputum-smear positivity and the presence of cavities on chest radiography), and previous history of tuberculosis treatment (which was a 3-category variable: no previous tuberculosis treatment, previous tuberculosis treatment with first-line drugs, and previous treatment with second-line drugs). Missing values were imputed for the 5 covariates used in multivariable analyses. For imputation, we used the mean from the other members of the same cohort to which the individual belonged if more than half the cohort members had values for that variable, or the mean value from all analyzed individuals. In sensitivity analyses, probabilistic imputation was used [20] for missing values. All statistical analyses were performed using SAS.
RESULTS
Study Selection, Participants, and DST Methods
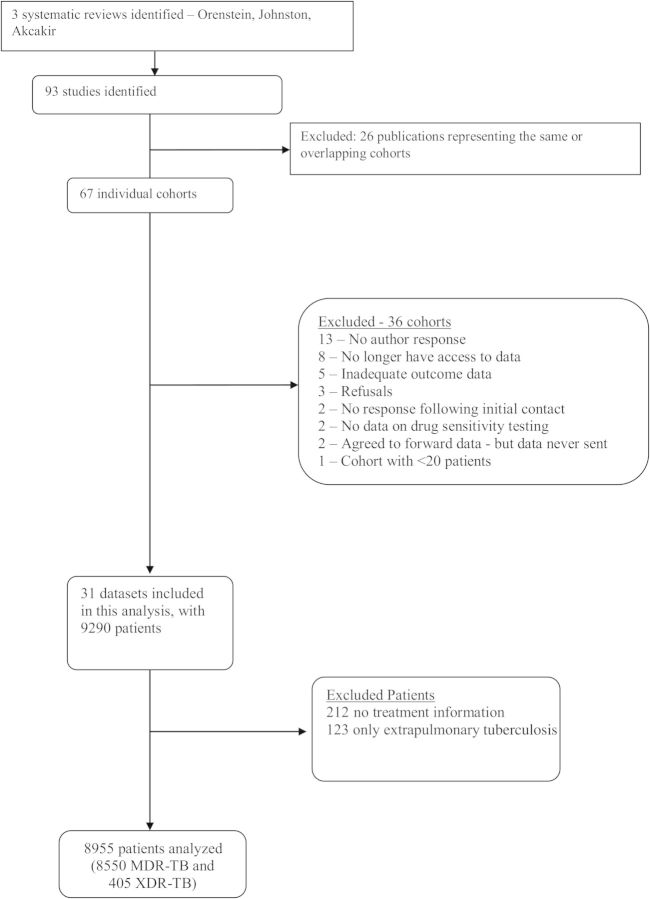
The final individual patient dataset comprised 9290 patients from 31 centers [21–53]. After excluding 123 patients with only extrapulmonary tuberculosis and 212 with no information on treatment outcome, a total of 8955 patients were included in this analysis: 8550 with MDR tuberculosis and 405 with XDR tuberculosis (Figure 1). Overall, the mean age was 39 years and 68% were male; 60% had had previous treatment with first-line tuberculosis drugs, and 11% with second-line drugs. Extensive disease, defined as cavities on chest radiography and/or acid-fast bacilli smear positive, was present in 72%. HIV serology was positive in 12% of patients, but only 1.3% of these patients were placed on antiretroviral therapy during tuberculosis treatment (Table 1).
Figure 1.
Flowchart of study selection. Abbreviations: MDR-TB, multidrug-resistant tuberculosis; XDR-TB, extensively drug-resistant tuberculosis.
Table 1.
Demographic and Pretreatment Clinical Characteristics of Patients Analyzed
| Characteristic | All Patients (N = 8955) |
Patients With Second-line DST Results (n = 8359)a |
Patients Without DST for SLDs (n = 596)b |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Age, y, mean | 39 | … | 39 | … | 35 | … |
| Sex | ||||||
| Female | 2837 | 31 | 2633 | 31 | 204 | 34 |
| Male | 6115 | 68 | 5723 | 68 | 392 | 66 |
| Unknown | 3 | 1 | 3 | 1 | 0 | … |
| History of tuberculosis treatment | ||||||
| None | 2082 | 23 | 1972 | 24 | 110 | 18 |
| Prior FLD | 5392 | 60 | 5084 | 61 | 308 | 52 |
| Prior SLD | 973 | 11 | 797 | 9 | 176 | 30 |
| Unknown | 508 | 5 | 506 | 6 | 2 | 0 |
| HIVc | ||||||
| Positive | 1091 | 12 | 1080 | 13 | 11 | 2 |
| Negative | 6572 | 73 | 6044 | 71 | 528 | 89 |
| Unknown | 1292 | 14 | 1235 | 15 | 57 | 9 |
| Site of disease | ||||||
| Pulmonary | 8476 | 95 | 7918 | 94 | 558 | 94 |
| Both | 242 | 3 | 221 | 3 | 21 | 3 |
| Unknown | 237 | 2 | 220 | 3 | 17 | 3 |
| Extensive diseased | ||||||
| Extensive | 6485 | 72 | 5997 | 72 | 488 | 82 |
| Not extensive | 2295 | 26 | 2188 | 26 | 107 | 18 |
| Unknown | 175 | 1 | 174 | 2 | 1 | 0 |
| Drug resistance | ||||||
| Pyrazinamide | 2641 | 29 | 2599 | 31 | 42 | 7 |
| Ethambutol | 3955 | 44 | 3856 | 46 | 99 | 17 |
| Streptomycin | 3972 | 44 | 3762 | 45 | 210 | 35 |
| Kanamycin or amikacin | 1745 | 19 | 1745 | 21 | … | … |
| Capreomycin | 606 | 7 | 606 | 7 | … | … |
| Fluoroquinolones | 894 | 10 | 894 | 11 | … | … |
| Ethionamide or prothionamide | 1712 | 19 | 1712 | 20 | … | … |
| Cycloserine | 472 | 5 | 472 | 6 | … | … |
| PAS | 1064 | 12 | 1064 | 11 | … | … |
Abbreviations: DST, drug susceptibility testing; FLD, first-line drug; HIV, human immunodeficiency virus; PAS, para-aminosalicylic acid; SLD, second-line drug.
a Patients with at least 1 result of DST to any second-line tuberculosis drug (other than streptomycin).
b Patients without any results of DST for second-line tuberculosis drugs.
c Only 15 patients on antiretrovirals, 14 who had second-line DST.
d Extensive disease defined as acid-fast bacilli smear positive and/or cavities on chest radiography.
