Abstract
Background.
Multimorbidity increases with aging, but risk factors beyond age are unknown.
Objective.
To investigate the association of inflammatory and anabolic hormonal biomarkers with presence and prospective development of multimorbidity.
Methods.
Nine-year longitudinal study of 1018 participants aged 60 years or older (InCHIANTI Study). Multimorbidity was evaluated at baseline and follow-up visits as number of diagnosed diseases from a predefined list of 15 candidate chronic conditions, defined according to standard clinical criteria. Linear mixed models were used to test cross-sectional and longitudinal associations between candidate biomarkers and multimorbidity.
Results.
At baseline, multimorbidity was significantly higher in older participants (p < .001) and higher IL-6, IL-1ra, TNF-α receptor II (TNFAR2), and lower dehydroepiandrosterone sulfate were associated with higher number of diseases, independent of age, sex, body mass index, and education. The rate of longitudinal increase in number of chronic diseases was significantly steeper in participants who were older at baseline (p < .001). In addition, higher baseline IL-6 and steeper increase of IL-6 levels were significantly and independently associated with a steeper increase in multimorbidity over time (p < .001 and p = .003, respectively). Sensitivity analyses, performed using 15 different models obtained by removing each of 15 conditions included in the original list of candidate diseases, confirmed that results were not driven by any specific condition.
Conclusions.
Accumulation of chronic diseases accelerates at older ages and in persons with higher baseline levels and steeper increase over time of IL-6. High IL-6 and increase in IL-6 may serve as early warning sign to better target interventions aimed at reducing the burden of multimorbidity.
Key Words: Multimorbidity, Inflammation, Interleukin-6, Aging, Chronic diseases.
The number of older persons affected by multiple chronic diseases is progressively increasing and caring for them poses a number of scientific and organizational challenges for health care systems around the world (1–3). The coexistence of multiple diseases in the same person is usually referred to as multi morbidity; if one condition is the focus, then the term comorbidity is more often used (4). Because aging is the strongest risk factor for many chronic diseases, including cardiovascular diseases, type 2 diabetes, cancer, and dementia, multimorbidity is considered an important landmark of poor health status in older people, resulting recently increasing interest among gerontology and clinical geriatric researchers in this topic (5). Moreover, it is well-established that multimorbidity increases with age and, independent of age, it is strongly associated with frailty, disability, hospitalization, and mortality (6). However, little is known about risk factors for multimorbidity beyond age. Understanding the nature of such risk factors may shed light on the mechanisms by which some individuals tend to develop multiple and apparently unrelated chronic diseases as they age (7). Epidemiological and clinical studies have found that older persons often show a “low-grade chronic proinflammatory state” (8) and a “multiple hormonal dysregulation” (9) characterized by high levels of serum cytokines and low levels of anabolic hormones, respectively. Both conditions are risk factors for chronic diseases and predict a variety of adverse health outcomes, including frailty, disability, and mortality. Thus, it is reasonable to hypothesize that individuals with chronic inflammation and/or hormonal dysregulation are more likely to be affected by or to develop multimorbidity. Yet, this hypothesis has not been formally tested.
This study aims to investigate the relationship of levels of inflammatory markers and anabolic hormones with multimorbidity in the participants of the InCHIANTI study to identify cross-sectional correlates and predictors of future development of multimorbidity over a 9-year follow-up.
Methods
Participants
The present analysis used data from the Invecchiare in Chianti (Aging in the Chianti Area, InCHIANTI) study, a longitudinal population-based study of older people living in the Chianti area, Tuscany, Italy. The study was designed to identify factors contributing to mobility decline and disability in older persons. A detailed description of the sampling procedures and data collection methods has been previously published (10). In brief, participants were randomly selected from the city registries of Greve in Chianti and Bagno a Ripoli using a multistage sampling method. Baseline data were collected in 1998–2000; the 3-year follow-up took place in 2001–2003, the 6-year follow-up in 2004–2006, and the 9-year follow-up in 2007–2009. The Italian National Research Council on Aging (INRCA) Ethical Committee ratified the entire study protocol and participants provided written consent to participate.
Of the 1203 participants at least 60 years old at enrollment, 1,018 participants attended at least one visit over the follow-up period and were included in the analysis presented here. Of these, 683 participants were still alive and provided data at the 9-year follow-up.
Multimorbidity
Both at baseline and at follow-up visits, multimorbidity was evaluated as number of diagnosed diseases, which affect each participant, from a predefined list of candidate chronic conditions (see as in what follows).
