Abstract
Apoptosis is a process that affects life span and health. Mice with liver-specific disruption of the growth hormone receptor (GHR) gene (ie, Ghr gene) liver-specific growth hormone receptor knockout [LiGHRKO] mice), as opposed to mice with global deletion of the Ghr gene (GHRKO; Ghr−/−), are characterized by severe hepatic steatosis and lack of improved insulin sensitivity. We have previously shown that levels of proapoptotic factors are decreased in long-lived and insulin-sensitive GHRKO mice. In the current study, expression of specific apoptosis-related genes was assessed in brains, kidneys, and livers of male and female LiGHRKO and wild-type mice using real-time PCR. In the brain, expression of Caspase 3, Caspase 9, Smac/DIABLO, and p53 was decreased in females compared with males. Renal expression of Caspase 3 and Noxa also decreased in female mice. In the liver, no differences were seen between males and females. Also, no significant genotype effects were detected in the examined organs. Lack of significant genotype effect in kidneys contrasts with previous observations in GHRKO mice. Apparently, global GHR deletion induces beneficial changes in apoptotic factors, whereas liver-specific GHR disruption does not. Furthermore, sexual dimorphism may play an important role in regulating apoptosis during liver-specific suppression of the somatotrophic signaling.
Key Words: Apoptosis, Growth hormone receptor (GHR), GHR gene disruption, Knockout mice.
Apoptosis, or programmed cell death, is one of the most crucial physiological processes that affects life span; it is, in part, responsible for the development and health of multicellular organisms (reviewed in ref. 1). There are two main apoptotic signaling pathways: intrinsic (mitochondrial) and extrinsic. The intrinsic pathway (involving p53, Caspase 9, and bax) is initiated by different factors within the cell (eg, free radicals, DNA damage, and hypoxia) (2–4). The extrinsic pathway (involving Caspase 8) is related to interactions mediated by death receptors, which are members of the tumor necrosis factor receptor gene superfamily (5). Disturbances in the regulation of apoptosis can lead to numerous diseases, such as cancer, neurodegenerative disorders, and autoimmune diseases (1).
Caspase 3 is a main executioner caspase and is responsible for destroying cells and inducing apoptosis, whereas Caspase 9 activates the effector Caspase 3. Smac/DIABLO (second mitochondria-derived activator of caspase/direct IAP-binding protein with low pI) is a mitochondrial protein that potentiates apoptosis after being released from mitochondria into the cytosol (6,7). Apoptotic protease-activating factor-1 (Apaf-1) forms a multiprotein complex (called the apoptosome) with procaspase 9 and cytochrome c (8,9). It leads to activation of Caspase 9 and caspase cascade and, finally, to activation of Caspase 3. p53 protein is a well-known tumor suppressor and an important factor that activates the intrinsic apoptotic pathway. NOXA is a member of the bcl-2 family and is described as a p53 target gene, serving as a candidate mediator of p53-induced apoptosis (10). Caspase 8 is an initiator caspase, indispensable for induction of the extrinsic apoptotic signaling pathway (11). Bax (bcl-2–associated X protein) is one of the main proapoptotic, and bcl-2 is one of the antiapoptotic bcl-2 family proteins (12). It is well known that the benefits of apoptosis rely on the removal of abnormal cells. However, the entire role of apoptosis in physiology/pathophysiology and potentially in life-span regulation remains unclear.
Mice with targeted disruption of the growth hormone (GH) receptor (GHR) gene (Ghr gene) globally (GHRKO; GHR knockout; Ghr−/−) (13) are dwarf, obese, insulin sensitive, and long lived (14). We have shown that these mice are characterized by decreased gene expression and/or protein level of numerous proapoptotic factors, including Caspase 3, Caspase 9, Caspase 8, Smac/DIABLO, bax, and Apaf-1 in the kidneys (and skeletal muscles as well) ((15–17) and reviewed in ref. 18). These alterations in levels of the proapoptotic factors were not further improved by calorie restriction, another potential life-extending intervention (17). Similarly, calorie restriction did not change Caspase 3 and Caspase 9 activities in the brain cortices of Fischer 344 rats (19). Moreover, GHRKO mice have increased levels of key regulators of mitochondrial biogenesis (18,20,21) and decreased thyroid follicle size (22) with mild thyroid hypofunction. These features of GHRKOs and other mice strains with altered GH action suggest the crucial role of GH-induced intracellular signaling in life-span regulation (reviewed in ref. 23).
Many of GH’s numerous physiological effects are mediated by insulin-like growth factor-1 (IGF-1), which is generated primarily in the liver and acts systemically. However, IGF-1 is also produced in other tissues acting in an autocrine or paracrine manner. Thus, to better understand how the GH/IGF-1 axis regulates physiological processes in selected tissues and/or organs, mice with tissue-specific GHR deletions recently have been generated ((24–28), List et al., unpublished data). One of these strains is mice with selective deletion of GHR in the liver (liver-specific growth hormone receptor knockout [LiGHRKO] mice). These mice have decreased body size and body fat, severely reduced levels of circulating IGF-1, and concurrently higher GH plasma levels, due to disruption of somatotrophic signaling in the liver ((24), List et al., unpublished data). However, of particular interest, LiGHRKO mice show an absence of improved insulin sensitivity and severe hepatic steatosis ((24), List et al., unpublished data) compared with GHRKO mice. Fasting blood glucose is elevated in both sexes of LiGHRKO animals, whereas fasting insulin is higher in males relative to controls. Male LiGHRKOs have normal glucose tolerance and mild insulin resistance, and females are glucose intolerant and insulin resistant (List et al., unpublished data).