Among the 31 included studies, 27 reported results of DST to PZA and EMB, and 26 studies reported methods and results of DST to second-line drugs. Solid media were more commonly used. Methods of DST and critical concentrations for first-line (Supplementary Table 1) and second-line tuberculosis drugs (Supplementary Table 2) used in the laboratories of the participating centers are detailed in the Supplementary Data.
Association of DST Results and Treatment Outcomes
Compared with failure/relapse, use of each of the drugs analyzed was associated with significantly higher odds of treatment success when the M. tuberculosis isolate was susceptible compared with resistant to that specific drug (Table 2). Similar results were found when death was included as part of the unsuccessful outcomes (ie, success vs failure/relapse/death) (Table 3).
Table 2.
Treatment Outcomes (Cure/Complete Versus Failure/Relapse) According to Drug-Specific Susceptibility Testing Result Among Patients With Multidrug-Resistant and Extensively Drug-Resistant Tuberculosis Who Took That Drug
| Drug Used | No. Analyzed |
OR of Treatment Success if Susceptible to the Drug Used (Cure/Complete vs Failure/Relapse); Reference = Resistant to the Drug Used |
||
|---|---|---|---|---|
| Resistant (No.) | Susceptible (No.) | Unadjusted OR (95% CI) | Adjusted OR (95% CI)a | |
| Pyrazinamide | 485 | 1061 | 2.0 (1.3–3.1) | 1.9 (1.3–2.9) |
| Ethambutol | 512 | 1110 | 1.8 (1.2–2.6) | 1.7 (1.2–2.4) |
| Streptomycinb | 196 | 468 | 1.9 (1.1–3.2) | 1.7 (1.0–3.0) |
| Kanamycin or amikacinb | 151 | 2106 | 3.9 (2.0–7.3) | 3.4 (1.7–6.9) |
| Capreomycinb | 172 | 684 | 2.3 (1.4–3.7) | 2.4 (1.4–4.0) |
| Ofloxacinb | 299 | 3116 | 5.3 (3.5–8.2) | 4.6 (2.7–8.0) |
| Levofloxacin and other later-generation quinolonesb | 125 | 325 | 3.5 (1.8–7.0) | 3.2 (1.6–6.7) |
| Ethionamide or prothionamide | 651 | 2184 | 2.4 (1.9–3.1) | 2.3 (1.8–3.0) |
| Cycloserine | 213 | 2893 | 2.3 (1.5–3.3) | 2.2 (1.5–3.3) |
| PAS | 228 | 1342 | 2.2 (1.5–3.0) | 2.0 (1.3–3.1) |
Bold values indicate statistically significant results.
Abbreviations: CI, confidence interval; OR, odds ratio; PAS, para-aminosalicylic acid.
a Models adjusted for age, sex, extent of disease, past history of treatment with first- and second-line drugs, and human immunodeficiency virus (HIV) coinfection. The numbers of missing values for each covariate that was imputed were as follows: age, 25; sex, 3; extent of disease, 175 (1.9%); past treatment with first-line drugs, 508 (5.7%); past treatment with second-line drugs, 852 (9.5%); HIV coinfection, 1292 (14.3%).
b Patients who received >1 quinolone or an injectable drug were excluded from this analysis.
Table 3.
Treatment Outcomes (Cure/Complete Versus Failure/Relapse/Death) According to Drug-Specific Susceptibility Testing Result Among Patients With Multidrug-Resistant and Extensively Drug-Resistant Tuberculosis Who Took That Drug
| Drug Used | No. Analyzed |
OR of Treatment Success if Susceptible to the Drug Used (Cure/Complete vs Failure/Relapse/Death); Reference = Resistant to the Drug Used |
||
|---|---|---|---|---|
| Resistant (No.) | Susceptible (No.) | Unadjusted OR (95% CI) | Adjusted OR (95% CI)a | |
| Pyrazinamide | 741 | 1300 | 1.6 (1.3–2.0) | 1.6 (1.3–2,1) |
| Ethambutol | 858 | 1335 | 1.7 (1.1–2.4) | 1.6 (1.1–2.4) |
| Streptomycinb | 243 | 552 | 1.9 (1.2–2.8) | 1.9 (1.3–2.8) |
| Kanamycin or amikacinb | 191 | 2600 | 2.5 (1.5–4.1) | 2.3 (1.4–3.8) |
| Capreomycinb | 190 | 817 | 1.5 (1.0–2.4) | 1.7 (1.1–2.7) |
| Ofloxacinb | 372 | 3687 | 4.1 (2.8–6.1) | 3.8 (2.4–6.0) |
| Levofloxacin or other later-generation fluoroquinolonesb | 145 | 351 | 3.4 (1.9–6.2) | 3.0 (1.6–5.4) |
| Ethionamide or prothionamide | 826 | 2557 | 2.2 (1.8–2.7) | 2.1 (1.7–2.6) |
| Cycloserine | 250 | 3397 | 1.9 (1.3–2.8) | 1.9 (1.3–2.4) |
| PAS | 284 | 1580 | 1.9 (1.4–2.6) | 1.8 (1.3–2.5) |
Bold values indicate statistically significant results.
Abbreviations: CI, confidence interval; OR, odds ratio; PAS, para-aminosalicylic acid.
a Models adjusted for age, sex, extent of disease, history of treatment with first- and second-line drugs, and human immunodeficiency virus (HIV) coinfection. The numbers of missing values for each covariate that was imputed were as follows: age, 25; sex, 3; extent of disease, 175 (1.9%), past treatment with first-line drugs, 508 (5.7%); past treatment with second-line drugs, 852 (9.5%); HIV coinfection, 1292 (14.3%).
b Patients who received >1 quinolone or an injectable drug were excluded from this analysis.
The estimated association of resistance and drug effect did not vary importantly across studies in most cases. The estimated heterogeneity of parameter estimates was nonzero and statistically significant only for ethambutol when the unsuccessful outcome was failure/relapse/death. The estimate was nonzero and statistically significant for kanamycin and ofloxacin for failure/relapse (data not shown in tabular form).
Assessment of Potential Confounding
Use of a certain drug despite in vitro resistance to that drug may be associated with worse outcomes simply because fewer treatment options were available—because of associated resistance to other drugs, or fewer second-line drugs available at a given center. To assess this, we performed several analyses.