Chronic Diseases
At baseline and follow-ups we ascertained the presence of 15 chronic conditions (11), predefined in the study design and characterized by high prevalence and high risk of disability in older adults. Most of them (hypertension, diabetes, ischemic heart disease, congestive heart failure, stroke, chronic obstructive pulmonary disease, cancer, Parkinson’s disease, hip fracture, and lower extremities joint disease) were defined using standard criteria that combined information from self-reported medical history, medication use, medical documents, and a clinical medical examination. In addition, anemia was defined as hemoglobin <12g/dL in women and <13g/dL in men (12); chronic kidney disease was defined as glomerular filtration rate estimated using the Cockroft–Gault equation <30mL/L (although a threshold of 60mL/L is usually considered indicative of chronic kidney disease, we selected a lower cutoff because it has been shown that in older populations the Cockroft–Gault equation constantly underestimates the true glomerular filtration rate) (13); peripheral arterial disease was defined as ankle-brachial index measured by Doppler stethoscope <0.9 (14); cognitive impairment was defined as age and education corrected-Mini Mental State Examination score <24 and depression was defined as a score of 20 or greater of the Center for Epidemiological Studies-Depression Scale (15).
Laboratory Measures
For all the tested inflammatory markers and anabolic hormones, laboratory measures used in this analysis were performed in serum samples collected at baseline visit in 1998. For IL-6, measures performed in serum samples collected at follow-up visits were used in the present analysis.
Inflammatory markers.—
Interleukin (IL)-6, IL-1β, IL-1 receptor antagonist (IL-1ra), and IL-10 were measured in duplicate by high-sensitivity enzyme-linked immunoabsorbent assays (BIOSOURCE, international, Camarillo, CA). TNF-α was measured using multiplex technology (Human Serum Adipokine Panel B LINCOplex kit; Linco Research, Inc., St Charles, MO). Interleukin-18, soluble TNF-α receptor I (sTNFAR1), and II (sTNFAR2) were measured using an ultra-sensitive quantitative sandwich enzyme-linked immunoassay (ELISA) (R&D Systems, Inc, Minneapolis, MN). An IL-15 was measured using a chemiluminescent immunoassay (Human IL-15 Chemiluminescent Immunoassay, R&D Systems). High-sensitivity C reactive protein (CRP) was measured by ELISA and colorimetric competitive immunoassay that uses purified protein and polyclonal anti-CRP antibodies (Calbiochem, San Diego, CA). Assay precisions and detectable limits for these measures were reported earlier (16,17).
Based on previous literature, “high” IL-6 was defined as equal to or higher than 3.5 pg/mL and “high” CRP as equal to or higher than 3 µg/mL. Because clinically relevant thresholds for the other inflammatory markers are not available, we used the upper tertile to define “high” levels.
Hormones.—
Dehydroepiandrosterone sulfate (DHEAS), total insulin-like growth factor-1 (IGF-1), and total testosterone concentrations were measured in duplicate by immunoradiometric assays, using commercial reagents (Diagnostic Systems Laboratories Corporation, Webster, TX). Concentrations of bioavailable testosterone (serum-free and albumin-bound testosterone, but not sex hormone-binding globulin) were calculated using the Vermeulen formula. Estradiol levels were measured in the Laboratory of the University of Parma using ultrasensitive RIA (DSL-4800, Chematil, Angri (SA), Italy). Assay precisions and detectable limits for these measures were reported previously (18,19).
Because all of these hormones decline with aging and low levels have been associated with adverse outcomes (9), “low” levels of anabolic hormones were defined as values lower than the sex-specific threshold of the lower tertile in the analyzed population.
Covariates
Body mass index (BMI) was calculated as measured weight in kilograms divided by measured height in meters squared (kg/m2). Education was expressed as years of school attendance.
Statistical Analysis
The baseline characteristics of the sample were reported as mean ± standard deviation (SDs), median, and interquartile range (IQR) or percentage according to different number of diseases and compared using age, sex, and BMI adjusted linear and logistic regression models (Table 1). Variables with extremely skewed distribution were log-transformed before the analysis. Linear mixed models were performed to test the association of baseline age and baseline values of biomarkers, dichotomized in “high” and “low” as described earlier, with higher cross-sectional number of diseases and steeper increase in number of diseases over the follow-up. Linear mixed models were also used to test the hypothesis that, independent of baseline IL-6, a faster increase over the follow-up in IL-6 would predict a steeper increase in number of diseases over the same time frame. Finally, sensitivity analyses were performed using 15 different models, obtained by removing each time one of 15 conditions included in the original list of candidate diseases. Results from sensitivity analyses were summarized as median and IQR of β coefficients and p values across the 15 regression models.
Table 1.