Results by Fan coworkers (24) and List coworkers (unpublished data) show significant alterations in weights of different organs (eg, brain, kidneys, and liver) between control and LiGHRKO mice, suggesting potential differences in apoptosis. Moreover, our previous studies report a decrease of proapoptotic factors in kidneys of global GHRKO mice compared with control animals (16,17). Additionally, analysis of expression of these apoptosis-related genes in the brains is also related to the reported preservation of cognitive function in aging GHRKO mice (14).
For all these reasons, we have set out to determine the effect of liver-specific Ghr gene disruption on expression of the apoptosis-related genes (Caspase 3, Caspase 9, Smac/DIABLO, Apaf-1, Caspase 8, Noxa, Bcl-2, Bax, p53) in brains, kidneys, and livers of LiGHRKO mice compared with wild-type (WT) animals.
Materials and Methods
Animals
Mice carrying the GHR “floxed” allele were generated according to the previously described method (28). Liver-specific GHRKO mice (FFCx) and floxed littermate controls (FFxx) were generated by breeding conditional floxed GHRflox/flox mice to B6.Cg-Tg(alb-cre)21Mgn/J transgenic mice purchased from Jackson Laboratories (Bar Harbor, ME) (List et al., unpublished data). Brains, kidneys, and livers from approximately 22-month-old male and female WT and liver-specific GHRKO (LiGHRKO) mice were kindly provided by Dr. M. B. Stout (Mayo Clinic, Rochester, MN). The animals comprised four (4) experimental groups: wild-type males (WT-male; seven animals), liver-specific GHR knockout males (LiGHRKO-male; eight animals), wild-type females (WT-female; eight animals), and liver-specific GHR knockout females (LiGHRKO-female; eight animals).
To statistically analyze differences between males and females (a potential significant gender effect), we pooled all males (WT-male and LiGHRKO-male mice) and all females (WT-female and LiGHRKO-female mice) (see the Results section). Similarly, for analyzing differences between WT and LiGHRKO mice (a potential significant genotype effect), we pooled all WT animals (WT-male and WT-female mice) and all LiGHRKOs (LiGHRKO-male and LiGHRKO-female mice) (see the Results section).
RNA Extraction and cDNA Transcription
RNA was extracted from the homogenates of the examined tissues using a miRNeasy Mini Kit (Qiagen) in accordance with the manufacturer’s instruction. RNA quantity and quality were analyzed using a NanoDrop 1000 Spectrophotometer (Thermo Scientific). Reverse transcription was performed, and complementary DNA was synthesized using an iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer’s instruction.
Real-Time PCR
Real-time PCR was carried out using the StepOne Real-Time PCR System Instrument (Life Technologies) with iQ SYBR Green Supermix (Bio-Rad Laboratories). The three steps of the PCR included: denaturation at 94°C for 2 minutes, annealing at 62°C for 30 seconds with fluorescence reading, and extension at 72°C for 30 seconds. In addition, a melting curve was done for each reaction to evaluate the potential of nonspecific products. β2-Microglobulin (B2M), which was previously validated in our laboratory as the most appropriate gene for normalizing the data (17,18,29), was used as a housekeeping gene. Gene expression was assessed by measuring steady state levels of mRNA. Relative expression from real-time PCR was calculated using the equation 2A −B/2C −D (where A = cycle threshold [C t] number for the gene of interest in the first control sample; B = C t number for the gene of interest in the analyzed sample; C = C t number for the housekeeping gene in the first control sample; D = C t number for housekeeping gene in the analyzed sample). The first control was expressed as 1.00 by this equation, and all other samples were calculated in relation to this value. Then, the results in the control group (WT-males) were averaged. All other outputs were divided by the mean value of the relative expression in the control group to yield the fold change of the expression of genes of interest compared with the control group. For real-time PCR, the primers used are listed in Table 1.
Table 1.
Primers Used for Gene Expression Analyses
| Gene | GenBank Accession No. | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|---|
| β 2 - Microglobulin | NM_009735 | aagtatactcacgccaccca | aagaccagtccttgctgaag |
| Caspase 3 | NM_009810 | tgcagcatgctgaagctgta | gagcatggacacaatacacg |
| Caspase 8 | AJ007749 | accgagatcctgtgaatgga | tgctttcccttgttcctcct |
| Caspase 9 | NM_015733 | agcagagagtagtgaagctg | acacagacatcatgagctcc |
| Smac/DIABLO | NM_023232 | aagagctgcaccagaaagca | tctgactgtcaatggcagga |
| Apaf-1 | AF064071 | acaacgctctgctacacga | cacacagcactgtccttaca |
| Noxa | AB041230 | gccaatctgttttagggtga | cagaacaggcaacatccgtt |
| Bax | NM_007527 | ccaccagctctgaacagatc | cagcttcttggtggacgcat |
| Bcl-2 | NM_009741 | tgggatgcctttgtggaact | gagacagccaggagaaatca |
| p53 | AF151353 | tcacagtcggatatcagcct | acactcggagggcttcactt |
Statistical Analysis
The data are expressed as mean ± standard error of the mean. To evaluate the effects of the genotype and sex, we used two-way analysis of variance. For analyzing differences between group means, we used a Bonferroni post hoc test. p value less than .05 was considered significant. All statistical calculations were conducted using SPSS version 17.0 (SPSS, Chicago, IL) with α = .05. All graphs were created using Prism 4.02 (GraphPad Software, San Diego, CA).
