Abstract
Objectives.
Successful emotion regulation partly depends on our capacity to modulate emotional responses through the use of cognitive strategies. Age may affect the strategies employed most often; thus, we examined younger and older adults’ neural network connectivity when employing two different strategies: cognitive reappraisal and selective attention.
Method.
The current study used psychophysiological interaction analyses to examine functional connectivity with a region of anterior cingulate cortex (ACC) because it is a core part of an emotion regulation network showing relative structural preservation with age.
Results.
Functional connectivity between ACC and prefrontal cortex (PFC) was greater for reappraisal relative to selective attention and passive viewing conditions for both age groups. For younger adults, ACC was more strongly connected with lateral dorsolateral PFC, ventrolateral PFC, dorsomedial PFC, and posterior cingulate regions during reappraisal. For older adults, stronger connectivity during reappraisal was observed primarily in ventromedial PFC and orbitofrontal cortex.
Discussion.
Our results suggest that although young and older adults engage PFC networks during regulation, and particularly during reappraisal, the regions within these networks might differ. Additionally, these results clarify that, despite prior evidence for age-related declines in the structure and function of those regions, older adults are able to recruit ACC and PFC regions as part of coherent network during emotion regulation.
Key Words: Aging, Emotion regulation, fMRI, PPI, Reappraisal, Selective attention.
In spite of cognitive and physical declines, older adults experience positive emotional lives (Carstensen & Mikels, 2005). Although several theories have offered potential explanations for enhanced well-being in old age, one prevailing theory (Carstensen, Isaacowitz, & Charles, 1999) argues that older adults are more motivated to pursue emotion regulation goals for purposes of feeling good in the here and now. They are therefore motivated to reduce their reactivity to negative stimuli (Isaacowitz, Wadlinger, Goren, & Wilson, 2006; Kisley, Wood, & Burrows, 2007; Levenson, Carstensen, & Gottman, 1994; Mather et al., 2004) and to regulate their negative affective responses (Isaacowitz, Toner, & Neupert, 2009; Kliegel, Jäger, & Phillips, 2007; Phillips, Henry, Hosie, & Milne, 2008; Raes, Bruyneel, Loeys, Moerkerke, & De Raedt, 2013).
One of the most studied regulatory strategies is cognitive reappraisal. Cognitive reappraisal is a flexible regulatory strategy that involves reframing a situation or stimulus within the environment in order to change its meaning and thus its emotional impact. This strategy has been shown to draw on processes associated with cognitive control and executive functioning (Ochsner & Gross, 2005). Recent neuroimaging studies have identified a network of regions involved in active attempts to reappraise, including lateral and medial prefrontal cortex (PFC), as well as posterior parietal cortex (Kalisch, 2009; Ochsner et al., 2004; Morris, Leclerc, & Kensinger, in press; Silvers, Buhle, & Ochsner, in press; Winecoff, Labar, Madden, Cabeza, & Huettel, 2011).
Older age is associated with volumetric gray matter decline in a number of lateral and dorsal PFC regions implicated in cognitive reappraisal (Fjell et al., 2009; Grieve, Clark, Williams, Peduto, & Gordon, 2005; Raz et al., 2004), raising the question of whether older adults can recruit these regions as part of a functional network required for reappraisal or whether older adults may achieve less success at reappraisal (see Urry & Gross, 2010, for this debate). Few neuroimaging studies have directly examined age differences in the neural mechanisms employed when engaging in emotion regulation processes. The extant evidence suggests that (a) older adults activate lateral and medial PFC regions to a lesser extent than younger adults for purposes of hedonic regulation (i.e., downregulation of negative affect), and (b) this lesser activation may relate to older adults’ decreased regulation success when using a cognitively demanding reappraisal strategy (Opitz, Rauch, Terry, & Urry, 2012; Tucker, Feuerstein, Mende-Siedlecki, Ochsner, & Stern, 2012; Winecoff et al., 2011). Urry and Gross (2010) proposed that older adults use less cognitively demanding regulatory strategies, such as situation selection/modification or attentional deployment, rather than strategies such as reappraisal or suppression that rely heavily on cognitive control processing.
We recently examined the influence of specific emotion regulation strategies on general activation patterns in response to valenced video clips among a sample of younger and older adults (Allard & Kensinger, 2014). Participants viewed clips in a passive viewing condition and two regulation conditions (selective attention and reappraisal). Analyses focused on activation at the “peak” of the emotional film clip (the most salient portion of the clip) for negative videos. An age × regulation condition interaction revealed activity within bilateral dorsolateral PFC (DLPFC) and anterior cingulate cortex (ACC), because older adults, but not younger adults, disproportionately recruited these regions for reappraisal compared with selective attention. Thus, in contrast to previous studies showing diminished PFC engagement during older adults’ regulation attempts (Opitz et al., 2012; Winecoff et al., 2011), these results suggest older adults do rely on PFC regions when engaging reappraisal strategies. In fact, these findings may indicate that older adults rely even more heavily on cognitive control regions when implementing reappraisal compared with other strategies.
An open question from this study was why older adults disproportionately engaged PFC regions for reappraisal. We suggested that this recruitment could reflect older adults’ efforts to recruit all available processes for effectively downregulating their negative affect, especially when instructed to do so via a cognitively demanding strategy. However, this recruitment also could reflect the fact that the regions were not working as part of a coherent regulatory network, requiring greater activity to elicit even moderate changes in emotional experience. Thus, the goal of the current study was to examine the functional connectivity of emotion regulation circuitry using psychophysiological interaction (PPI) analysis to better understand age differences in the neural processes that underlie attempts at emotion regulation. Specifically, we examined functional connectivity with dorsocaudal ACC during emotion regulation. We chose this region for a few reasons. First of all, dorsocaudal ACC has been implicated as part of a core emotion regulation network (Banks, Eddy, Angstadt, Nathan, & Phan, 2007; Buhle et al., in press; Suri & Gross, 2012), and this region emerged in an initial subset of our analyses showing differential activity based on regulation strategy for younger and older adults. Furthermore, dorsocaudal ACC has been implicated as a hub of integration for both the experience and control of negative affect (Shackman et al., 2011). For instance, this region is involved in the experience/appraisal of negative emotion (Etkin, Egner, & Kalisch, 2011) while also being implicated in the selection of appropriate regulation strategies in healthy adult samples (Blair et al., 2012; Kalisch, Wiech, Critchley, & Dolan, 2006). Additionally, as compared with gray matter loss within many of the PFC regions implicated in emotion regulation, gray matter volume of the ACC might be relatively preserved in aging (Grieve et al., 2005). Therefore, the ACC seed region chosen for the present study provides a good basis for examining potential differences in connectivity within a coherent emotion regulation network as a function of age and strategy use.
