Abstract
HIV-associated neurocognitive disorders (HAND) are associated with deficits in prospective memory (PM; “remembering to remember”), conferring risk of daily functioning declines. However, self-perceptions of PM functioning are not reliably associated with PM performance in HIV, suggesting a possible deficit in awareness of PM abilities (meta-PM). Our study examined meta-PM in HAND and its correlates using self-predictions of laboratory-based PM performance. Performance-based PM abilities, self-reported prediction of PM performance, and PM complaints in everyday life were assessed in 49 individuals with HAND, 93 HIV+ without HAND (HIV+ noHAND), and 121 seronegative adults (HIV−). After controlling for group-level differences, HAND was associated with a greater number of PM symptoms in everyday life and worse PM performance when compared with both HIV+ noHAND and HIV− samples. Although HAND individuals reported somewhat lower predictions regarding their laboratory PM performance relative to the other study groups, they nevertheless exhibited significantly greater inaccurate overconfidence in time-based PM abilities. Within the HAND group, overconfidence in time-based meta-PM was associated with executive dysfunction and antiretroviral (ARV) nonadherence. HAND individuals evidenced a moderate deficit in awareness of PM functioning characterized by overconfidence in time-based PM abilities. Overconfidence in PM may result in absence of compensatory strategy use, and lead to increased errors in daily functioning (e.g., ARV nonadherence).
Keywords: Executive functions, Metacognition, Everyday functioning
Introduction
Prospective memory (PM), or “remembering to remember,” is a complex, unique cognitive skill that draws upon both retrospective memory and executive functions (Gupta, Paul Woods, Weber, Dawson, & Grant, 2010; Woods et al., 2006). For instance, in order to carry out a PM task, an individual must successfully complete a sequence of steps that integrate multiple cognitive demands (McDaniel & Einstein, 2000): form an intention (e.g., buy milk at the corner store on the way home from work), maintain the intention across time in the face of ongoing activities (e.g., driving home from work), monitor and detect the cue when appropriate (e.g., nearing the grocery store), and, finally, retrieve and carry out the intended task (e.g., buy milk). PM tasks are commonly characterized by event- or time-based cues, with the latter relying more heavily on internally based self-monitoring abilities (McDaniel & Einstein, 2000). For example, common time-based PM tasks may include remembering an appointment or when to take one's medication, while event-based PM tasks could be mailing a letter or picking up the dry cleaning when passing by the establishment. Not surprisingly, PM is a cognitive skill of strong ecological relevance, with impairment being linked to range of everyday functioning declines, including healthcare non-compliance (Woods et al., 2009; Zogg et al., 2010), unemployment (Woods, Weber, Weisz, Twamley, & Grant, 2011), dependence in activities of daily living (Pirogovsky, Woods, Vincent Filoteo, & Gilbert, 2012; Woods, Weinborn, Velnoweth, Rooney, & Bucks, 2012), and even lower quality of life (Doyle et al., 2013). Additionally, PM deficits are commonly observed in a variety of neurological populations, from mild cognitive impairment (Karantzoulis, Troyer, & Rich, 2009) and schizophrenia (Woods, Twamley, Dawson, Narvaez, & Jeste, 2007) to Parkinson's disease (Raskin et al., 2011) and HIV infection (Carey et al., 2006). Therefore, exploration of PM, characterizing its mechanisms, and identifying factors that may moderate PM abilities may have important implications for treatment and everyday outcomes across a range of clinical populations.
Like other complex cognitive skills, awareness of and ability to predict one's PM capacity (i.e., metacognition of PM, “meta-PM”) is also commonly inaccurate. For example, healthy adults are only moderately accurate in their everyday PM complaints or appraisals of PM abilities before performing a task (i.e., predictions; Devolder, Brigham, & Pressley, 1990). Nonetheless, among neurological populations, meta-PM evaluations are even more discrepant, with severity of meta-PM inaccuracy increasing with severity of PM impairment (Roche, Fleming, & Shum, 2002; Smith, Souchay, & Moulin, 2011). For example, individuals with traumatic brain injury tend to rate their PM abilities as comparable to their healthy peers, yet demonstrate impaired PM both on performance-based tasks (Knight, Harnett, & Titov, 2005) and when rated by a significant other (Roche, Fleming, & Shum, 2002). The strong relationship between PM and meta-PM abilities suggests that there may be some common neural pathways on which these abilities travel that, when disrupted, confer a greater risk for impairment in both abilities. Indeed, both metacognition (Stuss, 2011) and PM (Burgess, Scott, & Frith, 2001, 2003) rely heavily upon rostro-frontal (i.e., Brodmann's Area 10) networks, particularly for the strategic aspects of PM (e.g., monitoring; Oksanen, Waldum, McDaniel, & Braver, 2014; Stuss, 2011). Of note, although it has not been examined in the context of meta-PM, global metacognitive and metamemory inaccuracies are also associated with increased errors in daily life (Hart, Giovannetti, Montgomery, & Schwartz, 1998; Ownsworth, Quinn, Fleming, Kendall, Shum, 2010). For example, an individual who is not aware of a neurocognitive deficit (e.g., impaired memory) may engage in a task that he does not have the capacity to complete (e.g., medication taking) and does not employ the use of compensatory aids (e.g., alarm) due to his lack of awareness, resulting in an error (e.g., forgetting to take medication). Thus, neurologically compromised individuals who demonstrate PM impairments may also be at a greater risk for impaired metacognition, which may then result in even greater anosognostic-related errors in everyday life.
