We assessed the potential impact of GeneXpert MTB/RIF to make respiratory isolation decisions for inpatients undergoing evaluation for tuberculosis, compared with smear microscopy. GeneXpert had similar sensitivity and specificity for culture-positive tuberculosis and could substantially reduce time spent in isolation.
Keywords: GeneXpert, tuberculosis, infection control, molecular diagnostic techniques
Abstract
Background. Placing inpatients with presumed active pulmonary tuberculosis in respiratory isolation pending results of serial sputum acid-fast bacilli (AFB) smear microscopy is standard practice in high-income countries. However, this diagnostic strategy is slow and yields few tuberculosis diagnoses. We sought to determine if replacing microscopy with the GeneXpert MTB/RIF (Xpert) nucleic acid amplification assay could reduce testing time and usage of isolation rooms.
Methods. We prospectively followed inpatients at San Francisco General Hospital undergoing tuberculosis evaluation. We performed smear microscopy and Xpert testing on concentrated sputum, and calculated diagnostic accuracy for both strategies in reference to serial sputum mycobacterial culture. We measured turnaround time for microscopy and estimated hypothetical turnaround times for Xpert on concentrated and unconcentrated sputum. We compared median and total isolation times for microscopy to those estimated for the 2 Xpert strategies.
Results. Among 139 patients with 142 admissions, median age was 54 years (interquartile range [IQR], 43–60 years); 32 (23%) patients were female, and 42 (30%) were HIV seropositive. Serial sputum smear microscopy and a single concentrated sputum Xpert had identical sensitivity (89%; 95% confidence interval [CI], 52%–100%) and similar specificity (99% [95% CI, 96%–100%] vs 100% [95% CI, 97%–100%]). A single concentrated sputum Xpert could have saved a median of 35 hours (IQR, 24–36 hours) in unnecessary isolation compared with microscopy, and a single unconcentrated sputum Xpert, 45 hours (IQR, 35–46 hours).
Conclusions. Replacing serial sputum smear microscopy with a single sputum Xpert could eliminate most unnecessary isolation for inpatients with presumed tuberculosis, greatly benefiting patients and hospitals.
(See the Editorial Commentary by Shah on pages 1361–3.)
Transmission of tuberculosis in healthcare settings poses a risk to vulnerable patients and healthcare workers. In the 1990s, multiple nosocomial outbreaks affecting patients and healthcare workers in the United States were attributed to delayed diagnosis and inadequate respiratory protections in hospitals [1–4]. In response, the US Centers for Disease Control and Prevention issued “Guidelines for Preventing the Transmission of Mycobacterium tuberculosis in Health-Care Facilities,” recommending administrative measures for systematically screening patients for active tuberculosis, use of personal respiratory protection devices by healthcare workers, and respiratory isolation of patients presenting with signs and symptoms of tuberculosis pending serial negative microscopic examination of sputum smears for acid-fast bacilli (AFB) [5]. These principles have proven effective in reducing the frequency and duration of exposure to patients with infectious tuberculosis in emergency departments and inpatient facilities [6–15].
Unfortunately, this approach is inefficient at identifying tuberculosis patients in low-burden settings. Rapid diagnostic algorithms based on clinical symptoms are nonspecific, and sputum smear microscopy requires several days to complete and is insufficiently sensitive to detect all cases of infectious tuberculosis [16–19]. Furthermore, negative-pressure respiratory isolation facilities are costly and the need for airborne precautions can delay tests and procedures done in hospital areas without this capability. In addition, patients may experience opportunity costs such as lost income, lost housing if residing in shelters, and lost positions in substance abuse and behavioral treatment programs. They may also experience stigma [20]. These costs and delays are unnecessary for most patients, as only a small proportion of those placed in respiratory isolation actually have tuberculosis. A rapid testing strategy allowing patients who test negative to be removed from respiratory isolation more quickly would benefit patients and healthcare facilities.
In 2008, Campos et al found that performing the GenProbe M. tuberculosis direct nucleic acid amplification test (NAAT) (Hologic, San Diego, California) on a single sputum sample could differentiate inpatients with from those without infectious tuberculosis as accurately and up to 2 days sooner than serial sputum smear microscopy [21]. Unfortunately, this strategy has not been further evaluated nor widely implemented, in part because of concerns about cost, uncertain reliability, and limited impact on clinical decisions [22–28]. Recently, however, there has been renewed interest in molecular testing following US Food and Drug Administration (FDA) approval of a novel, semiautomated NAAT, GeneXpert MTB/RIF (Cepheid MTB/RIF, Sunnyvale, California; hereafter referred to as “Xpert”), for diagnosis of active pulmonary tuberculosis, based on its high sensitivity and specificity for smear-positive tuberculosis and moderate sensitivity for smear-negative tuberculosis [29, 30]. Several recent analyses suggest that use of NAATs in general, and particularly of Xpert, could be cost saving compared with serial sputum microscopy for triage of inpatients into and out of respiratory isolation during evaluation for active pulmonary tuberculosis [22, 23, 31, 32]. Here, we add to that evidence with prospective observational data on the diagnostic accuracy and predicted turnaround time for Xpert in an inpatient setting in a low-burden country. We use these data to estimate the potential impact of Xpert on respiratory isolation usage.