Results
In the brain, gene expression of Caspase 3, Caspase 9, Smac/DIABLO, and p53 decreased in females compared with males (p = .02, p = .04, p = .03, p = .01, respectively) (Figure 1A–C and H). Thus, a significant sex effect was detected. Moreover, Noxa decreased in brains of WT-females compared with WT-males (p = .02 with significant Genotype × Gender interaction: p = .04) (Figure 1E). There were no differences in Apaf-1, Caspase 8, and Bcl-2 between male and female brains (Figure 1D, F, and G). Interestingly, there were no differences in mRNA expression for any examined apoptosis-related factors between the brains of WT and LiGHRKO mice (Caspase 3, Caspase 9, Smac/DIABLO, Apaf-1, Caspase 8, Noxa, Bcl-2, and p53: p = .55, p = .59, p = .27, p = .36, p = .82, p = .43, p = .30, p = .66, respectively) (lack of significant genotype effect), although there appeared to be a weak (but not statistically significant) tendency for expression levels of the examined apoptosis-related genes to increase in female LiGHRKO mice compared with WT-females (Figure 1).
Figure 1.
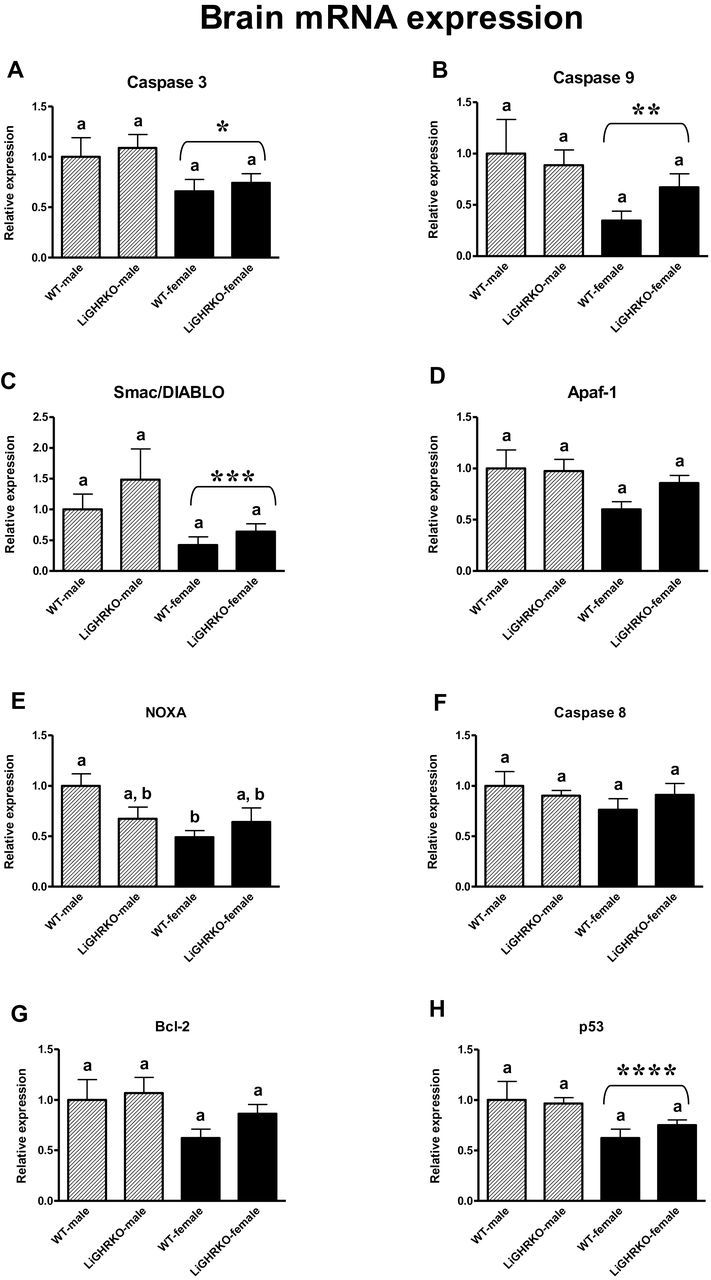
Brain mRNA expression of Caspase 3 (A), Caspase 9 (B), Smac/DIABLO (C), Apaf-1 (D), Noxa (E), Caspase 8 (F), Bcl-2 (G), and p53 (H) in male and female of wild-type (WT) and liver-specific growth hormone receptor knockout (LiGHRKO) mice. The data from real-time PCR were normalized by the housekeeping gene β2-Microglobulin (B2M) and expressed as the relative expression. Values are means ± SEM. (a and b) Values that do not share the same letter in the superscript are statistically significant (p < .05). *p = .02 vs male mice (the significance for sex), **p = .04 vs male mice (the significance for sex), ***p = .03 vs male mice (the significance for sex), ****p = .01 vs male mice (the significance for sex).
In kidney tissue, a decrease in expression of two apoptosis-related genes (Caspase 3 and Noxa) was observed in females compared with male mice, showing a significant effect of sex (p = .04 and p = .01, respectively) (Figure 2A and E). Moreover, renal Caspase 8 mRNA level decreased in WT-females compared with WT-males (p = .00 with significant Genotype × Gender interaction: p = .01) (Figure 2F). Apaf-1 showed a tendency for reduced expression in female kidneys (p = .09) (Figure 2D). mRNA levels of renal Caspase 9, Smac/DIABLO, Bcl-2, and Bax did not show a sex effect (Figure 2B, C, G, and H). Also, genotype did not significantly affect kidney mRNA levels of the examined apoptosis-related genes (Caspase 3, Caspase 9, Smac/DIABLO, Apaf-1, Caspase 8, Noxa, Bcl-2, and Bax: p = .42, p = .30, p = .61, p = .80, p = .27, p = .41, p = .84, p = .87, respectively) (Figure 2).