Functional connectivity with the ACC was compared when younger (18–35) and older (55–85) adults (from Allard & Kensinger, 2014) were asked to use selective attention versus reappraisal strategies to reduce reactions while viewing negative (Given the large gender disparity in our sample, we controlled for gender in our initial group-level functional magnetic resonance imaging [fMRI] analyses. Controlling for gender did not affect our initial results. Thus, all analyses are collapsed across gender.) film clips. We chose to assess negative film clips during downregulation of negative affect, consistent with the focus of the majority of studies that have compared younger (see meta-analysis by Diekhof, Geier, Falkai, & Gruber, 2011) and older adults’ neural responsivity to negative inputs (particularly in the context of reappraisal: Opitz et al., 2012; Urry et al., 2006; Urry, van Reekum, Johnstone, & Davidson, 2009; van Reekum et al., 2007), as well as research involving our candidate ACC seed region in strategy selection during emotion regulation (Blair et al., 2012; Kalisch et al., 2006).
Method
Participants
Forty-five younger (25 female, range = 18–35 years; M = 23.40, SD = 4.39) and 42 older adults (25 female, range = 55–85; M = 69.21, SD = 8.62) participated in this study. Eleven younger adults and 12 older adults were excluded from subsequent analyses due to poor data quality (i.e., excessive motion artifacts: greater than ±5mm of head motion; <20 motion outliers within each scan run as determined by an artifact correction procedure) or a failure to complete all three functional scans. The final sample included 34 young (16 female; M = 23.79, SD = 4.33) and 30 older adults (20 female; M = 68.47, SD = 8.14) (Given the large gender disparity in our sample, we controlled for gender in our initial group-level fMRI analyses. Controlling for gender did not affect our initial results. Thus, all analyses are collapsed across gender.) who had no history of psychiatric, neurological, or learning disorders, nor any history or current use of psychiatric medication. Informed consent was obtained from all participants in accordance with the Boston College Institutional Review Board. All participants received $25/hr. for their participation.
Stimuli
Analyses focused on responses to a series of negative film clips. The clips were chosen by the researchers and included popular television programs, feature films, and documentaries. The film clips depicted a variety of sad/fear/disgust scenarios (e.g., a man comforting a dying dog; a woman being threatened on the phone; and a man digging through a messy toilet); (Each condition also included positive and neutral videos [six positive, six negative, and three neutral videos across all three conditions for a total of 45 videos over the entire experiment.). The connectivity analyses, however, only include the regulatory blocks with the negative videos; more information regarding the positive and neutral videos can be found in Allard and Kensinger (2014). A separate group of 14 younger and 14 older adults rated each clip on dimensions of valence and arousal. Ratings were made on a scale from 1 (highly unpleasant, non-arousing) to 9 (highly pleasant, highly arousing). Negative videos were matched on ratings of valence and arousal between younger and older adults: Negative valence (M young = 2.19, SD = 0.79; M old = 2.12, SD = 1.14; p = .76), negative arousal (M young = 7.05, SD = 0.79; M old = 7.19, SD = 0.69; p = .46). For each scan session, the condition in which a particular clip was presented (passive viewing, selective attention, or reappraisal condition) was alternated across participants. Stimulus presentation was accomplished using SR Research EyeLink 1000 software (Kanata, Ontario, Canada) during the scan session. This presentation program was used as part of a larger study where we obtained eye tracking data from participants in response to the film clips/regulation instructions; the eye tracking data are not reported here.
Procedure
Before entering the scanner, participants were instructed that they would view a series of emotional film clips during three task runs. Within each run, six negative clips were presented. Participants were told that they would be told how to view the film clips with a series of instructions presented while in the scanner. For the passive viewing condition, participants were instructed to “view the film clip naturally as if at home watching television.” When participants viewed the clips in the selective attention condition, they were instructed to “focus on areas of the screen that would help increase positive and decrease any negative feelings/reactions in response to the clips.” When participants viewed the clips in the reappraisal condition, they were provided with hedonic regulation instructions. When presented with the negative clips, a “Decrease Negative” prompt appeared; participants were instructed to achieve reappraisal by altering how they were thinking about the meaning of the clip. They were given examples utilizing both detached reappraisal (“Try to distance yourself from the events being portrayed by reminding yourself that what you are viewing is a fictional event; these are just actors portraying a role.”) and positive reappraisal (“Try to put a positive spin on the outcome of the event being portrayed. For example, if you see a clip of a car accident, try to imagine that no one was seriously injured/killed, and everyone walked away from the accident relatively unharmed.”). At the end of each scan run, participants rated their current mood on a scale from 1 (worst possible mood) to 10 (best possible mood). Additionally, participants provided self-report valence and arousal ratings for the film clips, outside the scanner, at the end of the fMRI session.