Baseline Characteristics of the Study Population (n = 1018) According to the Different Number of Chronic Diseases (InCHIANTI Study, 1998–2000)
| Number of Chronic Diseases | p Value | ||||
|---|---|---|---|---|---|
| No Disease (n = 213) | One Disease (n = 347) | Two or Three Diseases (n = 364) | Four or more Diseases (n = 94) | ||
| Age (y), mean (SD) | 70.7 (5.9) | 72.3 (6.9) | 75.4 (7.2) | 78.1 (7.2) | <.001* |
| Female sex, % | 45.5 | 57.1 | 61.4 | 63.8 | .002† |
| BMI (kg/m2), mean (SD) | 27.5 (3.5) | 27.2 (4.1) | 27.4 (4.0) | 28.6 (4.9) | .019‡ |
| Education (y), median (IQR) | 5.0 (4–7) | 5.0 (4–6) | 5.0 (3–5) | 4.5 (3–5) | .018§ |
| Inflammatory markers | |||||
| IL-6 (pg/mL), median (IQR) | 2.3 (1.8–3.4) | 2.7 (1.9–3.6) | 3.0 (2.0–4.1) | 3.9 (2.8–5.1) | <.001§ |
| IL-6 ≥ 3.5 pg/mL, % | 23.4 | 28.8 | 33.6 | 58.0 | <.001|| |
| CRP (µg/mL), median (IQR) | 2.4 (1.0–4.9) | 2.2 (1.9–4.7) | 2.7 (1.4–5.4) | 3.9 (1.9–9.8) | .003§ |
| CRP ≥ 3 µg/mL, % | 44.4 | 38.6 | 46.6 | 57.4 | .089|| |
| IL-1RA (pg/mL), median (IQR) | 125.8 (89.2–167.5) | 126.0 (94.1–169.7) | 136.8 (98.0–186.8) | 160.3 (118.1–223.4) | <.001§ |
| IL-1RA ≥ 161.92 pg/mL, % | 26.0 | 32.2 | 34.5 | 48.9 | <.001|| |
| IL-18 (pg/mL), median (IQR) | 378.6 (295.3–465.6) | 369.2 (287.9–453.6) | 376.5 (299.6–474.6) | 441.7 (346.1–553.8) | .009§ |
| IL-18 ≥ 2864.8 pg/mL, % | 29.4 | 29.7 | 33.5 | 53.2 | .002|| |
| TNFAR1 (pg/mL), median (IQR) | 1242.3 (1006.9–1526.6) | 1226.1 (1021.2–1531.5) | 1365.1 (1123.0–1677.6) | 1884.9 (1330.6–2373.6) | <.001§ |
| TNFAR1 ≥ 1514.9 pg/mL, % | 25.1 | 26.4 | 36.4 | 62.6 | <.009|| |
| TNFAR2 (pg/mL), median (IQR) | 2459.8 (2156.7–2771.2) | 2465.3 (2170.7–2924.7) | 2631.5 (2297.0–3099.6) | 3210.2 (2646.9–3715.9) | .001§ |
| TNFAR2 ≥ 2664.8 pg/mL, % | 20.7 | 27.3 | 37.6 | 63.7 | <.001|| |
| Hormones | |||||
| DHEAS (µg/dL), Median (IQR) | 84.2 (49.6–142.4) | 71.1 (43.3–115.4) | 58.8 (32.8–104.5) | 54.6 (33.5–89.3) | .001§ |
| DHEAS < 53.9 µg/dL for men and <44.7 µg/dL for women, % | 26.0 | 29.1 | 38.8 | 40.2 | .008|| |
Notes: BMI = body mass index; DHEAS = dehydroepiandrosterone sulfate; IL = interleukin; IQR = interquartile range; SD = standard deviation; TNFAR1 = TNF-α receptor I; TNFAR2 = TNF-α receptor II.
*p trend from sex and BMI adjusted linear regression model.
† p trend from age and BMI adjusted logistic regression model.
‡ p trend from age and sex adjusted linear regression model.
§ p trend from age, sex and BMI adjusted from linear regression using log-transformed variables (then back-transformed for data presentation).
|| p trend from age, sex and BMI adjusted logistic regression.
All analyses were performed using the SAS statistical package, version 9.3 (SAS institute Inc., Cary, NC).