METHODS
Study Patients
From March 2012 to March 2013, we performed a prospective observational study to measure the hypothetical clinical and public health impact of the Xpert assay on utilization and length of stay in inpatient respiratory isolation rooms. We used a hypothetical trial design, comparing the accuracy of diagnostic strategies and estimating the potential impact of each strategy on clinical decisions and patient-important outcomes [33]. We included consecutive patients admitted to the inpatient medical service at San Francisco General Hospital for evaluation for pulmonary tuberculosis. Patients submitted at least 2 sputum samples for AFB smear microscopy from 1 of 9 hospital wards (and the emergency department) on the order of a physician. Each ward is equipped with negative-pressure respiratory isolation rooms, in accordance with hospital, state, and federal guidelines [6]. However, San Francisco General Hospital policy allows discharge from respiratory isolation after only 2, rather than the usual 3, negative examinations of concentrated sputum smears, because the incremental yield of a third exam is low [34]. To complete the microbiological evaluation, clinicians are encouraged to order a third sputum sample for mycobacterial culture and speciation.
Procedures
The hospital microbiology laboratory performed microbiologic and Xpert testing according to standard protocols. A clinical laboratory scientist performed standard N-acetyl-l-cysteine (NALC)–sodium hydroxide (NaOH) sputum concentration (final NaOH concentration of 1.5%) for smear and mycobacterial culture [35]. Smears from concentrated pellets were stained with auramine–rhodamine and examined with fluorescence microscopy. Mycobacterial culture was performed using Middlebrook 7H11 solid media (Remel, Lenexa, Kansas) and BacT/Alert MP, a modified Middlebrook 7H9 liquid media (bioMérieux, Durham, North Carolina). A clinical laboratory scientist performed Xpert on a residual concentrated sputum pellet (0.5 mL) prepared from the first sputum specimen (or the second when the first pellet had a residual volume <0.5 mL or was unavailable). Staff were not blinded to test results, but in all but 2 smear-negative, Xpert-negative, culture-negative cases, microscopy results were reported prior to Xpert. In all cases, smear and Xpert results were available before culture results. Xpert results were not reported, as the assay was not FDA approved at the time of the study. The clinical laboratory scientist performed routine quality-control procedures (Supplementary Data).
Measurements and Statistical Analysis
We collected clinical and demographic information from electronic medical records and the microbiology laboratory database. We calculated diagnostic accuracy (sensitivity, specificity, and positive and negative predictive values) with exact binomial 95% confidence intervals (CIs) for smear microscopy and Xpert in reference to a gold standard of any positive mycobacterial culture among the first 3 sputum samples collected within 7 days of admission. We defined the smear examination as positive if there was any positive result among the first 2 sputum samples collected within 7 days of admission, and negative if there were at least 2 negative results. We excluded patients who had only 1 sputum smear examined, if negative, because the final smear status of these patients could not be determined.
We recorded the time from the written order for hospital admission to laboratory-documented completion of each of 4 key processes in testing inpatients for active pulmonary tuberculosis: (1) time to receipt of the sputum sample in the laboratory, (2) time to processing the sample and preparing smears, (3) time to reporting of smear results, and (4) time to discharge from the hospital. To capture the overall amount of time that patients with presumed tuberculosis consume hospital resources while awaiting results, we calculated the median interval between admission and each of these points for all patients.
Based on these measures, we estimated the hypothetical turnaround time for Xpert, based on several assumptions. We assumed that Xpert would be performed on a single “concentrated” (ie, NALC-NaOH processed and centrifuged) sputum pellet at the next standard testing time (3 hours after sputum concentration, performed once daily in the lab at 4:00 pm; the “concentrated Xpert strategy”). We assumed Xpert results would require 3 additional hours, including the time required to prepare the specimen (35 minutes for sample processing and loading into the testing cartridge, based on previous direct observation [31]), perform Xpert testing (1 hour and 40 minutes), and report the result to the managing clinician (45 minutes). Finally, we assumed that the results of the single Xpert test would immediately be used to discontinue (if negative) or renew (if positive) isolation orders. We measured the median hypothetical duration of isolation for patients without tuberculosis from admission to Xpert result. We compared this with the observed median time required to obtain the second negative smear result, which we also assumed would immediately be used to discontinue respiratory isolation orders. We defined the difference between these times as the median time in a respiratory isolation room saved with the Xpert strategy, a measure of the individual impact. We aggregated individual time differences to estimate total time saved during the 1-year study period, a measure of the public health impact.
We conducted a sensitivity analysis assuming that Xpert testing would be performed on “direct” (ie, unconcentrated) sputum collected during initial evaluation in the emergency department (“direct Xpert strategy”). We assumed that Xpert results would be available 3 hours after the receipt of sputum in the laboratory, including time for processing, testing, and reporting, as described above. We performed all analyses using Stata software, version 11.0 (StataCorp, College Station, Texas).
Ethics Approval
The University of California, San Francisco Committee on Human Research approved the study, and waived the requirement for informed consent on the grounds that the study posed minimal risk to patients. These results have been presented in abstract form [36].