In the current study, we chose to examine these relationships in the context of HIV disease because HIV-infected individuals are at risk for both time- and event-based PM impairment and report increased PM symptoms in daily life (Carey et al., 2006; Woods et al., 2007). Of clinical relevance, these HIV-associated PM deficits demonstrate moderate-to-large associations with poorer everyday functioning outcomes (e.g., antiretroviral, ARV, nonadherence; Contardo, Black, Beauvais, Dieckhaus, & Rosen, 2009; Woods et al., 2009). Whether awareness of PM functioning contributes to the adverse impact of HIV-associated PM deficits on daily activities is not known. One study to date has specifically explored the relationship between perceived and objective PM in the context of HIV disease. Woods and colleagues (2007) demonstrated that self-reported daily PM symptoms were not associated with performances on a laboratory-based PM task, but instead were most strongly linked to affective dysregulation (i.e., anxiety, depression, and fatigue). Such findings are commensurate with prior reports of a discrepancy between retrospective memory complaints and performance in a substantial proportion of HIV-infected individuals (e.g., up to 50% discrepant; Hinkin et al., 1996; Rourke, Halman, & Bassel, 1999).
Of note, the abovementioned studies may be limited by the method in which “metacognition” was operationalized (i.e., self-reported cognitive complaints in daily life vs. performance on a neuropsychological test). Cognitive complaints purport to measure individuals' difficulties in everyday life (e.g., endorsed “forgetting to take medication”), which may reflect beliefs in cognitive capacity or may simply reflect the non-cognitive complexities of daily life (e.g., ran out of money for medications). Therefore, in order to better characterize and explore insight into specific neurocognitive abilities, it may be informative to use prediction paradigms in which an individual is directly asked to assess his ability to complete a given task in the laboratory. Chiao and colleagues (2013) were the first to employ a prediction paradigm in order to study metacognition in HIV and, consistent with the aforementioned studies, found that greater HIV-associated neurocognitive impairment was associated with greater global metacognitive inaccuracies. Overall, it appears that both PM and metacognitive processes may be impaired in HIV infection, but there is more work needed in the assessment and characterization of a possible meta-PM deficit and how this may be associated with real-world functioning in this population. Therefore, in the current study, we aimed to (1) determine the possible presence and nature (e.g., under- or overconfidence) of a meta-PM deficit in individuals with HIV+ when compared with seronegatives; (2) expand the metacognitive literature in HIV infection by employing a prediction based paradigm (i.e., “How well do you think you will perform on this next task?”) instead of cognitive complaints; and (3) explore the cognitive and clinical correlates of such meta-PM abilities.
Methods
Participants
The current study was approved by the University of California, San Diego's Human Research Protections Program and all participants provided written consent prior to participation. A total of 253 participants who completed an NIH-funded R01 study examining PM in HIV were included in the current study. Participants were classified into one of three groups: (1) HIV seronegative comparison participants (HIV−, n = 150), (2) HIV+ participants who did not evidence an HIV-associated neurocognitive disorder (HIV+ no HAND, n = 131), and (3) HIV+ participants who met criteria for an HIV-associated neurocognitive disorder (HAND, n = 72). HAND diagnoses were based on results of a comprehensive neuropsychological evaluation (detailed below) consistent with the current Frascati criteria (Antinori et al., 2007). See Table 1 for study cohort characteristics.
Table 1.
Participant demographic and clinical characteristics (mean, SD; %, n; median, interquartile range)
| HIV-(n = 150) | HIV+ no HAND (n = 131) | HAND (n = 72) | p-value | |
|---|---|---|---|---|
| Age (years) | 43.4 (15.5) | 45.2 (13.2) | 46.3 (13.9) | .32 |
| Education (years) | 13.9 (2.3) | 13.7 (2.3) | 13.3 (2.6) | .23 |
| Gender (% M) | 70.0% | 84.0% | 87.5% | .002 |
| Ethnicity (% Caucasian) | 55.3% | 59.5% | 50.0% | .81 |
| LT affective disordera (%) | 37.3% | 69.5% | 56.1% | <.001 |
| LT substance use disorder (%) | 60.0% | 78.6% | 65.3% | .003 |
| AIDS (%) | – | 50.1% | 56.9% | .40 |
| Nadir CD4 | – | 204 (100, 332) | 162 (50, 328.8) | .11 |
| Current CD4 | – | 558 (384, 797.5) | 517 (376.8, 732) | .36 |
| Plasma VL Detectable | – | 19.7% (25) | 24.6% (17) | .42 |
| CSF VL detectable | – | 21.4% (21) | 12.7% (7) | .18 |
| % On antiretrovirals | – | 87.0% | 87.5% | .92 |
| Duration of infection (years) | – | 13.1 (4.0, 20.4) | 13.2 (3.8, 20.6) | .84 |
Notes: HAND, HIV-associated neurocognitive disorder; LT, lifetime; CSF, cerebral spinal fluid; VL, viral load.
aMet DSM-IV-TR criteria for lifetime major depressive disorder or generalized anxiety disorder.
Individuals were excluded from study participation if they met criteria for any of the following: a positive urine toxicology result for illicit substances (excluding marijuana) or a Breathalyzer test positive for alcohol on the day of testing; current drug or alcohol dependence within the past 30 days as determined by the Composite International Diagnostic Interview (CIDI version 2.1; World Health Organization, 1998) using DSM-IV-TR criteria (American Psychological Association, 1994), had a history of schizophrenia, other psychotic disorder or significant neurological disease (e.g., seizure, traumatic brain injury with loss of consciousness >15 min), or scored a verbal IQ estimate <70 on the Wechsler Test of Adult Reading (WTAR; Psychological Corporation, 2001). Of note, the R01 study from which participants were drawn utilized a discrepant age classification approach, such that no participants between the ages of 40 and 50 were enrolled.