Results
Baseline Characteristics of the Population
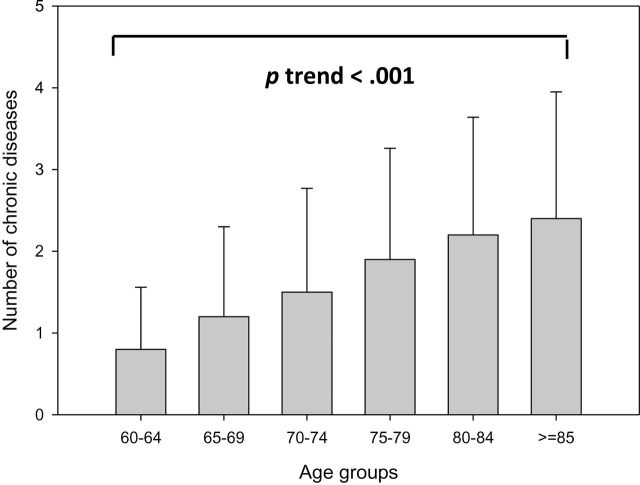
The baseline population included 1,018 participants, with mean age 73.6±7.2. Of these, 582 (57.2%) were women. The average number of chronic diseases increased with age (p < .001, Figure 1). In the univariate analyses, older age, female sex, higher BMI, and lower education were associated with higher multimorbidity (Table 1). After adjusting for age, sex, and BMI, high IL-6, CRP, IL-1RA, IL-18, TNFAR1, TNFAR2, and low DHEAS were each significantly associated with higher multimorbidity. Similar results, with the exception for CRP, were obtained when biomarkers were dichotomized as “high” versus “low” (see Method section). Other inflammatory markers (IL-1B, IL-10, IL-15, and TNFA) and anabolic hormones (total and bioavailable testosterone, estradiol, and IGF-1) were not associated with multimorbidity.
Figure 1.
Crude mean number of chronic diseases according to different age groups in the baseline population (InCHIANTI Study, 1998–2000).
Longitudinal Changes in Number of Chronic Diseases
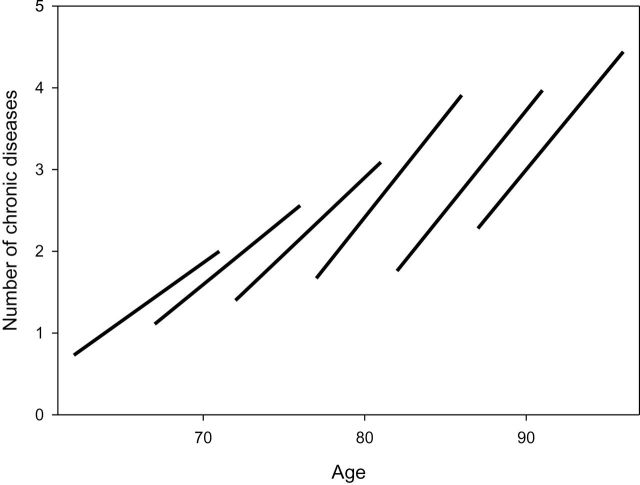
Intraindividual changes in average number of chronic diseases, limited to participants followed through the 9 year-follow-up (n = 683), are shown in Figure 2. Observing the crude data, it was evident that multimorbidity increased with aging in all age groups, but the rate of change was steeper in those who were older at baseline. A formal analysis performed on the entire study population using a linear mixed model confirmed the initial impression by showing a significant “age × time” interaction (p <.001) (see Model I in Table 2 and Supplementary Figure).
Figure 2.
Crude mean number of chronic diseases at baseline and at 9-year follow-up visit for participants with available data at 9-years follow-up (n = 683) and derived average trajectories of multimorbidity in different baseline age groups (InCHIANTI Study, 1998–2000, 2007–2009). The rough impression given by crude data that the increase in multimorbidity was steeper at older baseline ages was confirmed by liner mixed models (Model I, Table 2 and Supplementary Figure).
Table 2.