RESULTS
Study Patients
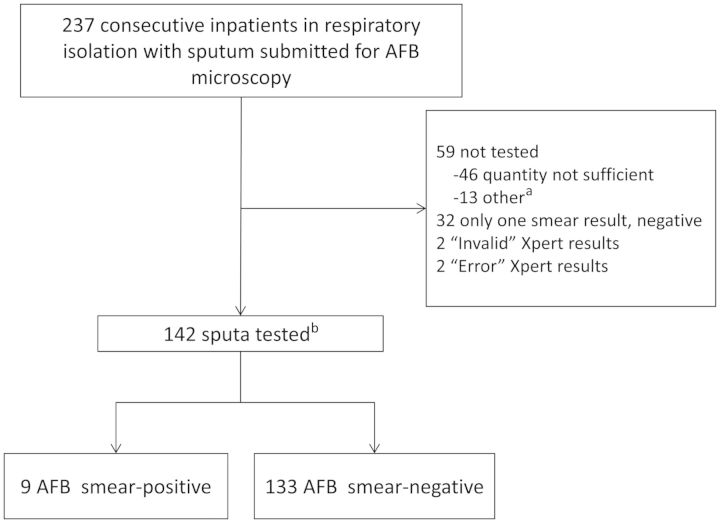
Among 237 admissions to the inpatient medical service for evaluation for pulmonary tuberculosis, there were 46 (19%) episodes in which the samples submitted were of insufficient quantity for Xpert testing after removal of the volumes required for routine smear microscopy and culture (Figure 1). Thirteen (5%) were not tested for other reasons, including delayed sample delivery and unavailability of the Xpert machine because of routine maintenance. In 2 (0.8%) instances, Xpert reported an “error”; in 2 others (0.8%), Xpert reported an “invalid” result; these were not repeated. We excluded 32 patients whose final smear status could not be determined because only 1 sputum sample was collected, which was smear negative. In total, 142 inpatient admissions were included; 3 patients provided specimens during 2 separate hospitalizations, with each episode analyzed independently.
Figure 1.
Study enrollment flow diagram. aIncludes 6 samples rejected for culture because >3 days had elapsed since collection, 4 samples that were not tested for reasons that were not documented, 2 specimens that arrived when the Xpert machine was not operating because it was undergoing routine maintenance, and 1 specimen that was not one of the first 2 samples collected. bThree patients provided specimens for the study on 2 separate admissions, with each episode analyzed independently. Abbreviations: AFB, acid-fast bacilli; Xpert, Gene Xpert MTB/RIF assay.
The median age of patients was 54 years (interquartile range [IQR], 43–60 years). Thirty-two (23%) patients were female. Forty-two (30%) were seropositive for human immunodeficiency virus, and 25 (18%) were homeless. Five (4%) died prior to hospital discharge (Table 1). Nine (6%) tested sputum AFB smear positive; 8 (89%) of these were culture positive for tuberculosis, and the other grew Mycobacterium abscessus from multiple sputum cultures. One AFB smear-negative patient had a positive tuberculosis culture, for a total of 9 (6%) tuberculosis culture–positive patients.
Table 1.
Clinical and Demographic Characteristics of Enrolled Patients
| Characteristic (n = 139)a | No. (%b) |
|---|---|
| Female sex | 32 (23) |
| Age, y, median (IQR) | 54 (43–60) |
| Race | |
| White | 45 (32) |
| Black | 35 (25) |
| Asian | 34 (24) |
| Other | 25 (18) |
| HIV seropositivec | 42 (30) |
| Sputum typed | |
| Expectorated | 54 (54) |
| Induced | 39 (39) |
| Tracheal aspirate | 7 (7) |
| Homelesse | 25 (18) |
| Died prior to discharge | 5 (4) |
| Length of stay, d, median (IQR) | 6 (4–10) |
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range.
a Three patients were admitted twice, giving 139 patients and 142 observations.
b Unless otherwise specified.
c Eight missing observations.
d Thirty-nine missing observations; data collected July 2012–March 2013.
e One missing observation.
Diagnostic Accuracy
Smear microscopy had moderately high sensitivity (8/9 [89%]; 95% CI, 52%–100%) and high negative predictive value (132/133 [99%]; 95% CI, 96%–100%; Tables 2 and 3), as well as high specificity (132/133 [99%]; 95% CI, 96%–100%) and moderately high positive predictive value (8/9 [89%]; 95% CI, 52%–100%). Xpert had equally high sensitivity for culture-positive tuberculosis (8/9 [89%]; 95% CI, 52%–100%) and negative predictive value (133/134 [99%]; 95% CI, 96%–100%), as well as perfect specificity (133/133 [100%]; 95% CI, 97%–100%) and positive predictive value (8/8 [100%]; 95% CI, 63%–100%). The one smear-negative, culture-positive patient was also Xpert negative. Seven patients with positive Xpert results tested negative for rifampin resistance mutations. One had an indeterminate rifampin resistance result; phenotypic drug susceptibility testing found the patient's isolate to be rifampin sensitive.
Table 2.