First, estimates were adjusted for the same clinical characteristics as in Tables 2 and 3, plus PZA resistance, or also PZA and/or fluoroquinolone resistance, or also PZA, fluoroquinolone, and/or second-line injectable resistance. As seen in Tables 4 and 5, even after these additional adjustments, odds of treatment success remained significantly greater if the isolate was sensitive to the drug in question with a few exceptions.
Table 4.
Treatment Outcomes (Cure/Complete Versus Failure/Relapse) According to Drug-Specific Susceptibility Testing Result Among Patients With Multidrug-Resistant and Extensively Drug-Resistant Tuberculosis Who Took That Drug: Additional Adjustment
| Drug Used (No. Given the Drug) | OR of Treatment Success if Susceptible to the Drug Used (Cure/Complete vs Failure/Relapse); Reference = Resistant to the Drug Used |
|||
|---|---|---|---|---|
| Adjusted for Clinical Characteristicsa, OR (95% CI) | Adjusted for Clinical Characteristicsa and PZA-Rb, OR (95% CI) | Adjusted for Clinical Characteristicsa and PZA-R/FQN-Rc, OR (95% CI) | Adjusted for Clinical Characteristicsa and PZA-R, FQN-R, and AMK-Rd, OR (95% CI) | |
| Pyrazinamide (1546) | 1.9 (1.3–2.9) | … | 1.7 (1.1–2.6) | 1.6 (1.1–2.4) |
| Ethambutol (1622) | 1.7 (1.2–2.4) | 1.5 (1.1–2.2) | 1.5 (1.1–2.1) | 1.4 (1.0–1.9) |
| Streptomycine (664) | 1.7 (1.0–3.0) | 1.7 (1.0–3.0) | 1.7 (1.0–2.9) | 1.5 (.9–2.6) |
| Kanamycin or amikacine (2257) | 3.4 (1.7–6.9) | 3.3 (1.6–6.6) | 2.8 (1.4–5.3) | … |
| Capreomycine (856) | 2.4 (1.4–4.0) | 2.4 (1.4–3.9) | 2.3 (1.3–3.9) | 2.0 (1.1–3.4) |
| Ofloxacine (3415) | 4.6 (2.7–8.0) | 4.8 (2.9–8.1) | … | 4.1 (2.5–6.9) |
| Levofloxacin or other later-generation fluoroquinolonese (450) | 3.2 (1.6–6.7) | 3.1 (1.5–6.6) | … | 3.1 (1.4–6.5) |
| Ethionamide or prothionamide (2835) | 2.3 (1.8–3.0) | 2.2 (1.7–3.0) | 1.8 (1.3–2.4) | 1,6 (1.2–2.1) |
| Cycloserine (3106) | 2.2 (1.5–3.3) | 2.1 (1.5–3.0) | 1.6 (1.1–2.5) | 1.5 (1.0–2.5) |
| PAS (1570) | 2.0 (1.3–3.1) | 2.0 (1.9–3.0) | 1.8 (1.2–2.8) | 1.7 (1.1–2.6) |
Bold values indicate statistically significant results.
Abbreviations: AMK-R, amikacin or kanamycin resistance; CI, confidence interval; FQN-R, fluoroquinolone resistance; OR, odds ratio; PAS, para-aminosalicylic acid; PZA-R, pyrazinamide resistance.
a Models adjusted for age, sex, extent of disease, past history of treatment with first- and second-line drugs, and human immunodeficiency virus (HIV) coinfection. The numbers of missing values for each covariate that was imputed were as follows: age, 25; sex, 3; extent of disease, 175 (1.9%); past treatment with first-line drugs, 508 (5.7%); past treatment with second-line drugs, 852 (9.5%); HIV coinfection, 1292 (14.3%).
b Model adjusted for clinical characteristics and for resistance to PZA.
c Model adjusted for clinical characteristics and for resistance to PZA and/or FQN.
d Model adjusted for clinical characteristics and for resistance to PZA, FQN, and/or AMK.
e Patients who received >1 quinolone or injectable were excluded from the analyses of effect of injectables or FQN.
Table 5.
Treatment Outcomes (Cure/Complete Versus Failure/Relapse/Death) According to Drug-Specific Susceptibility Testing Result Among Patients With Multidrug-Resistant and Extensively Drug-Resistant Tuberculosis Who Took That Drug: Additional Adjustments
| Drug Used (No. Given the Drug) | OR of Treatment Success if Susceptible to the Drug Used (Cure/Complete vs Failure/Relapse/Death);Reference = Resistant to the Drug Used |
|||
|---|---|---|---|---|
| Adjusted for Clinical Characteristicsa, OR (95% CI) | Adjusted for Clinical Characteristics and PZA-Rb, OR (95% CI) | Adjusted for Clinical Characteristics and PZA-R/FQN-Rc, OR (95% CI) | Adjusted for Clinical Characteristics and PZA-R, FQN-R, and AMK-Rd, OR (95% CI) | |
| Pyrazinamide (2041) | 1.6 (1.3–2,1) | … | 1.5 (1.1–1.9) | 1.4 (1.1–1.8) |
| Ethambutol (2193) | 1.6 (1.1–2.4) | 1.5 (1.1–2.8) | 1.4 (1.0–2.1) | 1.4 (.9–2.1) |
| Streptomycine (795) | 1.9 (1.3–2.8) | 1.9 (1.3–2.9) | 1.8 (1.2–2.7) | 1.6 (1.1–2.5) |
| Kanamycin or amikacine (2791) | 2.3 (1.4–3.8) | 2.2 (1.4–3.6) | 1.8 (1.2–2.8) | … |
| Capreomycine (1007) | 1.7 (1.1–2.7) | 1.6 (1.1–2.6) | 1.6 (1.0–2.5) | 1.3 (.8–2.1) |
| Ofloxacine (4059) | 3.8 (2.4–6.0) | 3.9 (2.5–6.2) | … | 3.4 (2.2–5.2) |
| Levofloxacin or other later-generation fluoroquinolonese (496) | 3.0 (1.6–5.4) | 2.9 (1.6–5.3) | … | 2.8 (1.7–4.8) |
| Ethionamide or prothionamide (3383) | 2.1 (1.7–2.6) | 2.1 (1.7–2.6) | 1.7 (1.3–2.1) | 1.5 (1.2–1.9) |
| Cycloserine (3647) | 1.9 (1.3–2.4) | 1.8 (1.2–2.7) | 1.4 (1.0–2.1) | 1.3 (.9–1.9) |
| PAS (1864) | 1.8 (1.3–2.5) | 1.8 (1.3–2.4) | 1.6 (1.2–2.2) | 1.5 (1.1–2.1) |
Bold values indicate statistically significant results.