Linear Mixed Models Testing the Association of Baseline Age and Baseline Biomarkers With Cross-sectional Multimorbidity and Longitudinal Increase in Number of Chronic Diseases Over the Follow-up (InCHIANTI Study: 1998–2000; 2001–2003; 2004–2006; 2007–2009)
| Number of Chronic Diseases | ||||||
|---|---|---|---|---|---|---|
| Model I | Model II | Sensitivity Analysis | ||||
| β (SE) | p Value | β (SE) | p Value | β Median (IQR) | p Value Median (IQR) | |
| Baseline age (y) | 0.059 (0.006) | <.001 | 0.05 (0.01) | <.001 | 0.05 (0.05–0.05) | <.001 |
| Women, % | 0.18 (0.08) | .032 | 0.19 (0.09) | .026 | 0.19 (0.14–0.22) | .028 (0.007–0.066) |
| Education, y | −0.04 (0.01) | .001 | −0.04 (0.01) | .002 | −0.04 (0.04–0.04) | .001 (0.001–0.002) |
| Time | 0.21 (0.01) | <.001 | 0.18 (0.01) | <.001 | 0.17 (0.16–0.17-0.09) | <.001 |
| IL-6 ≥ 3.5 pg/mL | 0.26 (0.09) | .006 | 0.25 (0.23–0.26) | .008 (0.005–0.011) | ||
| IL-1RA ≥ 161.92 pg/mL | 0.28 (0.09) | .002 | 0.27 (0.25–0.29) | .003 (0.001–0.006) | ||
| TNFAR2 ≥ 2664.8 pg/mL | 0.33 (0.09) | <.001 | 0.31 (0.29–0.32) | .001 (0.001–0.002) | ||
| DHEAS < 53.9 µg/dL (men), and <44.7 µg/dL (women) | 0.27 (0.09) | .002 | 0.27 (0.24–0.27) | .003 (0.002–0.006) | ||
| Baseline age (y) × time | 0.008 (0.001) | <.001 | 0.008 (0.001) | <.001 | 0.007 (0.007–0.008) | <.001 |
| IL-6 ≥ 3.5 pg/mL × time | 0.06 (0.01) | <.001 | 0.06 (0.05–0.06) | <.001 | ||
Notes: DHEAS = dehydroepiandrosterone sulfate; IL = interleukin; IQR = interquartile range; SE = standard error; TNFAR2 = TNF-α receptor II. High baseline levels of IL-6 were associated with a steeper increase in multimorbidity overtime (p value of interaction “IL-6 × time” < .001).
Association of Baseline Levels of Inflammatory Markers and Hormones With Baseline Multimorbidity and Longitudinal Changes in Number of Chronic Diseases
Further analyses were performed to test whether baseline levels of candidate inflammatory and hormonal biomarkers were independently associated with cross-sectional multimorbidity and longitudinal changes in number of chronic diseases over the follow-up. All biomarkers significantly associated with multimorbidity at the univariate analysis were tested in a linear mixed model adjusted for covariates (age, sex, education, and BMI) (shown in Supplementary Material) and, from this fully saturated model, a parsimonious model that included only variables with p value < .05 was derived (Model II in Table 2). In Model II, independent of age, sex, and education, baseline high IL-6, IL-1RA, TNFAR2, and low DHEAS (dichotomized as described earlier) were significantly associated with higher baseline multimorbidity. In addition, the interaction “IL-6 × time” was highly significant, meaning that individuals with higher levels IL-6 at baseline experienced a steeper increase in multimorbidity with age compared with those with normal values. Particularly, persons with higher baseline levels of IL-6 presented an average higher increase in number of diseases of about 0.06 per year (p < .001).
Association Between Longitudinal Increase in IL-6 Levels and Longitudinal Increase in Number of Chronic Diseases
Finally, we used linear mixed models to test the hypothesis that, independent of baseline IL-6, the rate increase over time in IL-6 would predict the rate of increase in multimorbidity over the same time frame. We found that a steeper increase of IL-6 was associated with a steeper increase in number of chronic diseases over the follow-up, after adjusting for age, sex, and education (p < .001). The association was still statistically significant after adjusting for baseline values of IL-6 (p = .003).
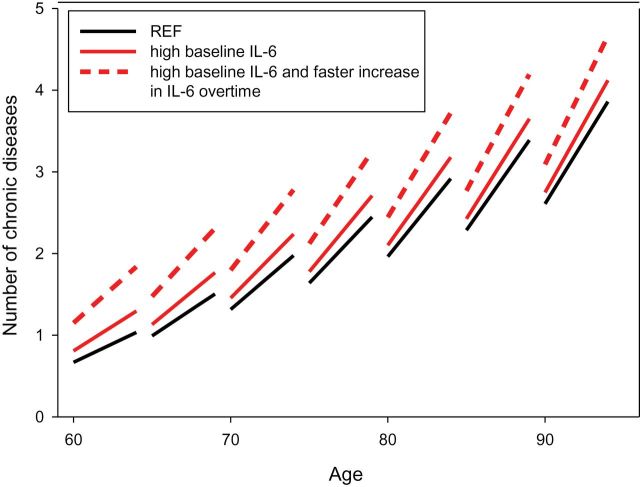
Figure 3 summarizes the “accelerative” effect of higher baseline IL-6 and steeper increase in IL-6 levels over time on the rate of longitudinal increase in number of chronic diseases. Particularly, the sample population was categorized according to high versus low baseline levels of IL-6 and upper tertile versus medium-lower tertiles of rate of increase in IL-6 overtime. Figure 3 shows that participants with high baseline levels and faster increase overtime in IL-6 presented a significant steeper increase in number of chronic diseases over the follow-up compared with those with high baseline levels but slower increase in IL-6 overtime (p = .019) and those with low baseline levels of IL-6 (reference group, REF, p < .001).