Objective Prospective Memory Assessment
The research version (Woods et al., 2008) of the Memory for Intentions Screening Test (MIST; Raskin, Buckheit, & Sherrod, 2010) was administered to measure objective PM capacities. The MIST comprises eight PM tasks, which are counterbalanced across delay interval (2 or 15 min), response type (verbal or physical action), and cue type (time or event based). Word search puzzles are provided as a distractor task in order to prevent overt rehearsal of the instructed intentions. Our analyses focused specifically on the time- (e.g., “In 15 min, tell me to check my mail”) and event-based (e.g., “When I show you the postcard, self-address it”) subscales of the MIST, which are scored on a range of 0–8 (higher scores reflect better performance). In order to analyze MIST scores on the same metric as our other assessments (see below), the time- and event-based subscales were transformed into sample-based z-scores (higher scores indicating better performance).
Prospective Memory Performance Prediction
Prior to completing the MIST, participants rated the likelihood that they would be able to successfully complete the time- and event-based PM tasks on a Likert-type scale (1 = “Very Likely” to 4 = “Very Unlikely”). Values for the time- and event-based PM predictions were also converted into sample-based z-scores in which higher values represented higher self-rated likelihood of completing the task.
Metacognition of Prospective Memory Variable
A meta-prospective memory (meta-PM) variable was created for both time- and event-based abilities by simply subtracting an individual's MIST time- or event-based performance z-score from his/her predicted time- or event-based z-score, respectively. In this manner, time- and event-based meta-PM values closest to zero represented more accurate predictions, lower meta-PM values indicated inaccurate underconfidence, and higher values represented inaccurate overconfidence in predictions.
Prospective Memory Complaints in Daily Life
All participants completed the Prospective and Retrospective Memory Questionnaire (PRMQ; Smith, Della Sala, Logie, & Maylor, 2000), which is a 16-item self-report inventory that measures the frequency of perceived memory difficulties that occur in everyday life (e.g., “How often do you decide to do something in a few minutes' time and then forget to do it?”) using a 5-point Likert-type scale (1 = “never” to 5 = “very often”). For the current study, only the PM subscale was evaluated (8 items), with total scores ranging from 8 to 40. For the purposes of our analyses, raw scores were transformed into population-based z-scores in which higher scores denoted elevated symptoms (i.e., lower functioning).
Other Neurocognitive Assessment
A comprehensive neuropsychological battery, designed to capture the primary domains affected by HIV infection, was administered to all participants. The following neuropsychological domains were assessed: (1) Executive functions: action fluency test (Woods et al., 2005), total rule violations from the Tower of London – Drexel University (TOL-DX; Culbertson & Zillmer, 2001) and total time on Trail Making Test (TMT) Part B (Reitan & Wolfson, 1985); (2) Learning: total unit score from the Logical Memory I (LM-I) subtest of the Wechsler Memory Scale – Third Edition (WMS-III; Psychological Corporation, 1997) and trials 1–5 total from the California Verbal Learning Test – Second Edition (CVLT-2; Delis, Kramer, Kaplan & Ober, 2001); (3) Delayed recall: total unit score from the LM-II subtest and the long delay free recall total from the CVLT-2; (4) Attention: total correct trials on the Digit Span subtest of the Wechsler Adult Intelligence Scale—Third Edition (WAIS-III; Psychological Corporation, 1997) and Trial 1 total from the CVLT-2; and (5) Processing speed: total execution time on the TOL-DX, total time on TMT Part A, and total correct on the Digit Symbol subtest of the WAIS-III. Classification of HAND was determined by converting raw test scores to demographically adjusted T-scores correcting for age, education, gender, and ethnicity, where appropriate. T-scores were then transformed into a global clinical rating (see Woods et al., 2004), ranging from 0 (above average) to 9 (severely impaired). Global clinical ratings >4 indicated a diagnosis of HAND.
Uncorrected sample-based NP z-scores were used for in all correlational analyses in order to capture an individual's absolute level of neurocognitive performance (i.e., not relative to one's age, education, etc.). Given that our meta-PM variable was based on uncorrected PM performance and prediction values (i.e., “How do you think you will perform?” vs. “How do you think you will perform compared with your peers?”), uncorrected neurocognitive scores are most consistent with these values. The NP domain-based z-scores outlined in Table 2 were created by simply averaging each of the individual test z-scores within a given domain.
Table 2.
Executive dysfunction is associated with greater inaccurate overconfidence in time-based PM among individuals with HAND (n = 72)
| Uncorrected domain and individual test scores (z-scores) | Time-based meta-PM (ρ) | p-value |
|---|---|---|
| Mean NP z-score | −.27 | .02 |
| Executive domain | −.26 | .03 |
| Action fluency | −.32 | .006 |
| Tower of London—rule violations | −.24 | .05 |
| TMT, Part B | −.11 | .35 |
| Learning domain | −.21 | .08 |
| WMS-III LM-I | −.20 | .09 |
| CVLT-2 Trials 1–5 | −.13 | .27 |
| Memory domain deficit score | −.15 | .22 |
| WMS-III LM-II | −.12 | .32 |
| CVLT-2 Long Delay Free Recall | −.09 | .44 |
| Attention domain deficit score | −.05 | .70 |
| Digit span | −.04 | .72 |
| CVLT-2 Trial 1 | −.04 | .72 |
| Processing speed domain deficit score | −.18 | .13 |
| ToL execution time | −.19 | .11 |
| TMT, Part A | −.12 | .33 |
| Digit symbol | −.03 | .78 |
Antiretroviral Adherence
Antiretroviral (ARV) adherence was monitored for all HIV+ participants via the Medication Event Monitoring System (MEMS; Aprex Corporation, Union City, CA) across a 30-day period. The MEMS caps provide a time and date stamp each time the pill bottle is opened, serving as a proxy for medication-taking behavior. Overall, adherence was derived as: [(no. of bottle openings)/(no. of prescribed doses)] × 100%. HIV+ participants were then dichotomized as “nonadherent” if overall ARV adherence was <90% (Woods et al., 2009).