Figure 2.
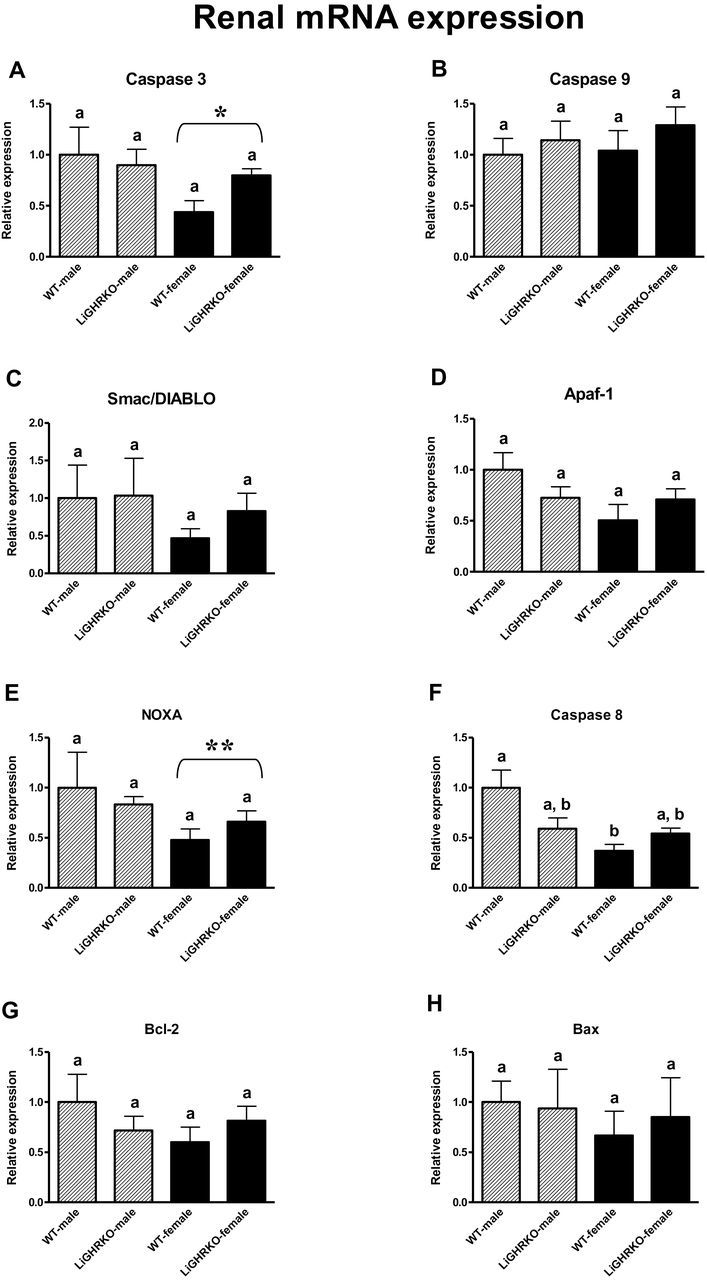
Renal mRNA expression of Caspase 3 (A), Caspase 9 (B), Smac/DIABLO (C), Apaf-1 (D), Noxa (E), Caspase 8 (F), Bcl-2 (G), and Bax (H) in male and female of wild-type (WT) and liver-specific growth hormone receptor knockout (LiGHRKO) mice. The data from real-time PCR were normalized by the housekeeping gene β2-Microglobulin (B2M) and expressed as the relative expression. Values are means ± SEM. (a and b) Values that do not share the same letter in the superscript are statistically significant (p < .05). * p = .04 vs male mice (the significance for sex), **p = .01 vs male mice (the significance for sex).
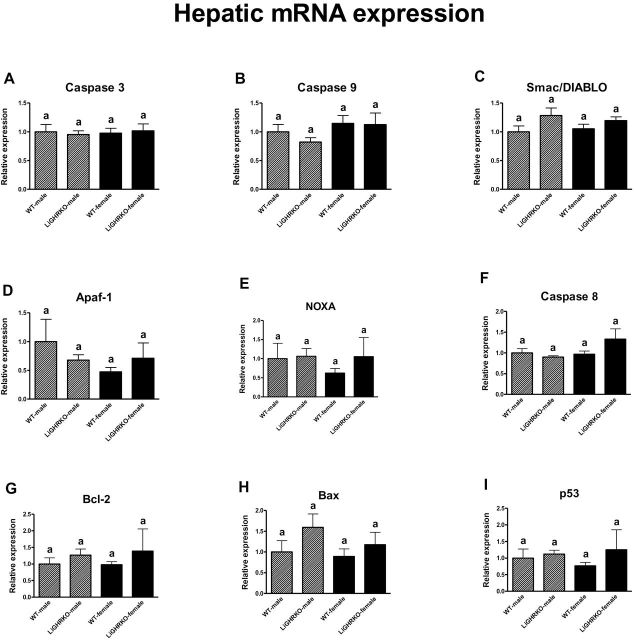
Intriguingly, liver showed no differences in the expression of apoptosis-related genes between males and females (Caspase 3, Caspase 9, Smac/DIABLO, Apaf-1, Caspase 8, Noxa, Bcl-2, Bax, and p53: p = .84, p = .11, p = .86, p = .25, p = .15, p = .56, p = .87, p = .40, p = .87, respectively) (Figure 3). Importantly and similar to the findings in the brains and kidneys, the mRNA level of the examined apoptosis-related genes was not significantly affected by genotype in livers (Caspase 3, Caspase 9, Smac/DIABLO, Apaf-1, Caspase 8, Noxa, Bcl-2, Bax, and p53: p = .98, p = .47, p = .38, p = .84, p = .34, p = .45, p = .30, p = .16, p = .33, respectively) (Figure 3).