Figure 3.
Linear mixed model estimated trajectories of longitudinal increase in number of chronic diseases over the follow-up in different baseline age groups, according to baseline values of IL-6 (high vs low) and rate of increase in IL-6 levels (upper tertile vs medium-lower). Participants with high baseline levels and faster increase overtime in IL-6 (dashed red line) presented a significant steeper increase in number of chronic diseases over the follow-up compared with those with high baseline levels but slower increase in IL-6 overtime (solid red line, p = .019) and those with low baseline levels of IL-6 (reference group (REF), dark line, p < .001) (InCHIANTI Study: 1998–2000; 2001–2003; 2004–2006; 2007–2009).
Sensitivity Analysis
Sensitivity analyses were also performed. In particular, we fitted 15 different models, each one based on a new value of number of diseases recalculated by eliminating one by one each of the 15 conditions included in the originallist of candidate diseases. The results, presented as median and IQR of β coefficients and p values (Table 2) across the 15 regression models, were consistent with the original analysis, suggesting that the results were not driven by a specific disease.
Discussion
Aging is by far the strongest risk factor for many chronic diseases, many of which have different pathophysiology. As a result, the prevalence of multimorbidity increases dramatically with age (20–27). Our study contributes to this literature by showing that the rise of multimorbidity with age is not linear but rather significantly accelerates at older ages. In addition, age-related proinflammatory state and decline in DHEAS are associated with higher cross-sectional multimorbidity and higher baseline IL-6 and steeper increase of IL-6 predict accelerated rise of multimorbidity with aging.
High baseline levels of IL-6, TNFAR2, IL-1RA or low baseline levels of DHEAS were associated with higher multimorbidity than participants with normal levels of the same markers. Moreover, individuals with baseline levels of IL-6 equal or higher than 3.5 pg/mL were more likely to experience a steeper increase in multimorbidity over the follow-up compared with those with normal values. In addition, independent of baseline IL-6, a steeper increase in IL-6 levels over the time of the study was associated with a steeper increase in number of chronic diseases.
These findings are consistent with the literature showing that the clustering of chronic diseases in some older individuals exceeds that expected from the effect of age alone (28).We recognize that cause–effect relationships cannot be fully inferred from epidemiological studies. However, the strength, consistency, and time relationship between IL-6 levels and multimorbidity provide strong support to the notion that chronic inflammation is a predisposing factor to the development of multiple diseases with aging.
Few studies have examined longitudinally the development of multimorbidity. Although Van den Akker and coworkers (21) found older age linearly associated with a higher incidence of multimorbidity, their observation was limited to a 1-year follow-up. Building on this observation, our findings based on a relatively large population, objective clinical findings, and a 9-year follow-up clearly show that the association of age and multimorbidity is not linear. As a consequence, the effect of age as risk factor for multimorbidity derived from cross-sectional data likely underestimates the true effect of age observed longitudinally. Such underestimation is probably due to individuals with high multimorbidity being removed from the population because of mortality, or because they are less likely to participate in follow-up visits (Figure 2).
A mild, chronic proinflammatory state is a typical phenotype associated with older age, and has been implicated in the pathogenesis of many age-related diseases, including cardiovascular diseases, diabetes, anemia, cancer, pulmonary diseases, osteoporosis, arthritis, Parkinsonism, and dementia (29–32). Many hypotheses raised to explain the mild proinflammatory status of aging include a dysregulation of the NF-kB pathway, impaired mitochondrial function, and impaired mitophagy leading to excessive production of reactive oxygen species, and accumulation of senescent cells with age can all lead to higher vulnerability to chronic diseases development and faster disease progression (7,30). In addition, the age-related increase in serum levels of inflammatory markers is highly correlated with obesity, insulin resistance, sarcopenia, osteopenia, and neurodegeneration. This is consistent with multiple studies showing that elevated levels of inflammatory markers predict increased risk of adverse health outcomes such as disability, hospitalization, and mortality in older adults (33–36).