Trial Structure
A black fixation cross was presented on the center of a gray screen for 10, 12, 14, 16, or 18 s. Each video was presented for 40 s (An instruction prompt was embedded within each video for 14 younger and 13 older adults participants across all three conditions, while reappraisal instructions were embedded within videos for all participants. When assessing results from the remaining 20 younger and 17 older adult participants who did not have visual prompts within the passive viewing and selective attention videos, results remained unchanged). During the passive viewing condition, the instruction “view” was presented on the bottom of the screen 4-s poststimulus onset and remained on the screen for for 3 s. This timing for the instruction phase has been used in previous studies (see Opitz et al., 2012). The same timing and instruction placement was used for the regulation conditions: “avoid negative” for negative videos in the selective attention condition and “decrease negative” in the reappraisal condition. The intertrial interval between videos in all conditions averaged to about 14 s (jittered between 10 and 18 s for each trial). The presentation order of the three conditions was varied across participants.
Data Acquisition and Analysis
Images were acquired on a 3 Tesla Siemens Trim Trio MRI scanner using a 12-channel head coil. Stimuli were projected onto a screen located at the back of the magnet bore, and participants viewed stimuli using a mirror attached to the head coil. T1-weighted localizer images and a T1-weighted inversion recovery echo-planar image required for autoalignment were collected. Anatomic data were collected with a multiplanar rapidly acquired gradient-echo (MEMPRAGE) sequence (repetition time [TR] = 2,200ms; echo time [TEs] = 1.64, 3.5, 5.36, 7.22; flip angle = 7°; field of view [FOV] = 256×256mm; slice thickness = 1mm, no gap; 1×1 × 1mm resolution. Functional images were collected using a T2*-weighted echo-planar imaging sequence with the following parameters: TR = 2,000ms, TE = 30ms, FOV = 216mm, flip angle = 85°. Thirty interleaved near axial slices were collected in a 3×3 × 3.6mm matrix (slice thickness = 3mm with a 20% skip).
Preprocessing and data analysis were conducted in SPM8 (Wellcome Department of Cognitive Neurology, London). Preprocessing steps were as follows: slice timing correction; motion correction using a six parameter, rigid body transformation algorithm; normalization to the Montreal Neurological Institute template (resampling at 2mm isotropic voxels); spatial smoothing using a 8mm full-width half maximum isotropic Gaussian kernel.
Neural activity contrasts for this participant sample have been reported previously (Allard & Kensinger, 2014), and so here we focus on functional connectivity analyses. PPI analyses (Friston et al., 1997) were conducted to examine the functional connectivity with a seed region placed within ACC. Analyses were implemented using the generalized PPI (gPPI) toolbox (McLaren, Ries, Xu, & Johnson, 2012). We based the seed region’s location off of a previous group-level analysis revealing activity within a region of dorsocaudal ACC (Talairach coordinate: 12 13 31). The use of gPPI allowed us to use the same coordinate peak for all participants; seed region localization was the same across all participants. To perform the PPI analyses, the deconvolved time series from a 6mm radius sphere around the seed location (see Figure 1A for localization of the seed region) was extracted for each participant. The effect of the PPI interaction term was then examined (calculating the ACC time series and a block vector representing the tasks of interest: selective attention > reappraisal; reappraisal > selective attention). Separate PPI analyses were conducted for younger and older adults. We examined significant clusters exhibiting stronger PPI-related ACC coupling in one strategy condition than the other within an a priori mask covering the bilateral amygdala and PFC (created using MARINA; Walter et al., 2003); (While the present paper focused on functional connectivity within PFC regions based on this masking procedure, we have provided whole-brain PPI results for the reported contrasts in Supplementary Table 1.). This mask was used to assess connectivity within regions typically associated with emotion regulation neural circuitry located within the PFC. Significant activation patterns were determined at a threshold of p < .001 (uncorrected) with at least five contiguous voxels in the cluster.
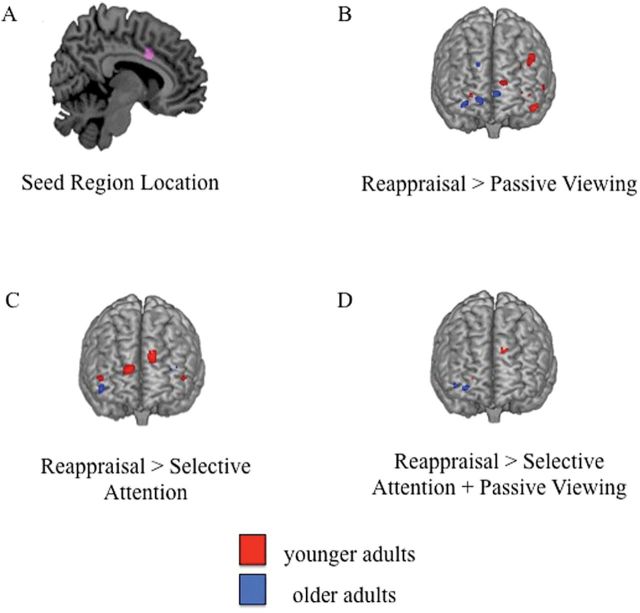
Figure 1.
(A) Seed region localization (pink) for all PPI analyses assessing young and older adults centered on a peak cluster in ACC (Talairach: 12 13 31). (B) Regions showing enhanced connectivity with the ACC seed region during reappraisal > passive viewing, (C) reappraisal > selective attention, and (D) reappraisal > passive viewing + selective attention (red: young adults; blue: older adults). PPI = psychophysiological interaction.
Results
Connectivity in Younger Adults
For younger adults, significantly stronger connectivity with the ACC was observed in the reappraisal relative to the passive viewing and selective attention conditions. When comparing reappraisal to passive viewing (Table 1, 1A; Figure 1B), functional connectivity was stronger for the reappraisal condition within several PFC regions, including left DLPFC and left ventrolateral PFC (VLPFC). When comparing reappraisal to selective attention (Table 1, 1B; Figure 1C), functional connectivity was stronger for reappraisal in regions of left and right VLPFC, left dorsomedial PFC (DMPFC), right medial PFC, and posterior cingulate. When examining the connectivity that was greater for reappraisal relative to both passive viewing and selective attention (Table 1, 1C; Figure 1D), results revealed significant connectivity within right DLPFC, right orbitofrontal cortex (OFC), and left DMPFC. At the required cluster extent and p value threshold, no regions showed enhanced functional connectivity to the ACC during the selective attention condition compared with either the passive viewing or reappraisal condition.