Psychiatric Assessment
The computerized CIDI was used to determine mood disorders and substance use diagnoses, which follow DSM-IV-TR criteria. Participants were classified as having a lifetime affective disorder if they met current and/or lifetime DSM-IV-TR criteria for major depressive disorder (MDD) or generalized anxiety disorder (GAD).
Additionally, all participants completed the profile of mood states (POMS; McNair, Lorr, & Droppleman, 1981). The POMS is a 65-item self-report questionnaire assessing current mood states in which participants rate affectively laden adjectives and brief phrases on a Likert-type scale ranging from 0 (“not at all”) to 4 (“extremely”). The Total Mood Disturbance score was used in the current analyses, which incorporates all items with higher values reflecting greater mood distress.
Results
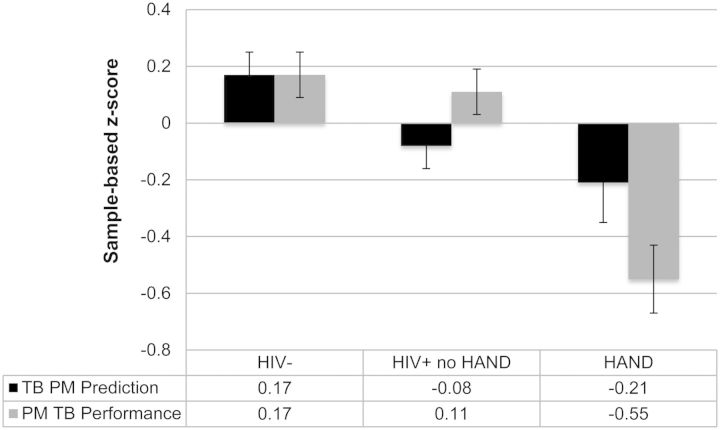
Given that gender, lifetime affective disorders (i.e., MDD and GAD), and lifetime substance use disorders differed across the groups, all between-group analyses included these factors as covariates. HAND individuals demonstrated worse time- (βs = 0.68–0.70, ps < .001) and event-based (βs = 0.38–0.54, ps < .02) objective PM performance, lower predictions of their time- (β = 0.38, p = .01) but not event-based (βs = 0.19–0.24, ps > .10) PM abilities, and greater time- (β = −1.7, p < .001) and event-based (βs = −1.17, ps < .001) PM symptoms in daily life compared with both HIV+ no HAND and seronegative comparison groups (see Fig. 1).
Fig. 1.
Prediction and subsequent performance on a TB PM task across study groups. HAND, HIV-associated neurocognitive disorder.
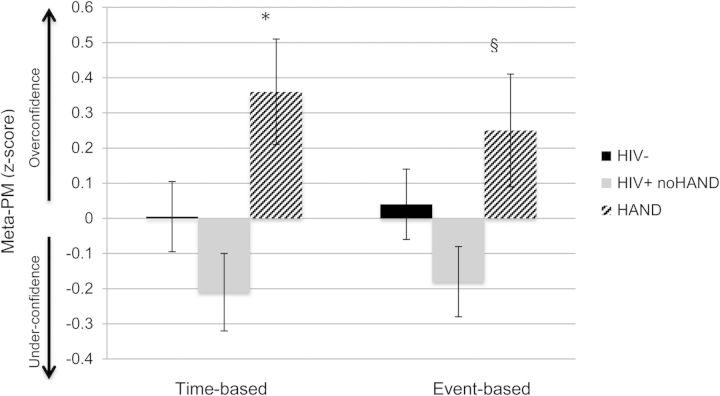
A multivariable linear regression model controlling for gender, and lifetime affective and substance use disorders showed a HAND group effect on time-based meta-PM (i.e., time-based PM prediction minus performance; Model: F(5, 344) = 2.3, p = .04). Specifically, individuals with HAND demonstrated greater inaccurate overconfidence in time-based PM abilities compared to HIV+ individuals no HAND (t = −2.9, p = .004), and showed a trend when compared with HIV− participants (p = .09; see Fig. 2). Additionally, HAND individuals tended to demonstrate greater overconfidence in event-based meta-PM abilities compared with HIV+ no HAND, though the effect did not reach statistical significance (β = −0.37, p = .056).
Fig. 2.
Individuals with HAND are inaccurately overconfident in time-based PM abilities. *HIV+ noHAND < HAND (p < .05), and HIV− <HAND (p = .09); §HIV+ noHAND < HAND (p = .056).
In order to categorize participants as over- or underconfident, we employed cut-points for the time-based meta-PM variable at ±1 SD of the sample-based z-score (i.e., >1 SD = “overconfidence” and <−1 SD = “underconfidence”). For those participants who fell within one SD on the meta-PM variable (i.e., “accurate” meta-PM), objective PM impairment was defined as a raw MIST score <5.5, which represents the −1 SD value for healthy participants on the MIST (Woods et al., 2008), in order to further characterize the “accurate” individuals. In this manner, four meta-PM groups emerged: (1) “accurate-normal:” meta-PM z-score within 1 SD of the mean and MIST performance within 1 SD; (2) “accurate-impaired:” meta-PM z-score within 1 SD of the mean and MIST performance below −1 SD; (3) “overconfident:” meta-PM z-score >1 SD of the mean; and (4) “underconfident:” meta-PM z-score <−1 SD of the mean. As illustrated in Fig. 3, as a whole, 43.6% of HAND individuals demonstrated inaccurate time-based meta-PM, with 35.2% being overconfident in their time-based PM abilities.
Fig. 3.
Prevalence and type of time-based meta-PM inaccuracies across study groups.