Contingency Tables Showing Diagnostic Accuracy Classifications for Culture-Positive Tuberculosis
| Sputum AFB Smear Microscopy (n = 142) |
Sputum Xpert (n = 142) |
||||
|---|---|---|---|---|---|
| Result | Culture-Positive (n = 9) | Culture-Negative (n = 133) | Result | Culture-Positive (n = 9) | Culture-Negative (n = 133) |
| Smear-positive (n = 9) | 8 | 1 | Xpert-positive (n = 8) | 8 | 0 |
| Smear-negative (n = 133) | 1 | 132 | Xpert-negative (n = 134) | 1 | 133 |
All smear-positive, culture-positive patients were also Xpert positive. The single smear-negative, culture-positive patient was also Xpert negative.
Abbreviations: AFB, acid-fast bacilli; smear, AFB smear microscopy; Xpert, GeneXpert MTB/RIF assay.
Table 3.
Diagnostic Accuracy Measures for Culture-Positive Tuberculosis (n = 142)
| Accuracy | Smear (95% CI) | Xpert (95% CI) |
|---|---|---|
| Sensitivity | 89% (52–100) | 89% (52–100) |
| Specificity | 99% (96–100) | 100% (97–100)a |
| Positive predictive value | 89% (52–100) | 100% (63–100)a |
| Negative predictive value | 99% (96–100) | 99% (96–100) |
Abbreviations: AFB, acid-fast bacilli; CI, confidence interval; smear, AFB smear microscopy; Xpert, GeneXpert MTB/RIF assay.
a One-sided 97.5% CI.
Processing Times
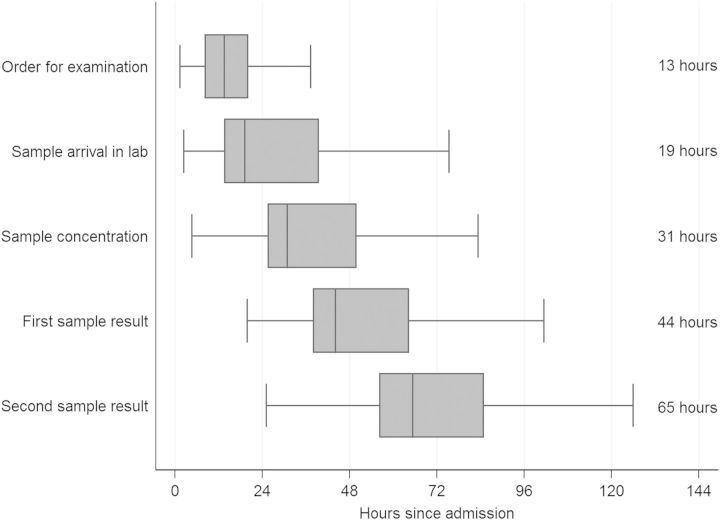
From hospital admission, it took a median of 13 hours (IQR, 8.2–20 hours) for a smear order to be placed (Figure 2), and 19 hours (IQR, 14–39 hours) for sputum to reach the laboratory. It took a median of 31 hours (IQR, 25–50 hours) from admission to the start of sputum processing, and 44 hours (IQR, 38–64 hours) until the first smear result was available. Overall, it took a median of 65 hours (IQR, 56–85 hours) from admission until a final smear diagnosis (first positive or second negative smear result) was obtained, and a median of 66 hours (IQR, 58–85 hours) to a final negative smear diagnosis (2 negative smear results).
Figure 2.
Horizontal boxplots showing distributions of completion times for each step in sputum examination by concentrated acid-fast bacilli smear microscopy for all inpatient evaluation episodes (n = 142). Horizontal boxplots show medians and interquartile ranges (IQRs), with whisker plots displaying lower and upper adjacent values (values inside 1.5 × IQR). In addition, the median values are provided as text at the right side of the plot. Three patients were admitted twice, giving 139 patients and 142 observations.
Concentrated Xpert Strategy
Hypothetically, if the laboratory protocol were to perform Xpert testing using concentrated sputum at the next available standard testing time (results available 3 hours after completion of sputum concentration), we estimated a median time from admission to Xpert result of 34 hours (IQR, 28–53 hours; Table 4). This would have reduced time spent in unnecessary respiratory isolation by a median of 35 hours (IQR, 24–36 hours). Aggregated across all 133 patients with negative tuberculosis cultures, this would have saved a total of 159 days (95% CI, 75–242 days) of unnecessary respiratory isolation during the 1-year study period.
Table 4.