Abbreviations: AMK-R, amikacin or kanamycin resistance; CI, confidence interval; FQN-R, fluoroquinolone resistance; OR, odds ratio; PAS, para-aminosalicylic acid; PZA-R, pyrazinamide resistance.
a Models adjusted for age, sex, extent of disease, past history of treatment with first- and second-line drugs, and human immunodeficiency virus (HIV) coinfection. The number of missing values for each covariate that were imputed were as follows: age, 25; sex, 3; extent of disease, 175 (1.9%); past treatment with first-line drugs, 508 (5.7%); past treatment with second-line drugs, 852 (9.5%); HIV coinfection, 1292 (14.3%).
b Model adjusted for clinical characteristics and for resistance to PZA.
c Model adjusted for clinical characteristics and for resistance to PZA and/or FQN.
d Model adjusted for clinical characteristics and for resistance to PZA, FQN, and/or AMK.
e Patients who received >1 quinolone or injectable were excluded from the analyses of effect of injectables or FQN.
Next, use of each drug when the isolate was resistant or sensitive to that drug was assessed according to whether the isolate was also resistant to another second-line drug. As seen in Table 6, the use of any of the drugs when resistant to those drugs was not associated with resistance to most of the other drugs, with a few exceptions. The most consistent finding was that when there was resistance to fluoroquinolones, then PZA, amikacin/kanamycin, ethionamide/prothionamide, and cycloserine were all more likely to have been used despite in vitro resistance to these agents. The other consistent finding was use of capreomycin, despite resistance, if the isolate was resistant to pyrazinamide, streptomycin, or amikacin/kanamycin.
Table 6.
Use of Tuberculosis Drugs When Resistant to That Drug, According to Whether Resistant or Sensitive to Other Drugs
| Drug | DST Result | PZA-Resistant Strains |
Ethambutol-Resistant Strains |
Streptomycin-Resistant Strains |
Amikacin/Kanamycin-
Resistant Strains |
Capreomycin-Resistant Strains |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | PZA Used | No. | EMB Used | No. | SM Used | No. | AMK Used | No. | CAP Used | ||
| Use of drug when MDR tuberculosis strain also resistant to: | |||||||||||
| PZA | Sensitive | … | … | 1196 | 22% | 1156 | 10% | 357 | 17% | 107 | 32% |
| Resistant | … | … | 1865 | 26% | 1733 | 7% | 884 | 21% | 380 | 47% | |
| EMB | Sensitive | 607 | 24% | … | … | 1239 | 11% | 311 | 17% | 134 | 43% |
| Resistant | 1863 | 35% | … | … | 2656 | 9% | 1351 | 19% | 471 | 48% | |
| SM | Sensitive | 815 | 36% | 1136 | 24% | … | … | 236 | 20% | 66 | 12% |
| Resistant | 1733 | 31% | 2656 | 25% | … | … | 1441 | 18% | 539 | 51% | |
| AMK/KAN | Sensitive | 1612 | 33% | 1351 | 24% | 2195 | 7% | … | … | 133 | 15% |
| Resistant | 884 | 34% | 2264 | 26% | 1441 | 9% | … | … | 467 | 55% | |
| CAP | Sensitive | 1377 | 35% | 2056 | 28% | 2434 | 5% | 892 | 17% | … | … |
| Resistant | 380 | 31% | 471 | 28% | 539 | 9% | 467 | 18% | … | … | |
| FQN | Sensitive | 1620 | 26% | 2461 | 17% | 2528 | 6% | 1042 | 17% | 399 | 47% |
| Resistant | 466 | 38% | 609 | 21% | 532 | 10% | 383 | 30% | 104 | 47% | |
| Ethionamide | Sensitive | 1647 | 34% | 1238 | 25% | 2038 | 7% | 805 | 15% | 263 | 49% |
| Resistant | 813 | 39% | 1259 | 28% | 1154 | 9% | 692 | 27% | 299 | 43% | |
| Cs | Sensitive | 2224 | 32% | 3260 | 23% | 87 | 5% | 3273 | 7% | 530 | 47% |
| Resistant | 217 | 37% | 337 | 30% | 178 | 15% | 265 | 12% | 55 | 38% | |
| PAS | Sensitive | 1663 | 32% | 2294 | 23% | 2202 | 6% | 906 | 15% | 295 | 49% |
| Resistant | 609 | 34% | 711 | 29% | 737 | 12% | 380 | 19% | 211 | 46% | |
| Quinolone-Resistant Strains |
Ethionamide-Resistant Strains |
Cycloserine-Resistant Strains |
PAS-Resistant Strains |
PAS-Resistant Strains |
|||||||
| No. | FQN Used | No. | ETH Used | No. | Cs Used | No. | PAS Used | ||||
| Use of drug when MDR tuberculosis strain also resistant to: | |||||||||||
| PZA | Sensitive | 273 | 74% | 505 | 58% | 148 | 67% | 340 | 34% | ||
| Resistant | 467 | 78% | 813 | 55% | 218 | 66% | 609 | 31% | |||
| EMB | Sensitive | 180 | 73% | 1259 | 57% | 103 | 58% | 283 | 27% | ||
| Resistant | 609 | 72% | 377 | 57% | 337 | 64% | 711 | 37% | |||
| SM | Sensitive | 288 | 76% | 392 | 64% | 149 | 71% | 325 | 31% | ||
| Resistant | 532 | 72% | 1154 | 50% | 265 | 67% | 737 | 33% | |||
| AMK/KAN | Sensitive | 448 | 73% | 886 | 57% | 250 | 68% | 656 | 35% | ||
| Resistant | 383 | 76% | 692 | 54% | 165 | 69% | 380 | 36% | |||
| CAP | Sensitive | 377 | 73% | 884 | 53% | 178 | 78% | 397 | 33% | ||
| Resistant | 104 | 85% | 299 | 49% | 55 | 75% | 211 | 25% | |||
| FQN | Sensitive | … | … | 979 | 55% | 215 | 60% | 681 | 37% | ||
| Resistant | … | … | 416 | 72% | 168 | 76% | 261 | 44% | |||
| Ethionamide | Sensitive | 368 | 78% | … | … | 186 | 63% | 572 | 33% | ||
| Resistant | 416 | 80% | … | … | 263 | 68% | 442 | 35% | |||
| Cs | Sensitive | 644 | 73% | 1322 | 56% | … | … | 817 | 35% | ||
| Resistant | 168 | 76% | 263 | 67% | … | … | 217 | 35% | |||
| PAS | Sensitive | 455 | 78% | 838 | 56% | 188 | 77% | … | … | ||
| Resistant | 261 | 73% | 442 | 63% | 217 | 63% | … | … | |||
Bold values indicate statistical significance of differences, from χ2 test: P < .001 (to account for multiple testing of 72 comparisons, only P values <.001 were considered significant and are shown).