Table 1.
Regions Exhibiting Increased Connectivity (Revealed by PPI Analyses) With a Seed Region in ACC
| Brain region | BA | x | y | z | K (one sample) | t (one sample) | t (group) | p (group) |
|---|---|---|---|---|---|---|---|---|
| A. Reappraisal > passive viewing: young adults | ||||||||
| Right precentral gyrus | 6 | 53 | 2 | 9 | 30 | 4.24 | 2.43 | .005 |
| Left middle frontal gyrus (DLPFC) | 8/9 | −44 | 14 | 42 | 258 | 4.12 | ||
| Left inferior frontal gyrus (VLPFC) | 45 | −46 | 14 | 9 | 86 | 4.13 | 3.45 | <.001 |
| 47 | −48 | 36 | −14 | 84 | 4.52 | 2.38 | .005 | |
| Left superior frontal gyrus | 9/10 | −10 | 59 | 17 | 16 | 3.73 | ||
| B. Reappraisal > selective attention: young adults | ||||||||
| Posterior cingulate gyrus | 31 | −6 | −33 | 37 | 47 | 3.76 | 3.32 | <.001 |
| Left inferior frontal gyrus (VLPFC) | 46 | −48 | 39 | 5 | 8 | 3.52 | 2.45 | .005 |
| Right middle frontal gyrus (OFC/VLPFC) | 10 | 46 | 49 | 7 | 21 | 3.85 | 2.17 | .009 |
| Left superior frontal gyrus (DMPFC) | 9 | −12 | 54 | 40 | 77 | 4.96 | ||
| Right superior frontal gyrus | 9/10 | 14 | 59 | 17 | 104 | 4.62 | 2.72 | .002 |
| C. Reappraisal > passive viewing + selective attention: young adult | ||||||||
| Right middle frontal gyrus (DLPFC) | 8 | 22 | 25 | 32 | 5 | 3.67 | ||
| Right middle frontal gyrus (OFC) | 10 | 24 | 47 | 1 | 8 | 4.00 | 2.40 | .005 |
| Left superior frontal gyrus (DMPFC) | 9 | −12 | 52 | 36 | 15 | 3.81 | 2.57 | .003 |
| D. Reappraisal > passive viewing: older adults | ||||||||
| Right postcentral gyrus | 1 | 16 | −29 | 47 | 15 | 4.48 | 1.95 | .014 |
| Left superior frontal gyrus | 6 | −20 | 4 | 50 | 8 | 3.63 | ||
| Right superior frontal gyrus (DMPFC) | 9 | 16 | 50 | 36 | 5 | 3.64 | ||
| Right medial frontal gyrus (OFC/VMPFC) | 10 | 16 | 56 | −1 | 25 | 4.15 | 3.23 | <.001 |
| Left medial frontal gyrus (OFC/VMPFC) | 10 | −4 | 61 | 6 | 29 | 4.14 | 2.71 | .002 |
| Right middle frontal gyrus (OFC) | 10/11 | 34 | 58 | −6 | 16 | 4.14 | 1.80 | .019 |
| E. Reappraisal > selective attention: older adults | ||||||||
| Right superior frontal gyrus | 6 | 20 | 4 | 50 | 6 | 3.81 | 2.46 | .004 |
| Left inferior/middle frontal gyrus (VLPFC/DLPFC) | 45/46 | −38 | 34 | 15 | 28 | 4.35 | ||
| Right middle frontal gyrus (OFC) | 10/11 | 46 | 50 | −4 | 28 | 3.91 | 1.77 | .02 |
| F. Reappraisal > passive viewing + selective attention: older adults | ||||||||
| Right middle frontal gyrus (OFC) | 10 | 44 | 48 | −2 | 6 | 3.70 | 2.30 | .006 |
| 10 | 30 | 60 | −5 | 9 | 3.76 | 2.18 | .008 | |
Note. x, y, z labels are converted to Talairach coordinates and region labels were looked up manually using the Talairach atlas (Talaraich & Tournoux, 1988). Coordinates are listed from posterior to anterior along the y axis. No significant clusters for the selective attention > passive, selective attention > reappraisal, or selective attention > passive viewing + reappraisal PPIs. The two, rightmost columns denote results for the between-groups t-tests comparing the PPI interaction within these clusters (e.g., for sections A, B, and C, regions are showing younger > older connectivity and vice versa for sections E, D, and F). If no values are included in a row, the results for that cluster were not significant, even at a liberal threshold of p < .05 (two tailed) are listed. PPI = psychophysiological interaction.
Connectivity in Older Adults
Similar to younger adults, older adults showed significantly stronger connectivity with the ACC in the reappraisal condition compared with the passive viewing and selective attention conditions. Reappraisal was associated with stronger ACC connectivity than passive viewing in right DMPFC and left and right ventromedial PFC (VMPFC)/OFC (Table 1, 1D; Figure 1B). As compared with selective attention, reappraisal was associated with stronger ACC connectivity within left VLPFC/DLPFC and right OFC (Table 1, 1E; Figure 1C). Finally, connectivity that was greater for reappraisal than for both passive viewing and selective attention emerged in two regions of right OFC (Table 1, 1F; Figure 1D). As with younger adults, at the required cluster extent and p value threshold, no regions were more strongly connected to the ACC for selective attention than for reappraisal or passive viewing.
Comparing Connectivity in Younger and Older Adults
Since both age groups showed enhanced connectivity with the ACC during reappraisal, we first examined whether there was any overlap in the specific regions that showed this enhancement. The separate contrasts for each age group, analyzed with our standard threshold of p < .001, were inclusively masked in a conjunction analysis. This conjunction analysis revealed no regions of overlap in the two age groups for any of the condition comparisons.