Next, we explored factors associated with time-based meta-PM among HAND participants. More severe global neurocognitive impairment was associated with greater inaccurate overconfidence in time-based PM (mean NP z-score: ρ = −0.27, p = .02). This effect appeared to be largely driven by executive dysfunction (executive functions domain: ρ = −0.26, p = .03), in which poorer performance on action fluency (ρ = −0.32, p = .006) and greater Tower of London rule violations (ρ = −0.24, p = .047) were associated with increased overconfidence in time-based meta-PM (see Table 2). Although poorer learning abilities showed a trend toward greater overconfidence, this effect did not reach significance (ps < .10). No other cognitive correlates were associated with time-based meta-PM (ps > .10). Additionally, no other demographic, psychiatric (POMS), substance use, HIV disease variables, or PM symptoms in daily life (i.e., PRMQ) were associated with time-based meta-PM in the HAND cohort (ps > .10).
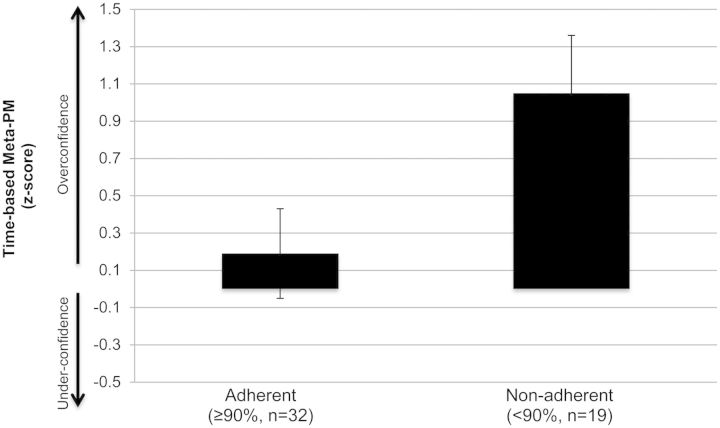
Of ecological relevance, overconfidence in time-based PM was associated with greater ARV nonadherence among HAND participants (<90%; Z = 4.9, p = .026, d = 0.64; see Fig. 4). Notably, those HAND participants who demonstrated ARV adherence versus nonadherence did not differ across demographics (age, education, gender, and ethnicity), severity of NP impairment (global NP rating), psychiatric (POMS, lifetime affective disorder diagnoses) or substance use factors, or severity of HIV disease (CD4 and nadir CD4 count, plasma or CSF viral load detectability, AIDS status, duration of infection; ps > .10), indicating that the observed effect was specific to time-based meta-PM.
Fig. 4.
Overconfidence in time-based PM predicts non-adherence to antiretroviral therapy among individuals with HAND.
Discussion
Consistent with prior studies, HIV+ individuals with global neurocognitive impairment (i.e., HAND) are particularly susceptible to objective PM deficits for both time- and event-based cues (Zogg et al., 2011). Interestingly, although these individuals also reported lower PM self-efficacy (i.e., more PM complaints in daily life and lower self-rated predictions for PM task performance), they were still inaccurately overconfident in their PM task predictions. Given that meta-PM abilities were comparable between HIV+ individuals without global impairment and seronegative controls, it appears that neurocognitive disruption, and not HIV infection alone, is driving the shift in metamemory accuracy. Of note, although the three study groups showed closer overall meta-PM accuracy rates (i.e., accurate normal or accurate impaired; 60.3% HIV−, 66.1% HIV+ no HAND, and 56.2% HAND), HAND participants demonstrated by far the largest proportion of overconfidence (i.e., over one-third of the cohort). Taken together, our findings suggest that while meta-PM inaccuracies exist in the general population, the nature of this disruption appears to be pronounced among individuals with HAND (i.e., larger proportion of overestimation).
Further characterizing the observed meta-PM deficit, it appeared that the discrepancy between predicted and actual PM abilities was most evident for time- rather than event-based PM tasks. One interpretation of this pattern of results is that time-based PM was simply more impaired among HAND individuals in this sample, which is consistent with the nature of PM impairment in the general HIV population (Morgan, Weber, Rooney, Grant, & Woods, 2012; Zogg et al., 2011), and therefore lead to poorer insight into such abilities. It is also possible that, by virtue of their deficits in executive functions, people with HAND experience greater difficulty accurately predicting future time-based performances, the exertion of which draws more heavily on strategic planning and monitoring skills than does event-based meta-PM. Evidence for this latter conceptualization is supported by our finding that greater severity of global neurocognitive impairment, driven by executive dysfunction, was associated with greater time-based PM overconfidence inaccuracy within the HAND cohort. Specifically, poorer internal search strategies (i.e., Action Fluency) and planning (i.e., Tower of London rule violations) appeared to have the strongest relationships with overconfidence in time-based PM. These findings are consistent with the metacognition literature which suggests that inaccurate overestimation of one's abilities is associated with poorer executive functions (Fernandez-Duque, Baird, & Posner, 2000), while underestimation is more strongly associated with mood symptoms (Hinkin et al., 1996). Of clinical and neurorehabilitation relevance, our findings suggest that compensatory strategies that support insight into strategic PM processes (e.g., salient cueing) may be particularly useful among individuals with HAND by reducing the executive functioning load of the task (Woods et al., 2014). These findings also converge with a recent study from Loft and colleagues (2014) demonstrating enhanced PM accuracy by reducing the speeded strategic demands of a PM task (i.e., introducing a delay immediately before PM task execution) in individuals with HIV. Taken together, neurorehabilitation techniques aimed at supporting the executive demands of a task and increasing self-monitoring skills in HAND (e.g., Goal Management Training) appear to be needed.