Figure 3.
Hepatic mRNA expression of Caspase 3 (A), Caspase 9 (B), Smac/DIABLO (C), Apaf-1 (D), Noxa (E), Caspase 8 (F), Bcl-2 (G), Bax (H), and p53 (I) in male and female of wild-type (WT) and liver-specific growth hormone receptor knockout (LiGHRKO) mice. The data from real-time PCR were normalized by the housekeeping gene β2-Microglobulin (B2M) and expressed as the relative expression. Values are means ± SEM. (a) Values that share the same letter in the superscript are not statistically significant.
Discussion
Based on the results of previous studies showing the decrease in the levels of proapoptotic factors in GHRKO mice (15–17), we hypothesized that the same kind of genetic intervention, although limited to the liver, may result in similar changes in the expression of these apoptosis-related genes in different tissues. Unexpectedly, hepatic deletion of GHR did not cause any differences in expression of these genes relative to control mice. In two other examined organs in which functional GHR was preserved, namely in brains and kidneys, there were also no differences between control and LiGHRKO mice. Thus, liver-specific GHR disruption did not lead to a decrease of proapoptotic factors level, a feature previously considering as potentially beneficial in global GHRKO mice (18). One could therefore hypothesize that the loss of GH signaling in the liver abolishes the potential beneficial profile of apoptosis-related factors observed in GHRKO mice, and that there is something unique about the aspect of global GHR−/− other than direct GH signaling that leads to the beneficial gene expression profile for GHRKO mice.
Importantly, the levels of certain apoptosis-related factors were previously analyzed in other mice characterized by marked longevity, namely the Ames dwarfs. Dhahbi coworkers (30) showed a decrease of Caspase 3 and an increase of bcl-2 gene expression in peripheral blood leukocytes when compared with WT animals. However, in another study, procaspase 3 protein levels unexpectedly increased in the kidney and liver of Ames dwarf mice (31). Therefore, these conflicting results emphasize the importance of further studies to elucidate the entire role of apoptosis, also in the circumstances of suppressed somatotrophic signaling.
Absence of the beneficial profile of the examined factors in LiGHRKO mice, compared with GHRKO animals, seems to be supported by characteristics of mice with specific GHR knockout in other tissues. For example, mice with GHR deletion in muscles (MuGHRKO) are characterized, in contrast to global GHRKO animals, by insulin resistance and glucose intolerance (25). Interestingly and unexpectedly, MuGHRKO mice, created with use of the muscle creatine kinase promoter/enhancer but not Mef-2c promoter/enhancer (as in the case of animals mentioned above (25)), are characterized by improved glucose metabolism (27). Another strain of tissue-specific GHRKO animals, fat-specific GHRKO (FaGHRKO) mice, show lack of glucose homeostasis improvement and absence of significant alterations in fasting blood glucose, serum insulin, and GH levels (28). Also, GHR disruption in pancreatic β-cells may impair insulin secretion (26). Thus, it seems that selective deletion of GHR in one organ or tissue may not duplicate the numerous beneficial effects of global GHR deletion. In support of this hypothesis, List coworkers (28) recently concluded that deletion of GHR in adipose tissue is not sufficient to increase adiponectin level. Increased levels of this important adipokine are widely considered beneficial and are observed in mice with global GHR knockout (32,33). Therefore, all of the above-mentioned observations support the hypothesis that global but not tissue-specific removal of GHR is indispensable for producing the extended life span and resistance to the development of cancer and diabetes seen in GHRKO mice (23). Similar findings have been reported for human Laron syndrome patients in which no cancer and a dramatic reduction in diabetes have been reported (34). Obviously, resolving this interesting phenomenon will require further experiments.
Although there were no differences in expression of apoptosis-related genes between control and LiGHRKO mice, there was notable difference between sexes. We found that female mice have decreased levels of proapoptotic gene mRNAs compared with male animals, suggesting a potential role of sexual dimorphism in the control of the apoptosis process. Actually, numerous previous studies seem to support this interesting hypothesis and confirm the decreased intensity of apoptosis (expressed as different apoptotic markers) in females compared with males in mice and other animals. Siegel coworkers (35) have shown that the baseline mRNA level of the X-linked inhibitor of apoptosis (XIAP; the primary endogenous inhibitor of caspases) is higher in female compared with male mice in the brain tissue. In another study, Caspase 3 activity had a tendency to increase in brain (and was increased in heart) of spontaneously hypertensive male rats (36). Also, the apoptotic area in the brain was increased in male Sprague-Dawley rats compared with females after experimentally induced focal cerebral infarction (37). These findings could be considered as consistent with our results showing the decrease of several proapoptotic factors in female brains. Decreased apoptosis in females compared with males was also demonstrated by Huang coworkers (38). The authors have analyzed expression of proapoptotic Bax and antiapoptotic Bcl-2 in rat hearts, showing lower Bax protein level, higher Bcl-2 mRNA and protein levels, and increased ratio of Bcl-2/Bax in females (38). Consistently, an increase in proapoptotic Caspase 3, Caspase 9, and Bax was observed in male rats’ hearts after arteriovenous shunt (39). The results of the studies by Hofmann-Lehmann coworkers (40) are also in accordance with our current observations, pointing to the decreased potential for apoptosis in females. Namely, the rate of apoptosis in peripheral blood lymphocytes, expressed as a percentage of apoptotic cells determined by flow cytometry, was lower in female cats compared with males (40). Thus, one could hypothesize that the decreased apoptosis in the female gender, observed in numerous species, may be related to obvious differences in sex hormone levels between males and females. In fact, the results of the study by Le May coworkers (41) have demonstrated the protection of β-cells from oxidative stress–induced apoptosis in mice by estradiol—the main sex hormone in females. Furthermore, some authors even postulate that differences between genders may be an effect of sex differences at the cellular level (42). Sexual dimorphism may also affect the transcriptional regulation of several imprinted genes (43).