Hypothetical Impact of Xpert Assay on Time to Testing Completion and Duration of Respiratory Isolation Among Patients With Negative Tuberculosis Cultures (n = 133)
| Impact | Smear Microscopy Strategya | Concentrated Xpert Strategyb | Direct Xpert Strategyc |
|---|---|---|---|
| Time to result, h, median (IQR) | 66 (58–85) | 34 (28–53) | 4.5 (2.9–10) |
| Time savings vs microscopy, h, median (IQR)d | … | 35 (24–36) | 45 (35–46) |
| Total time in isolation, days/y (95% CI) | 840 (116–1564) | 684 (0–1410) | 35 (31–39) |
| Total time savings vs control, days/y (95% CI) | … | 159 (75–242) | 258 (227–288) |
Abbreviations: AFB, acid-fast bacilli; CI, confidence interval; IQR, interquartile range; smear, AFB smear microscopy; Xpert, GeneXpert MTB/RIF assay.
a Collection of 2 sputum samples on separate days for N-acetyl-l-cysteine–sodium hydroxide (NALC-NaOH) concentrated acid-fast bacilli smear microscopy.
b Collection of 1 sputum sample for processing using NALC-NaOH concentration and testing by Xpert.
c Collection of 1 sputum sample for direct testing by Xpert.
d Savings reflect within-patient differences and are not equal to differences between medians for each strategy.
Direct Xpert Strategy
If the laboratory performed Xpert testing directly on unconcentrated sputum collected at initial evaluation in the emergency department (results available within 3 hours of arrival in the laboratory), median time from admission to Xpert result would have been 4.5 hours (IQR, 2.9–10 hours; Table 4). This would have reduced the time spent in unnecessary respiratory isolation by a median of 45 hours (IQR, 35–46 hours). Aggregated across all 133 patients with negative tuberculosis cultures, this would have saved a total of 258 days (95% CI, 227–288 days) of unnecessary respiratory isolation during the 1-year study period.
DISCUSSION
Respiratory isolation utilizes substantial hospital resources, and imposes significant opportunity costs in the form of lost hospital room availability and patient and provider time. Given the low diagnostic yield and high costs associated with isolating patients with possible tuberculosis in low-burden settings, and the negative impact that delayed triage of patients into and out of scarce respiratory isolation rooms has on the quality and efficiency of services delivered to patients, modifications of the current standard smear algorithm are needed [37]. We estimated the potential impact of Xpert for reducing time spent in unnecessary isolation based on observed local sputum processing times for smear microscopy, and conservative assumptions about sputum processing times for Xpert. Our findings suggest that using a single sputum Xpert test to guide inpatient management decisions could reduce the median duration of time in isolation by nearly 2 days, thereby saving hundreds of days per year in isolation room occupancy.
Our study confirms the previously described low yield of tuberculosis diagnoses among patients placed in respiratory isolation in low-burden settings [16–19]; only 6% of inpatients undergoing evaluation for pulmonary tuberculosis had cultures positive for tuberculosis. Furthermore, we have shown that standard sputum concentration services do not achieve rapid turnaround times, with patients waiting a median of almost 3 days before receiving a second negative smear result, which is necessary to have isolation discontinued. Using a single negative sputum Xpert result to exclude infectious tuberculosis could allow >90% of patients to be removed from isolation in <1 day. Our findings complement those of Lippincott et al, who found that the sensitivity of a 1-specimen Xpert strategy was identical to that of a serial smear microscopy strategy and could reduce median time in isolation by 46 hours [38]. We have previously shown the 1-specimen Xpert strategy to be highly cost saving in the inpatient setting [31]; time and cost savings would likely be even greater in hospitals that require 3 negative sputum smear results prior to discontinuation of respiratory isolation, rather than 2 results as at our hospital. Several other analyses have demonstrated that implementing Xpert and other NAATs for this purpose could result in considerable cost savings [22, 23, 32]. These data, combined with the well-established greater sensitivity of Xpert [30] and its benefits to patients in reduced time spent in isolation, should lead to a reevaluation of current guidelines, which recommend serial sputum testing with smear microscopy, NAAT, or combination of these assays for triage of respiratory isolation rooms [39].
We did not assess strategies using >1 Xpert test because their projected costs would be prohibitive in our public hospital setting. At a tuberculosis prevalence of 5%, the number needed to test with a second Xpert to find 1 additional smear-negative tuberculosis patient is around 200 [38]. At our institution, this would cost >$350 000 per incremental smear-negative, Xpert-positive patient identified [31]. About 90% of the incremental cost arises from the isolation room cost associated with waiting an additional 24 hours to collect a second sputum [38]; future studies might examine the feasibility, yield, and cost of doing >1 sputum Xpert in a single day.
Our study has a number of strengths. First, we provide data on the diagnostic accuracy of Xpert MTB/RIF in an inpatient population in a low-burden setting, where there have been few data to date. Furthermore, the added information on potential impact helps fill an important gap for evidence synthesis and policy making on the use of Xpert MTB/RIF for the indication of triage of inpatient respiratory isolation rooms [40, 41]. Finally, we add to the general literature on the impact of tuberculosis diagnostics in low-burden settings, which is limited [42], by providing an example of an efficient and innovative approach to generating data on potential impact using the hypothetical trial design [33, 43]. Given its simplicity, safety, and low cost, we hope that in the future more diagnostic studies will use this design to provide information on impact to enhance the value of these studies to policy makers.