Fluoroquinolones includes ofloxacin, levofloxacin or later-generation quinolones. Ethionamide includes ethionamide and prothionamide.
Abbreviations: AMK/KAN, amikacin or kanamycin; CAP, capreomycin; Cs, cycloserine; DST, drug susceptibility testing; EMB, ethambutol; ETH, ethionamide; FQN, fluoroquinolones; MDR, multidrug resistant; PAS, para-aminosalicylic acid; PZA, pyrazinamide; SM, streptomycin.
The use of PZA, EMB, fluoroquinolones, or second-line injectables despite in vitro resistance to the same drugs was seen in virtually all centers. There was no discernible association with use of other second-line drugs, or patterns of resistance to other second-line drugs (Supplementary Tables 4A–E). This suggests that limited availability of alternative drugs at the participating centers was not an explanation for the use of drugs despite in vitro resistance.
Finally, the effect of PZA, EMB, streptomycin, cycloserine, PAS, and capreomycin resistance was stratified by the critical concentrations used. If M. tuberculosis isolates were considered to be susceptible to PZA or EMB, the odds of success compared to failure/relapse were somewhat higher when the critical concentration values to distinguish susceptible from resistant were higher than recommended (Table 7). There was no difference in outcomes for the other drugs analyzed. Results were similar when success was compared with failure/relapse/death (Supplementary Table 3). Additional analyses stratified by performance of DST on solid or liquid media found no substantial or consistent difference in findings (results not shown in tabular form).
Table 7.
Treatment Outcomes (Cure/Complete Versus Failure/Relapse) According to Drug Susceptibility Testing Using Recommended or Higher Than Recommended Critical Concentrations Among Patients Who Took That Druga
| Recommended Critical Concentration |
Higher Critical Concentration |
|||
|---|---|---|---|---|
| DST for Drug | OR of Treatment Success if Susceptible to the Drug Used (Cure/Complete vs Failure/Relapse); Reference = Resistant to Drug Used |
OR of Treatment Success if Susceptible to the Drug Used (Cure/Complete vs Failure/Relapse); Reference = Resistant to Drug Used |
||
| No. Given the Drug, Unadjusted OR (95% CI) | No. Given the Drug, Adjusted ORb (95% CI) | No. Given the Drug, Unadjusted OR (95% CI) | No. Given the Drug, Adjusted ORb (95% CI) | |
| Pyrazinamide: | 1275 | 1275 | 68 | 68 |
| 21 studies at recommended and 2 studies at higher | 2.0 (1.4–3.0) | 2.0 (1.3–3.0) | 3.9 (1.0–16.2) | No convergencec |
| Ethambutol: | 1148 | 1148 | 185 | 185 |
| 17 studies at recommended and 3 studies at higher | 1.6 (1.1–2.3) | 1.5 (1.1–2.4) | 2.0 (.9–4.7) | 2.2 (1.0–5.3) |
| Streptomycin: | 197 | 197 | 434 | 434 |
| 7 studies at recommended and 12 at studies higher | 1.1 (.2–5.0) | No convergencec | 1.8 (.9–3.4) | 1.8 (.9–3.5) |
| Capreomycin: | 240 | 240 | 235 | 235 |
| 7 studies at recommended and 2 studies at higher | 3.5 (.8–15) | 4.7 (.9–25.0) | No convergencec | No convergencec |
| Cycloserine: | 2489 | 2489 | 215 | 215 |
| 11 studies at recommended and 5 studies at higher | 2.5 (1.2–4.6) | 2.3 (1.4–4.1) | 2.0 (.7–5.3) | No convergencec |
| PAS: | 782 | 782 | 163 | 163 |
| 5 studies at recommended and 6 studies at higher | 2.5 (1.6–4.0) | 2.2 (1.1–4.7) | 1.5 (.5–4.9) | No convergencec |
Bold values indicate statistically significant results.
Abbreviations: CI, confidence interval; DST, drug susceptibility testing; OR, odds ratio; PAS, para-aminosalicylic acid.
a All studies that either did not provide or used less than recommended critical concentrations were excluded from this analysis.
b Models adjusted for age, sex, extent of disease, past history of treatment with first- and second-line drugs, and human immunodeficiency virus (HIV) coinfection. The number of missing values for each covariate which were imputed were as follows: age, 25; sex, 3; extent of disease, 175 (1.9%); past treatment with first-line drugs, 508 (5.7%); past treatment with second-line drugs, 852 (9.5%); HIV coinfection, 1292 (14.3%).
c Multivariable models did not converge (too few observations and too much heterogeneity).
DISCUSSION
In this study, the impact of in vitro resistance to various second-line drugs on individual treatment outcomes was analyzed among 8955 patients from 31 centers located in countries in all WHO health regions. For all drugs tested, use of that drug was associated with higher odds of treatment success compared with failure and relapse, or compared with failure, relapse, and death if the isolate was susceptible rather than resistant to that drug. We did not find evidence that use of a drug when the isolate was known to be resistant to that drug was because of additional resistance or lack of access to certain drugs at some centers. These findings suggest that DST results, using current methods, can be useful for selection of tuberculosis drugs in individualized treatment of patients with MDR tuberculosis.
This study had a number of strengths. The most important was the size of the study population—8955 patients with MDR tuberculosis were included, making this the largest analysis of the clinical significance of DST for second-line drugs. To our knowledge, this is the first evidence of the association of DST results for second-line drugs and treatment outcomes. These analyses also represent an important extension of findings from the original 31 cohorts. No single cohort had adequate power to assess the utility of DST to individual drugs; compiling all patients into 1 large dataset provided much greater power for this analysis. In this regard, the results for group drugs 4 should be particularly useful, as there is very little evidence regarding clinical utility and validity of DST for this class of drugs [4].