However, it should be emphasized that in some studies, there was no evidence of decreased apoptosis in females compared with males. For example, Sanz coworkers (44) have demonstrated that there are no differences between males and females in different markers of apoptosis, such as Caspase 3 and Caspase 9 activities, as well as mono- and oligonucleosomes in the liver, heart, and skeletal muscles in mice. Presumably, the mouse strain used in the study (C57Bl/6J) or the age of the animals (10 months) could lead to results different from our findings. Additionally, in the studies performed in humans, a serum level of inhibitor of apoptosis—soluble cell-surface receptor that transduces apoptotic signals (sFas)—was higher in men compared with women (45). On the contrary, a stimulator of apoptosis—sFas ligand (sFasL)—and one of the components of apoptosome—cytochrome C—were lower (but without statistical significance) in men compared with women (45). Moreover, in the Sprague-Dawley rats, the antiapoptotic Bcl-2 level was increased in males compared with females under physiological circumstances (46). However, after cardiac ischemia-reperfusion, Bcl-2 decreased in males, and the apoptotic cell number was lower in females (46). Nevertheless, considering results of all previous studies together with the present findings, we believe that female sex could be considered the factor that may lead to the decrease of apoptotic intensity.
Numerous studies have consistently demonstrated clear sex differences in mice with global and local disruption of GH-induced signaling. For example, Berryman coworkers (47) reported significant differences based on sex for percent fat mass and absolute lean mass in GHRKO mice. Moreover, sex-specific alterations in the expression of xenobiotic metabolizing enzymes in mice with altered GH signaling were recently reported (48). In another study, total lean body mass was increased only in female FaGHRKOs compared with controls (28). Also, serum leptin and circulating interleukin-6 levels were changed only in female FaGHRKO mice (28). Similarly, in LiGHRKO mice, List coworkers (unpublished data) recently showed changes limited to female mice with hepatic GHR deletion (vs controls), such as increased local IGF-1 gene expression in subcutaneous and retroperitoneal white adipose tissue, increased circulating adipsin level, and decreased levels of circulating IGF-binding protein-5 (IGFBP-5) and IGFBP-7. All of the above-mentioned differences in the examined parameters between male and female mice are likely related to differences in GH secretion pattern. Namely, the plasma GH pattern in males is characterized by high GH pulses occurring with a specific periodicity (49). In contrast, GH secretion is less variable in females, with smaller GH pulses and higher interpulse levels (49). These alterations are presumably caused by sexually dimorphic network responses in pituitary GH (50).
In summary, LiGHRKO mice provide a very interesting experimental model for analyzing the role of liver-specific GH signaling (or lack thereof) in the control of physiological and pathophysiological processes, including apoptosis. It appears that the role of somatotrophic signaling in the regulation of these processes is much more complicated than once thought, and undoubtedly further studies are needed to determine all networks related to this crucial metabolic pathway. In this study, the expression of apoptosis-related genes is different between males and females in brains and kidneys but not in livers, and there is no effect of liver-specific knocking out of the Ghr gene. Therefore, while trying to explain the results of the present study, it is tempting to assume that sexual dimorphism may play an important role in the regulation of apoptosis in the circumstances of an altered somatotrophic signaling. However, the differences observed between particular tissues may suggest that there are still unknown mechanisms participating in the regulation of apoptosis, including, among others, GH-induced signaling and sex.
Funding
This work was supported by the National Institute on Aging (NIA) (AG031736, AG032290, AG19899) and Polish National Science Centre (DEC-2012/04/M/NZ4/00198) (507/1-107-05/507-10-050 of the Medical University of Lodz, Poland).
Conflict of Interest
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Acknowledgement
The authors would like to thank Amanda Bekoin for helping with the editing of the manuscript.