Our study has several limitations. First, because of the low number of tuberculosis cases, we have incomplete information about the sensitivity of Xpert compared with smear microscopy for our patients. Nevertheless, there is abundant evidence that the sensitivity of Xpert is substantially higher than that of smear microscopy, even in low-burden settings [30]. Moreover, the low prevalence of tuberculosis in this setting means that the negative predictive value of Xpert is high; providers can therefore be confident in management decisions based on a negative Xpert result. In addition, although we tested only concentrated specimens, and no direct specimens, limited evidence suggests that Xpert performs similarly on either specimen, especially in detecting or excluding smear-positive tuberculosis [29, 44]. It should be noted that negative test results, whether obtained using Xpert or microscopy, should complement and not replace clinical decision making. If a physician still believes there is risk of tuberculosis following a negative result, a patient should be kept in isolation while additional workup is pursued. A second limitation is that we excluded a large number of specimens of insufficient quantity for Xpert testing. This is not surprising, because the study algorithm required that Xpert be performed on residual sputum. In practice, a separate specimen would likely need to be collected to have sufficient quantity for both Xpert and mycobacterial cultures. If the direct method of Xpert testing were adopted, collecting 2 specimens would be necessary because a direct sputum sample is unsuitable for culture after Xpert sample reagent is added [45]. A direct sputum specimen would ideally be obtained at the time of the initial patient evaluation in the emergency department to minimize time to decisions about isolation. A third limitation is that because we examined hypothetical rather than actual turnaround times for Xpert testing, our estimates of impact may be optimistic. Should the direct Xpert strategy be considered for adoption, it would be important to evaluate the capacity of hospital laboratories to perform Xpert on specimens as they arrive, given local staffing levels and workflow requirements. Finally, an inherent limitation of hypothetical trials is that we cannot evaluate how the results of either strategy would ultimately be used in practice by clinicians [33, 43]. Whereas our previous analysis demonstrated that our approach would be highly cost saving [31], we assumed that clinicians would use Xpert in the same manner as smear. However, it is possible that clinicians would order Xpert tests more often than smear, given its ease and rapid turnaround time, potentially leading to increased costs. Well-defined testing criteria would therefore be important to limit the number of unnecessary Xpert tests. In addition, it would be useful to see implementation studies confirming that our predicted turnaround times can be achieved in practice. However, given the high clinical and operational plausibility, potential value for clinical decision making, and cost-effectiveness [31] that we have demonstrated, it would be reasonable to introduce either the direct or the concentrated Xpert strategy in actual practice, with close evaluation and monitoring to determine its real-world feasibility, acceptability, and impact.
In conclusion, implementing Xpert testing for inpatients with possible pulmonary tuberculosis is likely a high-impact strategy for reducing respiratory isolation of patients unlikely to have tuberculosis in low-burden settings. In addition to improving the care experience for inpatients, routine use of Xpert could result in great cost savings and improved patient flow through the hospital.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors thank the patients and staff of San Francisco General Hospital, especially Mary Clancy and Phong Pham in the Clinical Microbiology Laboratory; and Cepheid for donating the GeneXpert MTB/RIF cartridges.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the American Lung Association (grant number CG-197164 to J. L. D.), the University of California, San Francisco Center for AIDS Research (to J. L. D.), and the National Institutes of Health (grant numbers K23 AI080147 to J. L. D. and K23 HL094141 to A. C.). Cepheid donated all Xpert MTB/RIF cartridges and reagents.
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Daley CL, Small PM, Schecter GF, et al. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus: an analysis using restriction-fragment-length polymorphisms. N Engl J Med. 1992;326:231–5. doi: 10.1056/NEJM199201233260404. [DOI] [PubMed] [Google Scholar]
- 2.Dooley SW, Villarino ME, Lawrence M, et al. Nosocomial transmission of tuberculosis in a hospital unit for HIV-infected patients. JAMA. 1992;267:2632–4. [PubMed] [Google Scholar]
- 3.Di Perri G, Cazzadori A, Concia E, Bassetti D. Transmission of HIV-associated tuberculosis to health-care workers. Lancet. 1992;340:1412. doi: 10.1016/0140-6736(92)92601-b. [DOI] [PubMed] [Google Scholar]