These findings should be generalizable, as the patients were treated at 31 different centers, which were located in all WHO world regions, including some very resource-limited settings. Hence, local treatment practice, study populations, and strains of M. tuberculosis were highly variable. Treatment regimens also varied considerably at different centers, more than would be explained on the basis of different patient characteristics, including DST results. Instead, these differences may have reflected local medical opinions and beliefs. We did not find evidence that this was due to lack of availability of certain drugs, but some physicians may have considered the DST unreliable for second-line drugs or for PZA and EMB and thus not used these results to guide therapy. This quasi-experimental evidence from varying treatment approaches in many different centers, independent of patient characteristics and DST results, strengthens the value of these findings related to use or nonuse of certain drugs despite DST results.
However, this study also had important limitations. All the data available were derived from observational cohort studies, and therapy was individualized in most patients. Therefore, the use of certain drugs was likely to have been influenced by clinical characteristics such as disease severity, prior treatment, resistance patterns, and concomitant use of other drugs. To account for this, we adjusted in multivariate analysis for several factors, including HIV coinfection and severity of disease. However, we did not have data on the duration of treatment with each individual drug; therefore, we could not analyze the impact of length of treatment with each drug on odds of treatment success when the tuberculosis was susceptible or resistant to that drug.
Even after adjusting for patient characteristics and extent of drug resistance, residual confounding could remain, due to unmeasured differences between patients who received different therapy. This residual confounding would best be controlled by conducting multiple randomized clinical trials comparing the use or nonuse of each individual drug with randomization stratified by DST results and severity of disease. However, published evidence from randomized trials in MDR tuberculosis are very scanty—only two phase 2 trials have been published [54, 55], and no phase 3 trials have been published at all [56].
A second important limitation was the differences between (and even within) laboratories with regard to the DST methods and critical concentrations. Not every center tested all drugs, limiting the power of our analysis. This was particularly true for the analyses of the critical concentrations for each drug, as very few laboratories used higher critical concentrations, limiting power to analyze this question. Very few centers performed DST for group 5 drugs, so the clinical utility of DST for these drugs could not be assessed at all. Additional differences in laboratory techniques such as the pH of the media or incubation time can affect DST results [4–6], but we had no information about these methodological details.
In conclusion, DST for EMB, PZA, and many second-line tuberculosis drugs using currently available methods appears to provide useful information that should be used by clinicians in selecting drugs for MDR tuberculosis treatment. However, additional studies are needed to improve, standardize, and validate the laboratory methods and critical concentrations for these tests.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The Collaborative Group for Meta-analysis of Individual Patient Data in MDR-TB members are as follows: S. D. Ahuja, D. Ashkin, M. Avendano, R. Banerjee, M. Bauer, J. N. Bayona, M. C. Becerra, A. Benedetti, M. Burgos, R. Centis, E. D. Chan, C. Y. Chiang, H. Cox, L. D'Ambrosio, K. DeRiemer, N. H. Dung, D. Enarson, D. Falzon, K. Flanagan, J. Flood, M. L. Garcia-Garcia, N. Gandhi, R. M. Granich, M. G. Hollm-Delgado, T. H. Holtz, M. D. Iseman, L. G. Jarlsberg, S. Keshavjee, H. R. Kim, W. J. Koh, J. Lancaster, C. Lange, W. C. M. de Lange, V. Leimane, C. C. Leung, J. Li, D. Menzies, G. B. Migliori, S. P. Mishustin, C. D. Mitnick, M. Narita, P. O'Riordan, M. Pai, D. Palmero, S. K. Park, G. Pasvol, J. Pena, C. Pérez-Guzmán , M. I. D. Quelapio, A. Ponce-de-Leon, V. Riekstina, J. Robert, S. Royce, H. S. Schaaf, K. J. Seung, L. Shah, T. S. Shim, S. S. Shin , Y. Shiraishi , J. Sifuentes-Osornio, G. Sotgiu, M. J. Strand, P. Tabarsi, T. E. Tupasi, R. van Altena, M. Van der Walt, T. S. Van der Werf , M. H. Vargas, P. Viiklepp, J. Westenhouse, W. W. Yew, J. J. Yim.
Financial support. This work was supported in part by the Stop Tuberculosis Department of World Health Organization, through a grant from the US Agency for International Development. Funding for data gathering at participating centers came from the following agencies: in the state of California, from the Centers for Disease Control and Prevention (cooperative agreement funds); in Italy, from the European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreement FP7-223681; in Mexico (Veracruz), from the Mexican Secretariat of Health, the US National Institutes of Health (A135969 and K01TW000001), the Wellcome Trust (176W009), the Howard Hughes Medical Institute (55000632), and the Mexican Council of Science and Technology: SEP (2004-C01-47499, FOSSIS 2005-2 [14475, 87332]); and in South Africa, from the South African Medical Research Council. M. L. B. was supported by a scholarship from Conselho Nacional de Desenvolvimento Científico e Tecnológico, Science Without Borders program (200097/2012-1). D. M. was supported by a salary award from the Fonds de Recherche en Sante de Quebec.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Global tuberculosis report. 2013. Available at: http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf . Accessed 22 October 2013.