References
- 1. Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. 10.1080/01926230701320337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–290. 10.1146/annurev.cellbio.15.1.269 [DOI] [PubMed] [Google Scholar]
- 3. Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–731. dx..org/10.1016/j.ceb.2003.10.009 [DOI] [PubMed] [Google Scholar]
- 4. Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther. 2005;4:139–163. 10.4161/cbt.4.2.1508 [DOI] [PubMed] [Google Scholar]
- 5. Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. 10.1016/S0092-8674(01)00237-9 [DOI] [PubMed] [Google Scholar]
- 6. Srinivasula SM, Datta P, Fan XJ, Fernandes-Alnemri T, Huang Z, Alnemri ES. Molecular determinants of the caspase-promoting activity of Smac/DIABLO and its role in the death receptor pathway. J Biol Chem. 2000;275:36152–36157. 10.1074/jbc.C000533200 [DOI] [PubMed] [Google Scholar]
- 7. Henry-Mowatt J, Dive C, Martinou JC, James D. Role of mitochondrial membrane permeabilization in apoptosis and cancer. Oncogene. 2004;23:2850–2860. 10.1038/sj.onc.1207534 [DOI] [PubMed] [Google Scholar]
- 8. Zou H, Li Y, Liu X, Wang X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–11556. 10.1074/jbc.274.17.11549 [DOI] [PubMed] [Google Scholar]
- 9. Adrain C, Martin SJ. The mitochondrial apoptosome: a killer unleashed by the cytochrome seas. Trends Biochem Sci. 2001;26:390–397. 10.1016/S0968-0004(01)01844-8 [DOI] [PubMed] [Google Scholar]
- 10. Oda E, Ohki R, Murasawa H, et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–1058. 10.1126/science.288.5468.1053 [DOI] [PubMed] [Google Scholar]
- 11. Kruidering M, Evan GI. Caspase-8 in apoptosis: the beginning of “the end”? IUBMB Life. 2000;50:85–90. 10.1080/713803693 [DOI] [PubMed] [Google Scholar]
- 12. Coultas L, Strasser A. The role of the Bcl-2 protein family in cancer. Semin Cancer Biol. 2003;13:115–123. 10.1016/S1044-579X(02)00129-3 [DOI] [PubMed] [Google Scholar]
- 13. Zhou Y, Xu BC, Maheshwari HG, et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc Natl Acad Sci U S A. 1997;94:13215–13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. List EO, Sackmann-Sala L, Berryman DE, et al. Endocrine parameters and phenotypes of the growth hormone receptor gene disrupted (GHR-/-) mouse. Endocr Rev. 2011;32:356–386. 10.1210/er.2010-0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gesing A, Bartke A, Wang F, Karbownik-Lewinska M, Masternak MM. Renal pro-apoptotic proteins are reduced by growth hormone resistance but not by visceral fat removal. Biol Chem. 2011;392:475–481. 10.1515/BC.2011.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gesing A, Masternak MM, Wang F, Karbownik-Lewinska M, Bartke A. Deletion of growth hormone receptor gene but not visceral fat removal decreases expression of apoptosis-related genes in the kidney-potential mechanism of lifespan extension. Age (Dordr). 2012;34:295–304. 10.1007/s11357-011-9232-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gesing A, Masternak MM, Wang F, Lewinski A, Karbownik-Lewinska M, Bartke A. Decreased expression level of apoptosis-related genes and/or proteins in skeletal muscles, but not in hearts, of growth hormone receptor knockout mice. Exp Biol Med (Maywood). 2011;236:156–168. 10.1258/ebm.2010.010202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gesing A, Masternak MM, Lewinski A, Karbownik-Lewinska M, Kopchick JJ, Bartke A. Decreased levels of proapoptotic factors and increased key regulators of mitochondrial biogenesis constitute new potential beneficial features of long-lived growth hormone receptor gene-disrupted mice. J Gerontol A Biol Sci Med Sci. 2013;68: 639–651. 10.1093/gerona/gls231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shelke RR, Leeuwenburgh C. Lifelong caloric restriction increases expression of apoptosis repressor with a caspase recruitment domain (ARC) in the brain. FASEB J. 2003;17:494–496. 10.1096/fj.02-0803fje [DOI] [PubMed] [Google Scholar]
- 20. Gesing A, Masternak MM, Wang F, et al. Expression of key regulators of mitochondrial biogenesis in growth hormone receptor knockout (GHRKO) mice is enhanced but is not further improved by other potential life-extending interventions. J Gerontol A Biol Sci Med Sci. 2011;66:1062–1076. 10.1093/gerona/glr080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gesing A, Bartke A, Wang F, Karbownik-Lewinska M, Masternak MM. Key regulators of mitochondrial biogenesis are increased in kidneys of growth hormone receptor knockout (GHRKO) mice. Cell Biochem Funct. 2011;29:459–467. 10.1002/cbf.1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gesing A, Bartke A, Masternak MM, Lewiński A, Karbownik-Lewińska M. Decreased thyroid follicle size in dwarf mice may suggest the role of growth hormone signaling in thyroid growth regulation. Thyroid Res. 2012;5:7. 10.1186/1756-6614-5-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Junnila RK, List EO, Berryman DE, Murrey JW, Kopchick JJ. The GH/IGF-1 axis in ageing and longevity. Nat Rev Endocrinol. 2013;9:366–376. 10.1038/nrendo.2013.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fan Y, Menon RK, Cohen P, et al. Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J Biol Chem. 2009;284:19937–19944. 10.1074/jbc.M109.014308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mavalli MD, DiGirolamo DJ, Fan Y, et al. Distinct growth hormone receptor signaling modes regulate skeletal muscle development and insulin sensitivity in mice. J Clin Invest. 2010;120:4007–4020. 10.1172/JCI42447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu Y, Liu C, Sun H, et al. Growth hormone receptor regulates β cell hyperplasia and glucose-stimulated insulin secretion in obese mice. J Clin Invest. 