Previous studies suggest that inaccurate overconfidence in cognitive abilities is associated with decreased motivation and frequency of compensatory strategy use, and subsequently, increased errors in daily functioning (Hart, Giovannetti, Montgomery, & Schwartz, 1998; Ownsworth et al., 2010). Indeed, we found that greater time-based meta-PM overconfidence was associated with a higher likelihood of ARV nonadherence among HAND individuals, further extending this literature. Given that HIV-associated time-based PM deficits are already an established risk factor for ARV nonadherence (Woods et al., 2009), our finding provides an additional mechanism by which HAND may result in nonadherence. That is, not only is PM important for adherence, but also insights into such PM abilities are critical. Moving forward, however, it will be important to determine how this relationship transpires, especially in the context of compensatory strategy use. For example, it is not clear from our data if impaired time-based meta-PM alone is sufficient to result in nonadherence, or if these individuals additionally are not using compensatory strategies due to overconfidence, which is leading to nonadherence, or likely, a combination of these and other factors.
Additionally, this is only the second study to date that has employed a prediction paradigm in order to explore metacognition in the context of HIV infection (vs. examining cognitive complaints; Chiao et al., 2013), which may be both a more direct assessment of metacognition (e.g., do you have the capacity to complete this task?) and superior in distinguishing the nuances in over- versus underconfidence. With regard to the latter, when applying complaints (either cognitive or functional symptoms in daily life) as an indicator of self-awareness, inaccurate overconfidence can only be determined by the absence of reported symptoms which may result in poor variability of metacognitive classifications. On the other hand, prediction paradigms allow for a one-to-one subtraction of estimation from performance, which provides a more continuous spectrum across which these processes may be observed with enhanced granularity. Furthermore, prediction paradigms circumvent the complexities inherent when using complaints in daily life. That is, cognitive complaints do not distinguish whether the individual is reporting a complaint due to a perceived cognitive limitation or simply due to surrounding environmental factors (e.g., forgot an appointment because car broke down).
Our study is not without limitations. First, although we employed a prediction paradigm, which may increase sensitivity to measurement of overconfidence, we did not also include a “postdiction” assessment. Postdiction queries an individual to again rate their performance after having completed the given task, and may be particularly useful in assessing one's ability to self-monitor (i.e., incorporate the feedback of actual task performance during self-evaluation). The literature on metacognition in HIV would benefit from studies exploring all aspects of these self-reflection process (e.g., self-knowledge, task appraisal, self-monitoring) in order to determine which metacognitive mechanism(s) may be most impaired and important for health and daily outcomes in this population. Additionally, our sample size was too small within the HAND cohort to examine associations with time-based meta-PM as a 4-level categorical variable (i.e., underconfidence, overconfidence, accurate -normal, accurate impaired), which may be helpful particularly in further characterizing these under- and overconfident groups. However, the continuous meta-PM variable employed here did allow for more nuanced findings within the cohort as a whole.
In sum, over a third of the HAND individuals demonstrated inaccurate overconfidence in their time-based PM abilities, which had significant implications for ARV nonadherence in this group. Deficits in time-based meta-PM were more severe than those for event-based meta-PM in the HAND participants, which appears to reflect the disproportionate deficits in the strategic executive functions aspects needed for accurate time-based meta-PM. Future studies are needed to continue to explore the mechanisms important to metacognitive processes in HIV infection, and especially determine their impact on other areas of daily functioning. Additionally, our study supports that neurorehabilitation interventions aimed at improving metacognition among HAND individuals may be fruitful in decreasing everyday errors in this population.
Funding
This research was supported by National Institutes of Health grants R01-MH073419, P30-MH62512, T32-DA31098, F31-DA035708, and F31-DA034510. This study was also supported (in part) by a Foundation for Rehabilitation Psychology Dissertation Award.
Conflict of Interest
None declared.
Acknowledgements
The San Diego HIV Neurobehavioral Research Center (HNRC) group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Robert K. Heaton, PhD, Co-Director: Igor Grant, MD; Associate Directors: J. Hampton Atkinson, MD, Ronald J. Ellis, MD, PhD, and Scott Letendre, MD; Center Manager: Thomas D. Marcotte, PhD; Jennifer Marquie-Beck, MPH; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, MD, PhD (PI), Scott Letendre, MD, J. Allen McCutchan, MD, Brookie Best, Pharm. D, Rachel Schrier, PhD, Debra Rosario, MPH; Neurobehavioral Component: Robert K. Heaton, PhD (PI), J. Hampton Atkinson, MD, Steven Paul Woods, Psy. D, Thomas D. Marcotte, PhD, Mariana Cherner, PhD, David J. Moore, PhD, Matthew Dawson; Neuroimaging Component: Christine Fennema-Notestine, PhD (PI), Monte S. Buchsbaum, MD, John Hesselink, MD, Sarah L. Archibald, MA, Gregory Brown, PhD, Richard Buxton, PhD, Anders Dale, PhD, Thomas Liu, PhD; Neurobiology Component: Eliezer Masliah, MD (PI), Cristian Achim, MD, PhD; Neurovirology Component: David M. Smith, MD (PI), Douglas Richman, MD; International Component: J. Allen McCutchan, MD, (PI), Mariana Cherner, PhD; Developmental Component: Cristian Achim, MD, PhD; (PI), Stuart Lipton, MD, PhD; Participant Accrual and Retention Unit: J. Hampton Atkinson, MD (PI), Jennifer Marquie-Beck, MPH; Data Management and Information Systems Unit: Anthony C. Gamst, PhD (PI), Clint Cushman; Statistics Unit: Ian Abramson, PhD (PI), Florin Vaida, PhD (Co-PI), Reena Deutsch, PhD, Anya Umlauf, MS.