- 2.Caminero JA, Sotgiu G, Zumla A, Migliori GB. Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis. Lancet Infect Dis. 2010;10:621–9. doi: 10.1016/S1473-3099(10)70139-0. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis—2011 update. Available at: http://whqlibdoc.who.int/publications/2011/9789241501583_eng.pdf . Accessed 22 October 2013. [DOI] [PubMed]
- 4.Policy guidance on drug-susceptibility testing (DST) of second-line antituberculosis drugs. 2008. Available at: http://whqlibdoc.who.int/hq/2008/WHO_HTM_TB_2008.392_eng.pdf . Accessed 22 October 2013. [PubMed]
- 5.Kim SJ. Drug-susceptibility testing in tuberculosis: methods and reliability of results. Eur Respir J. 2005;25:564–9. doi: 10.1183/09031936.05.00111304. [DOI] [PubMed] [Google Scholar]
- 6.Kim SJ, Espinal MA, Abe C, et al. Is second-line anti-tuberculosis drug susceptibility testing reliable? Int J Tuberc Lung Dis. 2004;8:1157–8. [PubMed] [Google Scholar]
- 7.Kam KM, Sloutsky A, Yip CW, et al. Determination of critical concentrations of second-line anti-tuberculosis drugs with clinical and microbiological relevance. Int J Tuberc Lung Dis. 2010;14:282–8. [PubMed] [Google Scholar]
- 8.Ahuja SD, Ashkin D, Avendano M, et al. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Med. 2012;9:28. doi: 10.1371/journal.pmed.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falzon D, Jaramillo E, Schunemann HJ, et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J. 2011;38:516–28. doi: 10.1183/09031936.00073611. [DOI] [PubMed] [Google Scholar]
- 10.Johnston JC, Shahidi NC, Sadatsafavi M, Fitzgerald JM. Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and meta-analysis. PLoS One. 2009;4:e6914. doi: 10.1371/journal.pone.0006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orenstein EW, Basu S, Shah NS, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis. 2009;9:153–61. doi: 10.1016/S1473-3099(09)70041-6. [DOI] [PubMed] [Google Scholar]
- 12.Akçakır Y. Correlates of treatment outcomes of multidrug-resistant tuberculosis (MDR-TB): a systematic review and meta-analysis. Montréal, Canada: McGill University; 2010. [Google Scholar]
- 13.Laserson KF, Thorpe LE, Leimane V, et al. Speaking the same language: treatment outcome definitions for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2005;9:640–5. [PubMed] [Google Scholar]
- 14.Higgins JPT, Green G. John Wiley & Sons; 2008. Cochrane handbook for systematic reviews of interventions. Available at: http://www.cochrane-handbook.org. Accessed 30 April 2011. [Google Scholar]
- 15.Pinheiro JC, Bates DM. Approximations to the log-likelihood function in the nonlinear mixed-effects model. J Comput Graph Stat. 1995;4:12–35. [Google Scholar]
- 16.Thompson SG, Turner RM, Warn DE. Multilevel models for meta-analysis, and their application to absolute risk differences. Stat Methods Med Res. 2001;10:375–92. doi: 10.1177/096228020101000602. [DOI] [PubMed] [Google Scholar]
- 17.Turner RM, Omar RZ, Yang M, Goldstein H, Thompson SG. A multilevel model framework for meta-analysis of clinical trials with binary outcomes. Stat Med. 2000;19:3417–32. doi: 10.1002/1097-0258(20001230)19:24<3417::aid-sim614>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 19.Schabenberger O. Introducing the GLIMMIX procedure for generalized linear mixed models. 2011. Available at: http://www2.sas.com/proceedings/sugi30/196-30.pdf . Accessed 2 July 2014.
- 20.Yarandi HN. Handling missing data with multiple imputation using PROC MI in SAS. Available at: http://analytics.ncsu.edu/sesug/2002/ST14.pdf . Accessed 2 July 2014.
- 21.Avendano M, Goldstein RS. Multidrug-resistant tuberculosis: long term follow-up of 40 non-HIV-infected patients. Can Respir J. 2000;7:383–9. doi: 10.1155/2000/457905. [DOI] [PubMed] [Google Scholar]
- 22.Burgos M, Gonzalez LC, Paz EA, et al. Treatment of multidrug-resistant tuberculosis in San Francisco: an outpatient-based approach. Clin Infect Dis. 2005;40:968–75. doi: 10.1086/428582. [DOI] [PubMed] [Google Scholar]
- 23.Chan ED, Laurel V, Strand MJ, et al. Treatment and outcome analysis of 205 patients with multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2004;169:1103–9. doi: 10.1164/rccm.200308-1159OC. [DOI] [PubMed] [Google Scholar]
- 24.Chiang CY, Enarson DA, Yu MC, et al. Outcome of pulmonary multidrug-resistant tuberculosis: a 6-yr follow-up study. Eur Respir J. 2006;28:980–5. doi: 10.1183/09031936.06.00125705. [DOI] [PubMed] [Google Scholar]
- 25.Cox HS, Kalon S, Allamuratova S, et al. Multidrug-resistant tuberculosis treatment outcomes in Karakalpakstan, Uzbekistan: treatment complexity and XDR-TB among treatment failures. PLoS One. 2007;5:e13901. doi: 10.1371/journal.pone.0001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeRiemer K, Garcia-Garcia L, Bobadilla-del-Valle M, et al. Does DOTS work in populations with drug-resistant tuberculosis? Lancet. 2005;365:1239–45. doi: 10.1016/S0140-6736(05)74812-1. [DOI] [PubMed] [Google Scholar]
- 27.Escudero E, Pena JM, Alvarez-Sala R, Vazquez JJ, Ortega A. Multidrug-resistant tuberculosis without HIV infection: success with individualised therapy. Int J Tuberc Lung Dis. 2006;10:409–14. [PubMed] [Google Scholar]
- 28.Geerligs WA, Van Altena R, De Lange WCM, Van Soolingen D, Van Der Werf TS. Multidrug-resistant tuberculosis: long-term treatment outcome in the Netherlands. Int J Tuberc Lung Dis. 2000;4:758–64. [PubMed] [Google Scholar]
- 29.Granich RM, Oh P, Lewis B, Porco TC, Flood J. Multidrug resistance among persons with tuberculosis in California, 1994–2003. JAMA. 2005;293:2732–9. doi: 10.1001/jama.293.22.2732. [DOI] [PubMed] [Google Scholar]
- 30.Banerjee R, Allen J, Westenhouse J, et al. Extensively drug-resistant tuberculosis in California, 1993–2006. Clin Infect Dis. 2008;47:450–7. doi: 10.1086/590009. [DOI] [PubMed] [Google Scholar]
- 31.Holtz TH, Lancaster J, Laserson KF, Wells CD, Thorpe L, Weyer K. Risk factors associated with default from multidrug-resistant tuberculosis treatment, South Africa, 1999–2001. Int J Tuberc Lung Dis. 2006;10:649–55. [PubMed] [Google Scholar]