We then directly compared the PPI interaction effects of younger and older adults to examine whether the effect of condition on ACC connectivity was significantly stronger in one age group than another. Thus, the PPI interaction terms were directly compared in between-group t-test analyses (e.g., PPI interaction for reappraisal > passive viewing for younger adults compared with PPI interaction for reappraisal > passive viewing for older adults, etc.). As reported in Table 1, these analyses revealed very few regions showing heightened connectivity in one age group versus the other at a threshold of p < .001.
Given the ambiguity in interpreting the conjunction and group-contrast analyses, revealing neither exact overlap nor significant age distinction, we took another approach. We increased our threshold for assessment of age differences to p < .05 (two tailed) and examined whether the regions revealed in a PPI contrast for a single age group (e.g., for reappraisal > selective attention for younger adults) also showed a significant effect of age group when using a between-groups t-test. Regions that showed significant group differences in connectivity are denoted in the last two columns of Table 1; nearly all regions showed significant group differences at this more liberal threshold.
Mood and Video Ratings
Self-reported mood ratings after each scan run were submitted to a 2 (age: young, old) × 3 (condition: passive, selective attention, reappraisal) repeated measures analysis of variance (ANOVA). A main effect of condition emerged, F(2,124) = 4.37, p < .05, ηp 2 = 0.06, revealing that mood was higher after the reappraisal condition (M = 7.33, SD = 1.16) than after the passive viewing (M = 6.89, SD = 1.54) condition (p < .01, adjusting for multiple comparisons). No significant effects of age, or an age × condition interaction, were observed.
For stimulus ratings of negative videos, separate repeated measures ANOVAs were conducted for valence and arousal ratings. In terms of valence, a significant main effect of age emerged, F(1,60) = 6.46, p < .05, ηp 2 = .10, indicating that older adults rated the videos as less negative (M = 3.28, SD = 1.07) as compared with young adults (M = 2.69, SD = 0.73). However, significant effects of condition, and the two-way interaction, were not observed. For arousal ratings, no significant effects or interactions emerged, suggesting that regardless of condition, both younger and older adults rated the negative videos as similarly arousing.
Discussion
Using PPI analysis (Friston et al., 1997; McLaren et al., 2012), we observed ACC coupling to PFC regions during emotion regulation for both younger and older adults. This basic finding emphasizes that age does not prohibit the ability to recruit ACC as part of a network in the service of emotion regulation. Furthermore, older adults were able to recruit lateral and medial PFC regions into that network, and did so more robustly when using reappraisal rather than selective attention to regulate.
It is well known that there are declines in cognitive control ability in advanced age (Hedden & Gabrieli, 2004) and structural diminution of PFC gray matter used to support the cognitive control necessary for emotion regulation (namely lateral PFC; Fjell et al., 2009; Grieve et al., 2005; Raz et al., 2004). Indeed, previous studies (Opitz et al., 2012; Winecoff et al., 2011) have shown that older adults do not activate PFC regions to the same extent as younger adults when performing cognitively demanding regulation strategies (such as reappraisal). However, we have recently shown that older adults successfully recruit lateral and medial PFC to support attempts to reappraise negative stimuli (Allard & Kensinger, 2014). In contrast to conclusions derived from previous studies observing a lack of PFC recruitment during reappraisal for older adults, our prior study might actually suggest that older adults rely more heavily on cognitive control regions to implement reappraisal relative to other strategies. However, these findings did not address why we observed disproportionate PFC engagement among older adults during reappraisal. Functional connectivity analyses provide a method for addressing two possible outcomes: (a) older adults could be relying heavily on these PFC regions to effectively downregulate negative affect through a coherent network or (b) this recruitment could reflect that these regions were being activated independently of other nodes of a regulatory network, thus requiring disproportionate activity within these regions to influence any change in affective experience.
The present results are more in line with the first suggestion: even for older adults, PFC regions appear to be functionally connected as part of a coherent network during attempts at reappraisal. Functional connectivity with dorsocaudal ACC was observed in several medial and lateral PFC regions for older adults, particularly right VMPFC and medial and lateral OFC. Functional connectivity between these regions provides interesting insights into the processes older adults might be engaging during reappraisal tasks. While dorsocaudal ACC might be involved in the selection of a reappraisal tactic, some work suggests that certain areas of VMPFC might be used to implement a selected regulation strategy (Diekhof et al., 2011; Schiller & Delgado, 2010), and lateral OFC might be involved in inhibitory control processes used to dampen prepotent affective responses (Hooker & Knight, 2006). Thus, in the context of trying to engage a reappraisal strategy, older adults might be coactivating this network that involves the selection of the reappraisal strategy in dorsocaudal ACC, the implementation of that reappraisal strategy in VMPFC, and the inhibition of emotional reactivity (specifically negative reactivity) within lateral OFC.
Similar to older adults, younger adults also demonstrated stronger connectivity in the reappraisal relative to selective attention and passive viewing conditions. However, ACC–PFC connectivity was observed in a different set of regions. In particular, enhanced connectivity was observed primarily in VLPFC, DLPFC, and DMPFC. This network of regions has been observed in previous studies assessing reappraisal processes in younger adults (Buhle et al., in press; Morris et al., in press; Ochsner et al., 2004; Silvers et al., in press). Some of the regulatory processes observed in these regions might relate to manipulation of affective appraisals in working memory (DLPFC), selection and inhibition of these appraisals (VLPFC), and updating and monitoring of regulation success (DMPFC; Buhle et al., in press). Furthermore, some of these regions have been shown to be more active in younger relative to older adult samples during reappraisal tasks. Opitz and colleagues (2012) observed diminished DMPFC and VLPFC activation in a sample of older relative to younger adults in response to reappraisal instructions when viewing negative images. Additionally, diminished VLPFC activity among older adults was related to difficulty in decreasing negative responses to those images. Another study revealed similar results: older adults showed diminished activity within a region of VLPFC, and this corresponded to increased reports of negative affect in older compared with younger adults (Winecoff et al., 2011).