Levels of DHEAS and other anabolic hormones decline significantly with aging (37) and are associated with a higher risk of frailty (38) and mortality (19,39), and inversely related to inflammatory markers. This evidence suggests that the development of multiple chronic diseases, including those apparently not related to each other, could be a consequence of the same biological processes that characterize normal aging (ie, inflammation and hormonal dysregulation). The involvement of chronic inflammation as a common pathway in the development of some clusters of comorbidities referred to a specific index condition (ie, chronic obstructive pulmonary disease) has been previously demonstrated (40). Yet, the current study documents that age-related inflammation and decline in DHEAS are indicative of overall multimorbidity in the elderly. Moreover, higher baseline levels of IL-6 and a steeper increase in IL-6 levels over time are associated with an accelerated development of multimorbidity with aging. Consistent with our findings and using data from 3044 middle-aged London civil servants followed over 10 years, Akbaraly and coworkers (41) recently found that higher IL-6 levels (>2.0ng/L) were associated with half the odds ratio (OR: 0.53, CI: 0.38–0.74) of successful aging, defined as being free of major chronic disease and with optimal physical, mental, and cognitive functioning.
Our study has the important limitation that we did not consider information on disease severity in evaluating multimorbidity. However, we used strict thresholds for defining diseases to minimize the chance of overdiagnoses and severity classification systems that homogeneously quantify the impact of diseases on health are not currently available. This is probably why our numeric estimates of multimorbidity tend to be lower than those reported by other studies (42,43).
In conclusion, our study demonstrates that the association of aging with a higher risk of multimorbidity is nonlinear and even stronger than that was suggested earlier from cross-sectional analyses. High IL-6 and increase in IL-6 over time are early susceptibility signs to impending and evolving multimorbidity and may be used to target older individuals for interventions aimed at reducing the burden of multimorbidity.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org
Funding
The InCHIANTI study baseline (1998–2000) was supported as a “targeted project” (ICS110.1/RF97.71) by the Italian Ministry of Health and in part by the U.S. National Institute on Aging (contracts: 263 MD 9164 and 263 MD 821336); the InCHIANTI Follow-up 1 (2001–2003) was funded by the U.S. National Institute on Aging (contracts: N.1-AG-1-1 and N.1-AG-1-2111); the InCHIANTI Follow-ups 2 and 3 studies (2004–2010) were financed by the U.S. National Institute on Aging (contract: N01-AG-5-0002); supported in part by the Intramural Research Program of the National Institute on Aging, National Institutes of Health, Baltimore, Maryland. Dr. C.M.B. was supported by the Paul Beeson Career Development Award Program (NIA K23 AG032910, AFAR, The John A. Hartford Foundation, The Atlantic Philanthropies, The Starr Foundation, and an anonymous donor).
Supplementary Material
Acknowledgments
Dr. E.F. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1. Parekh AK, Barton MB. The challenge of multiple comorbidity for the US health care system. JAMA. 2010;303:1303–1304 doi:10.1370/afm.1391 [DOI] [PubMed] [Google Scholar]
- 2. American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. Guiding principles for the care of older adults with multimorbidity: an approach for clinicians. J Am Geriatr Soc. 2012;13:E1–E25 doi:10.1111/j.1532-5415.2012.04188.x. Epub 2012 Sep 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition–multimorbidity. JAMA. 2012;307:2493–2494 doi:10.1001/jama.2012.5265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van den Akker M, Buntinx F, Knottnerus JA. Comorbidity or multimorbidity: what’s in a name? A review of literature. Eur J Gen Pract. 1996;2:65–70. [Google Scholar]
- 5. Burch JB, Augustine AD, Frieden LA, et al. Advances in geroscience: impact on healthspan and chronic disease. J Gerontol A Biol Sci Med Sci. 2014;69(suppl 1):S1–S3 doi:10.1093/gerona/glu041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gijsen R, Hoeymans N, Schellevis FG, Ruwaard D, Satariano WA, van den Bos GA. Causes and consequences of comorbidity: a review. J Clin Epidemiol. 2001;54:661–674. [DOI] [PubMed] [Google Scholar]
- 7. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(suppl 1):S4–S9 doi:10.1093/gerona/glu057 [DOI] [PubMed] [Google Scholar]
- 8. Ferrucci L, Corsi A, Lauretani F, et al. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maggio M, Cattabiani C, Lauretani F, et al. The concept of multiple hormonal dysregulation. Acta Biomed. 2010;81:19–29. [PMC free article] [PubMed] [Google Scholar]
- 10. Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. [DOI] [PubMed] [Google Scholar]