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government. The authors thank Marizela V. Cameron for her efforts coordinating the study and Dr. Erin E. Morgan, Donald R. Franklin, Jr., and Stephanie Corkran for their assistance with data processing.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Antinori A., Arendt G., Becker J. T., Brew B. J., Byrd D. A., Cherner M., et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess P. W., Quayle A., Frith C. D. Brain regions involved in prospective memory as determined by positron emission tomography. Neuropsychologia. 2001;39(6):545–555. doi: 10.1016/s0028-3932(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Burgess P. W., Scott S. K., Frith C. D. The role of the rostral frontal cortex (area 10) in prospective memory: a lateral versus medial dissociation. Neuropsychologia. 2003;41(8):906–918. doi: 10.1016/s0028-3932(02)00327-5. [DOI] [PubMed] [Google Scholar]
- Carey C. L., Woods S. P., Rippeth J. D., Heaton R. K., Grant I. The HNRC Group. Prospective memory in HIV-1 infection. Journal of Clinical & Experimental Neuropsychology. 2006;28(4):536–548. doi: 10.1080/13803390590949494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao S., Rosen H. J., Nicolas K., Wendelken L. A., Alcantar O., Rankin K. P., et al. Deficits in self-awareness impact the diagnosis of asymptomatic neurocognitive impairment in HIV. AIDS Research and Human Retroviruses. 2013;29(6):949–956. doi: 10.1089/aid.2012.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contardo C., Black A. C., Beauvais J., Dieckhaus K., Rosen M. I. Relationship of prospective memory to neuropsychological function and antiretroviral adherence. Archives Clinical Neuropsychology. 2009;24:547–554. doi: 10.1093/arclin/acp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson W. C., Zillmer E. A. TOL-DX Tower of London – Drexel University. Chicago: Multihealth Systems; 2001. [Google Scholar]
- Delis D. C., Kramer J. H., Kaplan E., Ober B. A. The California Verbal Learning Test-2nd Edition. San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- Devolder P. A., Brigham M. C., Pressley M. Memory performance awareness in younger and older adults. Psychology and Aging. 1990;5(2):291. doi: 10.1037//0882-7974.5.2.291. [DOI] [PubMed] [Google Scholar]
- Doyle K. L., Loft S., Morgan E. E., Weber E., Cushman C., Johnston E., et al. HIV Neurobehavioral Research Program (HNRP) Group. Prospective memory in HIV-associated neurocognitive disorders (HAND): the neuropsychological dynamics of time monitoring. Journal of clinical and experimental neuropsychology. 2013;35(4):359–372. doi: 10.1080/13803395.2013.776010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Duque D., Baird J. A., Posner M. I. Executive attention and metacognitive regulation. Consciousness and Cognition. 2000;9:288–307. doi: 10.1006/ccog.2000.0447. [DOI] [PubMed] [Google Scholar]
- Gupta S., Paul Woods S., Weber E., Dawson M. S., Grant I. HIV Neurobehavioral Research Center (HNRC) Group. Is prospective memory a dissociable cognitive function in HIV infection? Journal of clinical and experimental neuropsychology. 2010;32(8):898–908. doi: 10.1080/13803391003596470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart T., Giovannetti T., Montgomery M. W., Schwartz M. F. Awareness of errors in naturalistic action after traumatic brain injury. Journal of Head Trauma Rehabilitation. 1998;13(5):16–28. doi: 10.1097/00001199-199810000-00004. [DOI] [PubMed] [Google Scholar]
- Hinkin C. H., van Gorp W. G., Satz P., Marcotte R., Durvasula R. S., Wood S., et al. Actual versus self-reported cognitive dysfunction in HIV-1 infection: Memory-metamemory dissociations. Journal of Clinical and Experimental Neuropsychology. 1996;18(3):431–443. doi: 10.1080/01688639608408999. [DOI] [PubMed] [Google Scholar]
- Juengst S., Skidmore E., Pramuka M., McCue M., Becker J. Factors contributing to impaired self-awareness of cognitive functioning in an HIV positive and at-risk population. Disability Rehabilitation. 2012;24(1):19–25. doi: 10.3109/09638288.2011.587088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karantzoulis S., Troyer A. K., Rich J. B. Prospective memory in amnestic mild cognitive impairment. Journal of the International Neuropsychological Society. 2009;15(03):407–415. doi: 10.1017/S1355617709090596. [DOI] [PubMed] [Google Scholar]
- Knight R. G., Harnett M., Titov N. The effects of traumatic brain injury on the predicted and actual performance of a test of prospective remembering. Brain Injury. 2005;19(1):19–27. doi: 10.1080/02699050410001720022. [DOI] [PubMed] [Google Scholar]
- Loft S., Doyle K. L., Naar-King S., Outlaw A. Y., Nichols S. L., Weber E., et al. Allowing brief delays in responding improves event-based prospective memory for younger adults living with HIV disease. Journal of Clinical and Experimental Neuropsychology. 2014;36(7):761–772. doi: 10.1080/13803395.2014.942255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel M. A., Einstein G. O. Strategic and automatic processes in prospective memory retrieval: A multiprocess framework. Applied Cognitive Psychology. 2000;14:S127–S144. [Google Scholar]
- McNair D. M., Lorr M., Droppleman L. F. Manual for the profile of mood states. San Diego, CA: Educational and Industrial Testing Services; 1981. [Google Scholar]