The observed regions showing differentially enhanced functional connectivity with the ACC for both younger and older adults during reappraisal might help us understand how younger and older adults are supporting cognitively challenging regulation tasks. Although the regions comprising the networks in younger and older adults are somewhat similar (e.g., strategy selection and inhibition), age appeared to affect the regions that showed the largest effect of reappraisal instruction. One reason for the age-related differences in network connectivity might relate to age-related structural integrity within the regions observed. While older age is associated with volumetric gray matter decline in several PFC regions, some regions maintain relative structural preservation with age. In fact, several of the regions observed in the older adults’ functional network in the present study seem to show this preservation. In addition to maintained structural integrity in dorsocaudal ACC (Grieve et al., 2005), relative preservation is also observed within VMPFC and lateral OFC for high-functioning older adults (Fjell et al., 2009). Conversely, more accelerated gray matter decline associated with older age has been revealed among regions observed in the younger adult network. For instance structural declines in DLPFC and DMPFC (Fjell et al., 2009; Grieve et al., 2005), as well as some regions of VLPFC (Raz et al., 2004), have been examined previously. Therefore, the reappraisal network specific to each age group might reflect the PFC regions that were more structurally/functionally intact, with younger adults relying more on DLPFC, VLPFC, and DMPFC and older adults perhaps benefitting from preservation in VMPFC and OFC regions.
While age-related differences in lateral–medial PFC network connectivity emerged, this distinction was not definitive. A region of left VLPFC was both more active in the reappraisal condition than the selective attention condition in our previous study (Allard & Kensinger, 2014) and also more strongly coupled with ACC in the reappraisal condition than in the selective attention condition in the present study (BA 45/46; Talairach coordinates −38 34 11 and −38 34 15, respectively). This pattern provides additional evidence that older adults are relying on a cogent PFC network to facilitate reappraisal processes and suggests that high levels of activity in our previous study did not arise from a failure of regions working together.
Even though both age groups demonstrated more coherent PFC connectivity with the ACC in response to reappraisal instructions than either selective attention or passive viewing instructions, questions remain as to whether the deployment of such strategies were effective in downregulating negative affect for both age groups. There is some tentative evidence that engaging reappraisal strategies might have been useful for helping participants improve positive affect/diminish negative affect. Both younger and older adults displayed significant mood increases after the reappraisal scan session relative to the passive viewing session. Additionally, while older adults reported more positive ratings of negative film clips relative to younger adults, both groups showed more positive ratings of the clips after the scan session than the group of participants who provided normed ratings for the videos used; this was the case for all negative videos, not just those appearing in the reappraisal condition, perhaps reflecting a general improvement in mood after regulation attempts. While conclusions of reappraisal success derived from a subset of self-reported measures should be taken with caution, these results provide some indication that the reappraisal strategies employed were at least partially beneficial in producing desired outcomes. However, future research should determine how functional brain networks used to support regulatory processes help predict more objective assessments of regulatory success.
Overall, the present results provide evidence that older adults, similar to younger adults, show enhanced PFC recruitment when instructed to reappraise their emotional responses (e.g., Allard & Kensinger, 2014), and this PFC recruitment reflects a coherent network of activity implicated in supporting older adults’ attempts at reappraisal. However, what might account for the PFC connectivity observed in our older adult sample when previous studies (Opitz et al., 2012; Winecoff et al., 2011) do not show enhanced PFC activation during reappraisal tasks? One possibility is that activity and connectivity are simply measuring different things. Although, in some contexts, older adults show less lateral and medial PFC activation during reappraisal than younger adults, older adults may still engage these PFC regions as part of a coherent network when asked to regulate via reappraisal. Thus, under-recruitment in certain task contexts should not be taken as evidence that a network does not exist.
It is also possible that the nature of the design and stimuli made it easier for us to reveal older adults’ network coherence during reappraisal. We employed a rich stimulus set of dynamic, emotional film clips, as opposed to the static IAPS images often used in studies of emotion regulation. Not only were these films temporally protracted (40 s clips), providing older adults time to implement regulatory strategies, they also might have been a particularly evocative stimulus set that enhanced older adults’ ability or motivation to utilize this strategy. Support for this possibility, that older adults can successfully use reappraisal when motivated to do so, comes from behavioral evidence suggesting that older adults are successful at downregulating responses to negative videos, and such regulatory processes might not come at the cost of diminished cognitive control processing for older adults (Scheibe & Blanchard-Fields, 2009).
Limitations and Future Directions
Certain limitations of the current study are noteworthy. First, we utilized a paradigm that focused on hedonic emotion regulation. Thus, our results are not generalizable to other aspects of regulatory experience that can be adaptive in certain situations (e.g., utilitarian emotion regulation; see Tamir, Ford, & Gilliam, 2013). Second, we only included two of several possible emotion regulation strategies, and the present findings do not necessarily extend to other forms of regulation (i.e., suppression). Third, as in many studies of cognitive reappraisal, we did not specify whether participants should use a positive or detached reappraisal strategy—in part because these strategies are often utilized together, and it can be difficult to control which one participants use. It is possible that older adults are more adept than younger adults at using positive reappraisal and less capable of employing detached reappraisal (see Shiota & Levenson, 2009 for a discussion). Conversely, it could be that young and older adults prefer different reappraisal tactics even though they are capable of implementing either. Fourth, we did not employ additional measures to objectively assess regulatory success. Although participants did report some modulation of emotional reactions after the reappraisal condition (through self-reported mood ratings), we cannot fully infer that the functional connectivity within these regions helped participants attain successful regulation; rather, what can be concluded is that these networks are recruited during attempts to regulate emotion.