2011;121:2422–2426. 10.1172/JCI45027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vijayakumar A, Wu Y, Sun H, et al. Targeted loss of GHR signaling in mouse skeletal muscle protects against high-fat diet-induced metabolic deterioration. Diabetes. 2012;61:94–103. 10.2337/db11-0814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. List EO, Berryman DE, Funk K, et al. The role of GH in adipose tissue: lessons from adipose-specific GH receptor gene-disrupted mice. Mol Endocrinol. 2013;27:524–535. 10.1210/me.2012-1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, et al. Effects of caloric restriction on insulin pathway gene expression in the skeletal muscle and liver of normal and long-lived GHR-KO mice. Exp Gerontol. 2005;40:679–684. 10.1016/j.exger.2005.06.003 [DOI] [PubMed] [Google Scholar]
- 30. Dhahbi J, Li X, Tran T, Masternak MM, Bartke A. Circulating blood leukocyte gene expression profiles: effects of the Ames dwarf mutation on pathways related to immunity and inflammation. Exp Gerontol. 2007;42:772–788. 10.1016/j.exger.2007.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kennedy MA, Rakoczy SG, Brown-Borg HM. Long-living Ames dwarf mouse hepatocytes readily undergo apoptosis. Exp Gerontol. 2003;38:997–1008. 10.1016/S0531-5565(03)00164-5 [DOI] [PubMed] [Google Scholar]
- 32. Berryman DE, List EO, Coschigano KT, Behar K, Kim JK, Kopchick JJ. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm IGF Res. 2004;14:309–318. 10.1016/j.ghir.2004.02.005 [DOI] [PubMed] [Google Scholar]
- 33. Masternak MM, Bartke A, Wang F, et al. Metabolic effects of intra-abdominal fat in GHRKO mice. Aging Cell. 2012;11:73–81. 10.1111/j.1474-9726.2011.00763.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3:70ra13. 10.1126/scitranslmed.3001845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Siegel C, Li J, Liu F, Benashski SE, McCullough LD. miR-23a regulation of X-linked inhibitor of apoptosis (XIAP) contributes to sex differences in the response to cerebral ischemia. Proc Natl Acad Sci U S A. 2011;108:11662–11667. 10.1073/pnas.1102635108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ren J. Influence of gender on oxidative stress, lipid peroxidation, protein damage and apoptosis in hearts and brains from spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 2007;34:432–438. 10.1111/j.1440-1681.2007.04591.x [DOI] [PubMed] [Google Scholar]
- 37. Jung HS, Park SW, Hwang SN, et al. Gender differences in expression of apoptosis, p53, and bcl-2 in delayed focal cerebral infarction in rats. Korean J Pathol. 2000;34:1–10. [Google Scholar]
- 38. Huang C, Gu H, Zhang W, Herrmann JL, Wang M. Testosterone-down-regulated Akt pathway during cardiac ischemia/reperfusion: a mechanism involving BAD, Bcl-2 and FOXO3a. J Surg Res. 2010;164:e1–11. 10.1016/j.jss.2010.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dent MR, Tappia PS, Dhalla NS. Gender differences in apoptotic signaling in heart failure due to volume overload. Apoptosis. 2010;15:499–510. 10.1007/s10495-009-0441-8 [DOI] [PubMed] [Google Scholar]
- 40. Hofmann-Lehmann R, Holznagel E, Lutz H. Female cats have lower rates of apoptosis in peripheral blood lymphocytes than male cats: correlation with estradiol-17beta, but not with progesterone blood levels. Vet Immunol Immunopathol. 1998;65:151–160. 10.1016/S0165-2427(98)00150-0 [DOI] [PubMed] [Google Scholar]
- 41. Le May C, Chu K, Hu M, et al. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci U S A. 2006;103:9232–9237. 10.1073/pnas.0602956103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Straface E, Gambardella L, Brandani M, Malorni W. Sex differences at cellular level: “cells have a sex”. Handb Exp Pharmacol. 2012;214:49–65. 10.1007/978-3-642-30726-3_3 [DOI] [PubMed] [Google Scholar]
- 43. Faisal M, Kim H, Kim J. Sexual differences of imprinted genes’ expression levels. Gene. 2014;533:434–438. 10.1016/j.gene.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sanz A, Hiona A, Kujoth GC, et al. Evaluation of sex differences on mitochondrial bioenergetics and apoptosis in mice. Exp Gerontol. 2007;42:173–182. 10.1016/j.exger.2006.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kavathia N, Jain A, Walston J, Beamer BA, Fedarko NS. Serum markers of apoptosis decrease with age and cancer stage. Aging (Albany NY). 2009;1:652–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen C, Hu LX, Dong T, et al. Apoptosis and autophagy contribute to gender difference in cardiac ischemia-reperfusion induced injury in rats. Life Sci. 2013;93:265–270. 10.1016/j.lfs.2013.06.019 [DOI] [PubMed] [Google Scholar]
- 47. Berryman DE, List EO, Palmer AJ, et al. Two-year body composition analyses of long-lived GHR null mice. J Gerontol A Biol Sci Med Sci. 2010;65:31–40. 10.1093/gerona/glp175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li X, Bartke A, Berryman DE, et al. Direct and indirect effects of growth hormone receptor ablation on liver expression of xenobiotic metabolizing genes. Am J Physiol Endocrinol Metab. 2013;305:E942–E950. 10.1152/ajpendo.00304.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. MacLeod JN, Pampori NA, Shapiro BH. Sex differences in the ultradian pattern of plasma growth hormone concentrations in mice. J Endocrinol. 1991;131:395–399. [DOI] [PubMed] [Google Scholar]
- 50. Sanchez-Cardenas C, Fontanaud P, He Z, et al. Pituitary growth hormone network responses are sexually dimorphic and regulated by gonadal steroids in adulthood. Proc Natl Acad Sci U S A. 2010;107:21878–21883. 10.1073/pnas.1010849107 [DOI] [PMC free article] [PubMed] [Google Scholar]