- Morgan E. E., Weber E., Rooney A. S., Grant I., Woods S. P. HIV Neurobehavioral Research Program (HNRP) Group. Longer ongoing task delay intervals exacerbate prospective memory deficits in HIV-associated neurocognitive disorders (HAND) Journal of clinical and experimental neuropsychology. 2012;34(4):416–427. doi: 10.1080/13803395.2012.654764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen K. M., Waldum E. R., McDaniel M. A., Braver T. S. Neural mechanisms of time-based prospective memory: Evidence for transient monitoring. PLoS ONE. 2014;9(3):e92123. doi: 10.1371/journal.pone.0092123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ownsworth T., Quinn H., Fleming J., Kendall M., Shum D. Error self-regulation following traumatic brain injury: A single case study evaluation of metacognitive skills training and behavioral practice interventions. Neuropsychological Rehabilitation. 2010;20(1):59–80. doi: 10.1080/09602010902949223. [DOI] [PubMed] [Google Scholar]
- Pirogovsky E., Woods S. P., Vincent Filoteo J., Gilbert P. E. Prospective memory deficits are associated with poorer everyday functioning in Parkinson's disease. Journal of the International Neuropsychological Society. 2012;18(06):986–995. doi: 10.1017/S1355617712000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychological Corporation. WAIS-III and WMS-III technical manual. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- Psychological Corporation. Wechsler test of adult reading. San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- Raskin S., Buckheit C., Sherrod C. Memory for intentions test (MIsT) Lutz: Psychological Assessment Resources; 2010. [Google Scholar]
- Raskin S. A., Woods S. P., Poquette A. J., McTaggart A. B., Sethna J., Williams R. C., et al. A differential deficit in time-versus event-based prospective memory in Parkinson's disease. Neuropsychology. 2011;25(2):201. doi: 10.1037/a0020999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R. M., Wolfson D. The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation. Tuscon, AZ: Neuropsychology Press; 1985. [Google Scholar]
- Roche N. L., Fleming J. M., Shum D. H. Self-awareness of prospective memory failure in adults with traumatic brain injury. Brain Injury. 2002;16(11):931–945. doi: 10.1080/02699050210138581. [DOI] [PubMed] [Google Scholar]
- Rourke S. B., Halman M. H., Bassel C. Neuropsychiatric correlates of memory-metamemory dissociations in HIV-infection. Journal of Clinical and Experimental Neuropsychology. 1999;21(6):757–768. doi: 10.1076/jcen.21.6.757.852. [DOI] [PubMed] [Google Scholar]
- Smith G., Della Sala S., Logie R. H., Maylor E. A. Prospective and retrospective memory in normal ageing and dementia: A questionnaire study. Memory. 2000;8:311–312. doi: 10.1080/09658210050117735. [DOI] [PubMed] [Google Scholar]
- Smith S. J., Souchay C., Moulin C. J. Metamemory and prospective memory in Parkinson's disease. Neuropsychology. 2011;25(6):734. doi: 10.1037/a0025475. [DOI] [PubMed] [Google Scholar]
- Stuss D. T. Functions of the frontal lobes: Relation to executive functions. Journal of the International Neuropsychological Society. 2011;17:759–765. doi: 10.1017/S1355617711000695. [DOI] [PubMed] [Google Scholar]
- Woods S. P., Rippeth J. D., Frol A. B., Levy J. K., Ryan E., Soukup V. M., et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. Journal of Clinical and Experimental Neuropsychology. 2004;26(6):759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
- Woods S. P., Morgan E. E., Marquie-Beck J., Carey C. L., Grant I., Letendre S. L. Markers of macrophage activation and axonal injury are associated with prospective memory in HIV-1 disease. Cognitive and behavioral neurology: official journal of the Society for Behavioral and Cognitive Neurology. 2006;19(4):217. doi: 10.1097/01.wnn.0000213916.10514.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods S. P., Dawson M. S., Weber E., Gibson S., Grant I., Atkinson J. H. Timing is everything: Antiretroviral nonadherence is associated with impairment in time-based prospective memory. Journal of the International Neuropsychological Society. 2009;15(01):42–52. doi: 10.1017/S1355617708090012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods S. P., Doyle K. L., Morgan E. E., Naar-King S., Outlaw A. Y., Nichols S. L., et al. Task importance affects event-based prospective memory performance in adults with HIV-associated neurocognitive disorders and HIV-infected young adults with problematic substance use. Journal of the International Neuropsychological Society. 2014;20(6):1–11. doi: 10.1017/S1355617714000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods S. P., Moran L. M., Dawson M. S., Carey C. L., Grant I. The HNRC Group. Psychometric characteristics of the Memory for Intentions Screening Test. The Clinical Neuropsychologist. 2008;22:864–878. doi: 10.1080/13854040701595999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods S. P., Scott J. C., Sires D. A., Grant I., Heaton R. K., Tröster A. I., et al. Action (verb) fluency: Test-retest reliability, normative standards, and construct validity. Journal of the International Neuropsychological Society. 2005;11:408–415. [PubMed] [Google Scholar]
- Woods S. P., Twamley E. W., Dawson M. S., Narvaez J. M., Jeste D. V. Deficits in cue detection and intention retrieval underlie prospective memory impairment in schizophrenia. Schizophrenia Research. 2007;90(1):344–350. doi: 10.1016/j.schres.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods S. P., Weber E., Weisz B. M., Twamley E. W., Grant I. Prospective memory deficits are associated with unemployment in persons living with HIV infection. Rehabilitation Psychology. 2011;56(1):77. doi: 10.1037/a0022753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods S. P., Weinborn M., Velnoweth A., Rooney A., Bucks R. S. Memory for intentions is uniquely associated with instrumental activities of daily living in healthy older adults. Journal of the International Neuropsychological Society. 2012;18(01):134–138. doi: 10.1017/S1355617711001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Composite international diagnostic interview (CIDI, version 2.1). Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- Zogg J. B., Woods S. P., Weber E., Doyle K., Grant I. the HNRP Group. Are time- and event-based prospective memory comparably affected in HIV infection? Archives of Clinical Neuropsychology. 2011;26:250–259. doi: 10.1093/arclin/acr020. [DOI] [PMC free article] [PubMed] [Google Scholar]