An additional limitation was that few age group differences in network connectivity were apparent at a threshold of p < .001. At a more liberal threshold (p < .05), younger adults demonstrated greater ACC–LPFC connectivity compared with older adults while older adults showed greater ACC–MPFC connectivity compared with younger adults. This pattern suggests that younger and older adults may be recruiting different PFC regions to varying degrees (perhaps based on which regions could be more efficiently recruited) as part of a functional emotion regulation network during reappraisal. Yet, given the high p value threshold used, these findings require replication and should be interpreted with caution.
Despite these limitations, the present study still emphasizes that—despite age-related structural and functional decline—older adults are capable of engaging a coherent network while performing specific cognitive emotion regulation strategies, and they can engage PFC and ACC regions together in the service of emotion regulation. Furthermore, the results reveal that reappraisal engages slightly different neural networks for younger and older adults when attempting to regulate negative affect. Our results suggest a need for future research to disentangle age differences in the neural underpinnings involved in executing a variety of cognitive emotion regulation strategies and to examine how functional connectivity between regions relates to tangible regulatory outcomes. This line of research might help to explain which strategies are going to be more or less effective for younger and older adults in achieving regulatory success and enhanced emotional well-being.
Supplementary Material
Supplementary material can be found at: http://psychsocgerontology.oxfordjournals.org/
Funding
This work was supported by the National Institutes of Mental Health (MH080833 to E. A. Kensinger); the Dana Foundation; the Searle Scholar Program; and funding from Boston College.
Supplementary Material
Acknowledgments
We would like to thank Halle Zucker, Jenny Wong, John Morris, Sarah Scott, Kelly Durham, Meghan Horn, John Ksander, and Nadia Hadarra for their assistance with participant recruitment and data collection, as well as Jaclyn Ford for helping with data analysis.
References
- Allard E. S., Kensinger E. A. (2014). Age-related differences in neural recruitment during the use of cognitive reappraisal and selective attention as emotion regulation strategies. Frontiers in Psychology, 5, 296. 10.3389/fpsyg.2014.00296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks S. J., Eddy K. T., Angstadt M., Nathan P. J., Phan K. L. (2007). Amygdala-frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience, 2, 303–312. 10.1093/scan/nsm029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair K. S., Geraci M., Smith B. W., Hollon N., DeVido J., Otero M, … Pine D. S. (2012). Reduced dorsal anterior cingulate cortical activity during emotional regulation and top-down attentional control in generalized social phobia, generalized anxiety disorder, and comorbid generalized social phobia/generalized anxiety disorder. Biological Psychiatry, 72, 476–482. 10.1016/j.biopsych.2012.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle J. T., Silvers J. A., Wager T. D., Lopez R., Onyemekwu G., Kober H, … Ochsner K. N.(2013). Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral Cortex. 10.1093/cercor/bht154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen L. L., Isaacowitz D. M., Charles S. T. (1999). Taking time seriously. A theory of socioemotional selectivity. The American Psychologist, 54, 165–181. 10.1037//0003-066X.54.3.165 [DOI] [PubMed] [Google Scholar]
- Carstensen L. L., Mikels J. A. (2005). At the intersection of emotion and cognition. Current Directions in Psychological Science, 14, 117–121. 10.1111/j.0963-7214.2005.00348.x [Google Scholar]
- Diekhof E. K., Geier K., Falkai P., Gruber O. (2011). Fear is only as deep as the mind allows: A coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. NeuroImage, 58, 275–285. 10.1016/j.neuroimage.2011.05.073 [DOI] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15, 85–93. 10.1016/j.tics.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell A. M., Walhovd K. B., Fennema-Notestine C., McEvoy L. K., Hagler D. J., Holland D, … Dale A. M. (2009). One-year brain atrophy evident in healthy aging. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29, 15223–15231. 10.1523/JNEUROSCI.3252-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K. J., Buechel C., Fink G. R., Morris J., Rolls E., Dolan R. J. (1997). Psychophysiological and modulatory interactions in neuroimaging. NeuroImage, 6, 218–229. [DOI] [PubMed] [Google Scholar]
- Grieve S. M., Clark C. R., Williams L. M., Peduto A. J., Gordon E. (2005). Preservation of limbic and paralimbic structures in aging. Human Brain Mapping, 25, 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T., Gabrieli J. D. (2004). Insights into the ageing mind: a view from cognitive neuroscience. Nature reviews. Neuroscience, 5, 87–96. [DOI] [PubMed] [Google Scholar]
- Hooker C. I., Knight R. T. (2006). Role of the orbitofrontal cortex in the inhibition of emotion. In Zald D. H., Ruach S. L. (Eds.), The orbitofrontal cortex. New York: Oxford University Press. [Google Scholar]
- Isaacowitz D. M., Toner K., Neupert S. D. (2009). Use of gaze for real-time mood regulation: Effects of age and attentional functioning. Psychology and Aging, 24, 989–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacowitz D. M., Wadlinger H. A., Goren D., Wilson H. R. (2006). Selective preference in visual fixation away from negative images in old age? An eye-tracking study. Psychology and Aging, 21, 40–48. 10.1037/a0017706 [DOI] [PubMed] [Google Scholar]
- Kalisch R. (2009). The functional neuroanatomy of reappraisal: Time matters. Neuroscience and Biobehavioral Reviews, 33, 1215–1226. 10.1016/j.neubiorev.2009.06.003 [DOI] [PubMed] [Google Scholar]
- Kalisch R., Wiech K., Critchley H. D., Dolan R. J. (2006). Levels of appraisal: A medial prefrontal role in high-level appraisal of emotional material. NeuroImage, 30, 1458–1466. [DOI] [PubMed] [Google Scholar]
- Kisley M. A., Wood S., Burrows C. L. (2007). Looking at the sunny side of life: Age-related change in an event-related potential measure of the negativity bias. Psychological Science, 18, 838–843. [DOI] [PubMed] [Google Scholar]