- 4.Edlin BR, Tokars JI, Grieco MH, et al. An outbreak of multidrug-resistant tuberculosis among hospitalized patients with the acquired immunodeficiency syndrome. N Engl J Med. 1992;326:1514–21. doi: 10.1056/NEJM199206043262302. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care facilities. MMWR Recomm Rep. 1994;43(RR-13):1–132. [PubMed] [Google Scholar]
- 6.Jensen PA, Lambert LA, Iademarco MF, Ridzon R. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings. MMWR Recomm Rep. 2005;54(RR-17):1–141. [PubMed] [Google Scholar]
- 7.Fridkin SK, Manangan L, Bolyard E, Jarvis WR. SHEA-CDC TB survey, part I: status of TB infection control programs at member hospitals, 1989–1992. Society for Healthcare Epidemiology of America. Infect Control Hosp Epidemiol. 1995;16:129–34. doi: 10.1086/647073. [DOI] [PubMed] [Google Scholar]
- 8.Fridkin SK, Manangan L, Bolyard E, Jarvis WR. SHEA-CDC TB survey, part II: efficacy of TB infection control programs at member hospitals, 1992.: Society for Healthcare Epidemiology of America. Infect Control Hosp Epidemiol. 1995;16:135–40. doi: 10.1086/647074. [DOI] [PubMed] [Google Scholar]
- 9.Fella P, Rivera P, Hale M, Squires K, Sepkowitz K. Dramatic decrease in tuberculin skin test conversion rate among employees at a hospital in New York City. Am J Infect Control. 1995;23:352–6. doi: 10.1016/0196-6553(95)90265-1. [DOI] [PubMed] [Google Scholar]
- 10.Louther J, Rivera P, Feldman J, Villa N, DeHovitz J, Sepkowitz KA. Risk of tuberculin conversion according to occupation among health care workers at a New York City hospital. Am J Respir Crit Care Med. 1997;156:201–5. doi: 10.1164/ajrccm.156.1.9611091. [DOI] [PubMed] [Google Scholar]
- 11.Maloney SA, Pearson ML, Gordon MT, Del Castillo R, Boyle JF, Jarvis WR. Efficacy of control measures in preventing nosocomial transmission of multidrug-resistant tuberculosis to patients and health care workers. Ann Intern Med. 1995;122:90–5. doi: 10.7326/0003-4819-122-2-199501150-00002. [DOI] [PubMed] [Google Scholar]
- 12.Stroud LA, Tokars JI, Grieco MH, et al. Evaluation of infection control measures in preventing the nosocomial transmission of multidrug-resistant Mycobacterium tuberculosis in a New York City hospital. Infect Control Hosp Epidemiol. 1995;16:141–7. doi: 10.1086/647075. [DOI] [PubMed] [Google Scholar]
- 13.Wenger PN, Otten J, Breeden A, Orfas D, Beck-Sague CM, Jarvis WR. Control of nosocomial transmission of multidrug-resistant Mycobacterium tuberculosis among healthcare workers and HIV-infected patients. Lancet. 1995;345:235–40. doi: 10.1016/s0140-6736(95)90228-7. [DOI] [PubMed] [Google Scholar]
- 14.Cook S, Maw KL, Munsiff SS, Fujiwara PI, Frieden TR. Prevalence of tuberculin skin test positivity and conversions among healthcare workers in New York City during 1994 to 2001. Infect Control Hosp Epidemiol. 2003;24:807–13. doi: 10.1086/502141. [DOI] [PubMed] [Google Scholar]
- 15.Blumberg HM, Watkins DL, Berschling JD, et al. Preventing the nosocomial transmission of tuberculosis. Ann Intern Med. 1995;122:658–63. doi: 10.7326/0003-4819-122-9-199505010-00003. [DOI] [PubMed] [Google Scholar]
- 16.Wilmer A, Bryce E, Grant J. The role of the third acid-fast bacillus smear in tuberculosis screening for infection control purposes: a controversial topic revisited. Can J Infect Dis Med Microbiol. 2011;22:e1–3. doi: 10.1155/2011/314686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tokars JI, McKinley GF, Otten J, et al. Use and efficacy of tuberculosis infection control practices at hospitals with previous outbreaks of multidrug-resistant tuberculosis. Infect Control Hosp Epidemiol. 2001;22:449–55. doi: 10.1086/501933. [DOI] [PubMed] [Google Scholar]
- 18.Scott B, Schmid M, Nettleman MD. Early identification and isolation of inpatients at high risk for tuberculosis. Arch Intern Med. 1994;154:326–30. [PubMed] [Google Scholar]
- 19.Wisnivesky JP, Henschke C, Balentine J, Willner C, Deloire AM, McGinn TG. Prospective validation of a prediction model for isolating inpatients with suspected pulmonary tuberculosis. Arch Intern Med. 2005;165:453–7. doi: 10.1001/archinte.165.4.453. [DOI] [PubMed] [Google Scholar]
- 20.Behr MA, Warren SA, Salamon H, et al. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet. 1999;353:444–9. doi: 10.1016/s0140-6736(98)03406-0. [DOI] [PubMed] [Google Scholar]
- 21.Campos M, Quartin A, Mendes E, et al. Feasibility of shortening respiratory isolation with a single sputum nucleic acid amplification test. Am J Respir Crit Care Med. 2008;178:300–5. doi: 10.1164/rccm.200803-381OC. [DOI] [PubMed] [Google Scholar]
- 22.Marks SM, Cronin W, Venkatappa T, et al. The health-system benefits and cost-effectiveness of using Mycobacterium tuberculosis direct nucleic acid amplification testing to diagnose tuberculosis disease in the United States. Clin Infect Dis. 2013;57:532–42. doi: 10.1093/cid/cit336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi HW, Miele K, Dowdy D, Shah M. Cost-effectiveness of Xpert(R) MTB/RIF for diagnosing pulmonary tuberculosis in the United States. Int J Tuberc Lung Dis. 2013;17:1328–35. doi: 10.5588/ijtld.13.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dylewski J. Nucleic acid amplification testing for the diagnosis of tuberculosis: not for all. Clin Infect Dis. 2009;49:1456–7. doi: 10.1086/644497. author reply 1457. [DOI] [PubMed] [Google Scholar]