- 32.Kim DH, Kim HJ, Park SK, et al. Treatment outcomes and long-term survival in patients with extensively drug-resistant tuberculosis. Am J Respir Crit Care Med. 2008;178:1075–82. doi: 10.1164/rccm.200801-132OC. [DOI] [PubMed] [Google Scholar]
- 33.Kim HR, Hwang SS, Kim HJ, et al. Impact of extensive drug resistance on treatment outcomes in non-HIV-infected patients with multidrug-resistant tuberculosis. Clin Infect Dis. 2007;45:1290–5. doi: 10.1086/522537. [DOI] [PubMed] [Google Scholar]
- 34.Kwon YS, Kim YH, Suh GY, et al. Treatment outcomes for HIV-uninfected patients with multidrug-resistant and extensively drug-resistant tuberculosis. Clin Infect Dis. 2008;47:496–502. doi: 10.1086/590005. [DOI] [PubMed] [Google Scholar]
- 35.Leimane V, Riekstina V, Holtz TH, et al. Clinical outcome of individualised treatment of multidrug-resistant tuberculosis in Latvia: a retrospective cohort study. Lancet. 2005;365:318–26. doi: 10.1016/S0140-6736(05)17786-1. [DOI] [PubMed] [Google Scholar]
- 36.Lockman S, Kruuner A, Binkin N, et al. Clinical outcomes of Estonian patients with primary multidrug-resistant versus drug-susceptible tuberculosis. Clin Infect Dis. 2001;32:373–80. doi: 10.1086/318489. [DOI] [PubMed] [Google Scholar]
- 37.Masjedi MR, Tabarsi P, Chitsaz E, et al. Outcome of treatment of MDR-TB patients with standardised regimens, Iran, 2002–2006. Int J Tuberc Lung Dis. 2008;12:750–5. [PubMed] [Google Scholar]
- 38.Migliori GB, Besozzi G, Girardi E, et al. Clinical and operational value of the extensively drug-resistant tuberculosis definition. Eur Respir J. 2007;30:623–6. doi: 10.1183/09031936.00077307. [DOI] [PubMed] [Google Scholar]
- 39.Mitnick C, Bayona J, Palacios E, et al. Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. N Engl J Med. 2003;348:119–28. doi: 10.1056/NEJMoa022928. [DOI] [PubMed] [Google Scholar]
- 40.Munsiff SS, Ahuja SD, Li J, Driver CR. Public-private collaboration for multidrug-resistant tuberculosis control in New York City. Int J Tuberc Lung Dis. 2006;10:639–48. [PubMed] [Google Scholar]
- 41.Narita M, Alonso P, Lauzardo M, Hollender ES, Pitchenik AE, Ashkin D. Treatment experience of multidrug-resistant tuberculosis in Florida, 1994–1997. Chest. 2001;120:343–8. doi: 10.1378/chest.120.2.343. [DOI] [PubMed] [Google Scholar]
- 42.O'Riordan P, Schwab U, Logan S, et al. Rapid molecular detection of rifampicin resistance facilitates early diagnosis and treatment of multi-drug resistant tuberculosis: case control study. PLoS One. 2008;3:0003173. doi: 10.1371/journal.pone.0003173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palmero DJ, Ambroggi M, Brea A, et al. Treatment and follow-up of HIV-negative multidrug-resistant tuberculosis patients in an infectious diseases reference hospital, Buenos Aires, Argentina. Int J Tuberc Lung Dis. 2004;8:778–84. [PubMed] [Google Scholar]
- 44.Park SK, Lee WC, Lee DH, Mitnick CD, Han L, Seung KJ. Self-administered, standardized regimens for multidrug-resistant tuberculosis in South Korea. Int J Tuberc Lung Dis. 2004;8:361–8. [PubMed] [Google Scholar]
- 45.Perez-Guzman C, Vargas MH, Martinez-Rossier LA, Torres-Cruz A, Villarreal-Velarde H. Results of a 12-month regimen for drug-resistant pulmonary tuberculosis. Int J Tuberc Lung Dis. 2002;6:1102–9. [PubMed] [Google Scholar]
- 46.Quy HT, Cobelens FG, Lan NT, Buu TN, Lambregts CS, Borgdorff MW. Treatment outcomes by drug resistance and HIV status among tuberculosis patients in Ho Chi Minh City, Vietnam. Int J Tuberc Lung Dis. 2006;10:45–51. [PubMed] [Google Scholar]
- 47.Schaaf HS, Shean K, Donald PR. Culture confirmed multidrug resistant tuberculosis: diagnostic delay, clinical features, and outcome. Arch Dis Child. 2003;88:1106–11. doi: 10.1136/adc.88.12.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shin SS, Pasechnikov AD, Gelmanova IY, et al. Treatment outcomes in an integrated civilian and prison MDR-TB treatment program in Russia. Int J Tuberc Lung Dis. 2006;10:402–8. [PubMed] [Google Scholar]
- 49.Shiraishi Y, Nakajima Y, Katsuragi N, Kurai M, Takahashi N. Resectional surgery combined with chemotherapy remains the treatment of choice for multidrug-resistant tuberculosis. J Thorac Cardiovasc Surg. 2004;128:523–8. doi: 10.1016/j.jtcvs.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 50.Tupasi TE, Gupta R, Quelapio MI, et al. Feasibility and cost-effectiveness of treating multidrug-resistant tuberculosis: a cohort study in the Philippines. PLoS Med. 2006;3:e352. doi: 10.1371/journal.pmed.0030352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uffredi ML, Truffot-Pernot C, Dautzenberg B, Renard M, Jarlier V, Robert J. An intervention programme for the management of multidrug-resistant tuberculosis in France. Int J Antimicrob Agents. 2007;29:434–9. doi: 10.1016/j.ijantimicag.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 52.Yew WW, Chan CK, Chau CH, et al. Outcomes of patients with multidrug-resistant pulmonary tuberculosis treated with ofloxacin/levofloxacin-containing regimens. Chest. 2000;117:744–51. doi: 10.1378/chest.117.3.744. [DOI] [PubMed] [Google Scholar]
- 53.Yew WW, Chan CK, Leung CC, et al. Comparative roles of levofloxacin and ofloxacin in the treatment of multidrug-resistant tuberculosis: preliminary results of a retrospective study from Hong Kong. Chest. 2003;124:1476–81. doi: 10.1378/chest.124.4.1476. [DOI] [PubMed] [Google Scholar]
- 54.Gler MT, Skripconoka V, Sanchez-Garavito E, et al. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med. 2012;366:2151–60. doi: 10.1056/NEJMoa1112433. [DOI] [PubMed] [Google Scholar]
- 55.Diacon AH, Donald PR, Pym A, et al. Randomized pilot trial of eight weeks of bedaquiline (TMC207) treatment for multidrug-resistant tuberculosis: long-term outcome, tolerability, and effect on emergence of drug resistance. Antimicrob Agents Chemother. 2012;56:3271–6. doi: 10.1128/AAC.06126-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mitnick CD, Castro KG, Harrington M, Sacks LV, Burman W. Randomized trials to optimize treatment of multidrug-resistant tuberculosis. PLoS Med. 2007;4:e292. doi: 10.1371/journal.pmed.0040292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.