- Kliegel M., Jäger T., Phillips L. H. (2007). Emotional development across adulthood: Differential age-related emotional reactivity and emotion regulation in a negative mood induction procedure. International Journal of Aging & Human Development, 64, 217–244. [DOI] [PubMed] [Google Scholar]
- Levenson R. W., Carstensen L. L., Gottman J. M. (1994). The influence of age and gender on affect, physiology, and their interrelations: A study of long-term marriages. Journal of Personality and Social Psychology, 67, 56–68. [DOI] [PubMed] [Google Scholar]
- Mather M., Canli T., English T., Whitfield S., Wais P., Ochsner K, … Carstensen L. L. (2004). Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychological Science, 15, 259–263. [DOI] [PubMed] [Google Scholar]
- McLaren D. G., Ries M. L., Xu G., Johnson S. C. (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. NeuroImage, 61, 1277–1286. 10.1016/j.neuroimage.2012.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. A., Leclerc C. M., Kensinger E. A.(in press). Effects of valence and divided attention on cognitive reappraisal processes. Social, Cognitive, and Affective Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K. N., Gross J. J. (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9, 242–249. [DOI] [PubMed] [Google Scholar]
- Ochsner K. N., Ray R. D., Cooper J. C., Robertson E. R., Chopra S., Gabrieli J. D., Gross J. J. (2004). For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage, 23, 483–499. [DOI] [PubMed] [Google Scholar]
- Opitz P. C., Rauch L. C., Terry D. P., Urry H. L. (2012). Prefrontal mediation of age differences in cognitive reappraisal. Neurobiology of Aging, 33, 645–655. 10.1016/j.neurobiolaging.2010.06.004 [DOI] [PubMed] [Google Scholar]
- Phillips L. H., Henry J. D., Hosie J. A., Milne A. B. (2008). Effective regulation of the experience and expression of negative affect in old age. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 63, P138–P145. [DOI] [PubMed] [Google Scholar]
- Raes A. K., Bruyneel L., Loeys T., Moerkerke B., De Raedt R. (2013). Mindful attention and awareness mediate the association between age and negative affect. The Journals of Gerontology B: Psychological and Social Sciences. 10.1093/geronb/gbt074 [DOI] [PubMed] [Google Scholar]
- Raz N., Gunning-Dixon F., Head D., Rodrigue K. M., Williamson A., Acker J. D. (2004). Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: Replicability of regional differences in volume. Neurobiology of Aging, 25, 377–396. [DOI] [PubMed] [Google Scholar]
- Scheibe S., Blanchard-Fields F. (2009). Effects of regulating emotions on cognitive performance: What is costly for young adults is not so costly for older adults. Psychology and Aging, 24, 217–223. 10.1037/a0013807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D., Delgado M. R. (2010). Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends in Cognitive Sciences, 14, 268–276. 10.1016/j.tics.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman A. J., Salomons T. V., Slagter H. A., Fox A. S., Winter J. J., Davidson R. J. (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews. Neuroscience, 12, 154–167. 10.1038/nrn2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers J. A., Buhle J.T., Ochsner K. N.(in press). The neuroscience of emotion regulation: Basic mechanisms and their role in development, aging, and psychopathology. In Ochsner K. N., Kosslyn S. M. (Eds.), The handbook of cognitive neuroscience, Vol. 1 New York: Oxford University Press. [Google Scholar]
- Shiota M. N., Levenson R. W. (2009). Effects of aging on experimentally instructed detached reappraisal, positive reappraisal, and emotional behavior suppression. Psychology and Aging, 24, 890–900. 10.1037/a0017896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri G., Gross J. J. (2012). Emotion regulation and successful aging. Trends in Cognitive Sciences, 16, 409–410. 10.1016/j.tics.2012.06.007 [DOI] [PubMed] [Google Scholar]
- Talaraich J., Tournoux P. (1988). Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers, Inc. [Google Scholar]
- Tamir M., Ford B. Q., Gilliam M. (2013). Evidence for utilitarian motives in emotion regulation. Cognition & emotion, 27, 483–491. 10.1080/02699931.2012.715079 [DOI] [PubMed] [Google Scholar]
- Tucker A. M., Feuerstein R., Mende-Siedlecki P., Ochsner K. N., Stern Y. (2012). Double dissociation: Circadian off-peak times increase emotional reactivity; aging impairs emotion regulation via reappraisal. Emotion (Washington, D.C.), 12, 869–874. 10.1037/a0028207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry H. L., Gross J. J. (2010). Emotion regulation in older age. Current Directions in Psychological Science, 19, 352–357. 10.1177/0963721410388395 [Google Scholar]
- Urry H. L., van Reekum C. M., Johnstone T., Davidson R. J. (2009). Individual differences in some (but not all) medial prefrontal regions reflect cognitive demand while regulating unpleasant emotion. NeuroImage, 47, 852–863. 10.1016/j.neuroimage.2009.05.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry H. L., van Reekum C. M., Johnstone T., Kalin N. H., Thurow M. E., Schaefer H. S, … Davidson R. J. (2006). Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 26, 4415–4425. 10.1523/JNEUROSCI.3215-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Reekum C. M., Johnstone T., Urry H. L., Thurow M. E., Schaefer H. S., Alexander A. L., Davidson R. J. (2007). Gaze fixations predict brain activation during the voluntary regulation of picture-induced negative affect. NeuroImage, 36, 1041–1055. 10.1016/j.neuroimage.2007.03.052 [DOI] [PubMed] [Google Scholar]
- Walter B., Blecker C., Kirsch P., Sammer G., Schienle A., Stark R., Vaitl D. (2003). MARINA: An easy to use tool for the creation of masks for region of interest analyses [abstract]. Presented at the 9th International Conference on Functional Mapping of the Human Brain, June 19–22, 2003, New York, NY Available on CD-Rom in NeuroImage, 19(2). [Google Scholar]
- Winecoff A., Labar K. S., Madden D. J., Cabeza R., Huettel S. A. (2011). Cognitive and neural contributors to emotion regulation in aging. Social Cognitive and Affective Neuroscience, 6, 165–176. 10.1093/scan/nsq030 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.