- 25.Dorman SE. Coming-of-age of nucleic acid amplification tests for the diagnosis of tuberculosis. Clin Infect Dis. 2009;49:55–7. doi: 10.1086/599038. [DOI] [PubMed] [Google Scholar]
- 26.Guerra RL, Hooper NM, Baker JF, et al. Use of the amplified Mycobacterium tuberculosis direct test in a public health laboratory: test performance and impact on clinical care. Chest. 2007;132:946–51. doi: 10.1378/chest.06-2959. [DOI] [PubMed] [Google Scholar]
- 27.Conaty SJ, Claxton AP, Enoch DA, Hayward AC, Lipman MC, Gillespie SH. The interpretation of nucleic acid amplification tests for tuberculosis: do rapid tests change treatment decisions? J Infect. 2005;50:187–92. doi: 10.1016/j.jinf.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Dowdy DW, Maters A, Parrish N, Beyrer C, Dorman SE. Cost-effectiveness analysis of the Gen-Probe amplified Mycobacterium tuberculosis direct test as used routinely on smear-positive respiratory specimens. J Clin Microbiol. 2003;41:948–53. doi: 10.1128/JCM.41.3.948-953.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–15. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. Xpert(R) MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;1 doi: 10.1002/14651858.CD009593.pub3. CD009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Millman AJ, Dowdy DW, Miller CR, et al. Rapid molecular testing for TB to guide respiratory isolation in the U.S.: a cost-benefit analysis. PLoS One. 2013;8:e79669. doi: 10.1371/journal.pone.0079669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park PH, Holland DP, Wade A, Goswami ND, Bissette D, Stout JE. Public health costs for tuberculosis suspects in Wake County, North Carolina, United States. Int J Tuberc Lung Dis. 2013;17:759–63. doi: 10.5588/ijtld.12.0739. [DOI] [PubMed] [Google Scholar]
- 33.Lord SJ, Irwig L, Bossuyt PM. Using the principles of randomized controlled trial design to guide test evaluation. Med Decis Making. 2009;29:E1–12. doi: 10.1177/0272989X09340584. [DOI] [PubMed] [Google Scholar]
- 34.Mase SR, Ramsay A, Ng V, et al. Yield of serial sputum specimen examinations in the diagnosis of pulmonary tuberculosis: a systematic review. Int J Tuberc Lung Dis. 2007;11:485–95. [PubMed] [Google Scholar]
- 35.Kent PT, Kubica GP. Atlanta, GA: Centers for Disease Control and Prevention; Public health mycobacteriology. A guide for the level III laboratory, 1985. [Google Scholar]
- 36.Chaisson LH, Haller B, Roemer M, et al. Impact of automated MTB PCR on triage of inpatient respiratory isolation beds: a hypothetical trial. Am J Respir Crit Care Med. 2013;187:A5335. [Google Scholar]
- 37.Handel DA, Hilton JA, Ward MJ, Rabin E, Zwemer FL, Jr, Pines JM. Emergency department throughput, crowding, and financial outcomes for hospitals. Acad Emerg Med. 2010;17:840–7. doi: 10.1111/j.1553-2712.2010.00814.x. [DOI] [PubMed] [Google Scholar]
- 38.Lippincott CK, Miller MB, Popowitch EB, Hanrahan CF, Van Rie A. Xpert MTB/RIF assay shortens airborne isolation for hospitalized patients with presumptive tuberculosis in the United States. Clin Infect Dis. 2014;59:186–92. doi: 10.1093/cid/ciu212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention. Availability of an assay for detecting Mycobacterium tuberculosis, including rifampin-resistant strains, and considerations for its use — United States, 2013. MMWR Morb Mortal Wkly Rep. 2013;62:821–4. [PMC free article] [PubMed] [Google Scholar]
- 40.Nicolau I, Ling D, Tian L, Lienhardt C, Pai M. Research questions and priorities for tuberculosis: a survey of published systematic reviews and meta-analyses. PLoS One. 2012;7:e42479. doi: 10.1371/journal.pone.0042479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schunemann HJ, Oxman AD, Brozek J. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336:1106–10. doi: 10.1136/bmj.39500.677199.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brunet L, Minion J, Lienhardt C, Pai M. Mapping the landscape of tuberculosis diagnostic research. Am J Respir Crit Care Med. 2010:A2255. [Google Scholar]
- 43.Bossuyt PM, Lijmer JG, Mol BW. Randomised comparisons of medical tests: sometimes invalid, not always efficient. Lancet. 2000;356:1844–7. doi: 10.1016/S0140-6736(00)03246-3. [DOI] [PubMed] [Google Scholar]
- 44.Kurbatova EV, Kaminski DA, Erokhin VV, et al. Performance of Cepheid Xpert MTB/RIF and TB-Biochip MDR in two regions of Russia with a high prevalence of drug-resistant tuberculosis. Eur J Clin Microbiol Infect Dis. 2013;32:735–43. doi: 10.1007/s10096-012-1798-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Banada PP, Sivasubramani SK, Blakemore R, et al. Containment of bioaerosol infection risk by the Xpert MTB/RIF assay and its applicability to point-of-care settings. J Clin Microbiol. 2010;48:3551–7. doi: 10.1128/JCM.01053-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.