- 11. Wallace RB, Salive ME. The dimensions of multiple chronic conditions: where do we go from here? A commentary on the special issue of preventing chronic disease. Prev Chronic Dis. 2013;10:E59 doi: 10.5888/pcd10.130104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Woodman R, Ferrucci L, Guralnik J. Anemia in older adults. Curr Opin Hematol. 2005;12:123–128. [DOI] [PubMed] [Google Scholar]
- 13. Lamb EJ, Wood J, Stowe HJ, O’Riordan SE, Webb MC, Dalton RN. Susceptibility of glomerular filtration rate estimations to variations in creatinine methodology: a study in older patients. Ann Clin Biochem. 2005;42(Pt 1):11–18. [DOI] [PubMed] [Google Scholar]
- 14. McDermott MM, Fried L, Simonsick E, Ling S, Guralnik JM. Asymptomatic peripheral arterial disease is independently associated with impaired lower extremity functioning: the women’s health and aging study. Circulation. 2000;101:1007–1012. [DOI] [PubMed] [Google Scholar]
- 15. Milaneschi Y, Bandinelli S, Penninx BW, et al. The relationship between plasma carotenoids and depressive symptoms in older persons. World J Biol Psychiatry. 2012;13:588–598 doi: 10.3109/15622975.2011.597876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. [DOI] [PubMed] [Google Scholar]
- 17. Varadhan R, Yao W, Matteini A, et al. Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. J Gerontol A Biol Sci Med Sci. 2014;69:165–173 doi:10.1093/gerona/glt023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maggio M, Lauretani F, Ceda GP, et al. Relationship between low levels of anabolic hormones and 6-year mortality in older men: the aging in the Chianti Area (InCHIANTI) study. Arch Intern Med. 2007;167:2249–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maggio M, Ceda GP, Lauretani F, et al. SHBG, sex hormones, and inflammatory markers in older women. J Clin Endocrinol Metab. 2011;96:1053–1059 doi:10.1210/jc.2010-1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schellevis FG, van der Velden J, van de Lisdonk E, van Eijk JT, van Weel C. Comorbidity of chronic diseases in general practice. J Clin Epidemiol. 1993;46:469–473. [DOI] [PubMed] [Google Scholar]
- 21. van den Akker M, Buntinx F, Metsemakers JF, Roos S, Knottnerus JA. Multimorbidity in general practice: prevalence, incidence, and determinants of co-occurring chronic and recurrent diseases. J Clin Epidemiol. 1998;51:367–375. [DOI] [PubMed] [Google Scholar]
- 22. Walker AE. Multiple chronic diseases and quality of life: patterns emerging from a large national sample, Australia. Chronic Illn. 2007;3:202–218. [DOI] [PubMed] [Google Scholar]
- 23. Marengoni A, Winblad B, Karp A, Fratiglioni L. Prevalence of chronic diseases and multimorbidity among the elderly population in Sweden. Am J Public Health. 2008;98:1198–1200 doi:10.2105/AJPH.2007.121137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Uijen AA, van de Lisdonk EH. Multimorbidity in primary care: prevalence and trend over the last 20 years. Eur J Gen Pract. 2008;14(suppl 1):28–32 doi:10.1080/13814780802436093 [DOI] [PubMed] [Google Scholar]
- 25. Schäfer I, Hansen H, Schön G, et al. The influence of age, gender and socioeconomic status on multimorbidity patterns in primary care. First results from the multicare cohort study. BMC Health Serv Res. 2012;12:89 doi:10.1186/1472-6963-12-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Oostrom SH, Picavet HS, van Gelder BM, et al. Multimorbidity and comorbidity in the Dutch population – data from general practices. BMC Public Health. 2012;12:715 doi:10.1186/1471-2458-12-715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fortin M, Stewart M, Poitras ME, Almirall J, Maddocks H. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med. 2012;10:142–151 doi: 10.1370/afm.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–2268. [DOI] [PubMed] [Google Scholar]
- 29. Ferrucci L, Ble A, Bandinelli S, Lauretani F, Suthers K, Guralnik JM. A flame burning within. Aging Clin Exp Res. 2004;16:240–243. [DOI] [PubMed] [Google Scholar]
- 30. Chung HY, Cesari M, Anton S, et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30 doi:10.1016/j.arr.2008.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Prasad S, Sung B, Aggarwal BB. Age-associated chronic diseases require age-old medicine: role of chronic inflammation. Prev Med. 2012;54(suppl):S29–S37 doi:10.1016/j.ypmed.2011.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Howcroft TK, Campisi J, Louis GB, et al. The role of inflammation in age-related disease. Aging (Albany NY). 2013;5:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Volpato S, Guralnik JM, Ferrucci L, et al. Cardiovascular disease, interleukin-6, and risk of mortality in older women: the women’s health and aging study. Circulation. 2001;103:947–953. [DOI] [PubMed] [Google Scholar]
- 34. Bruunsgaard H, Ladelund S, Pedersen AN, Schroll M, Jørgensen T, Pedersen BK. Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clin Exp Immunol. 2003;132:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baune BT, Rothermundt M, Ladwig KH, Meisinger C, Berger K. Systemic inflammation (Interleukin 6) predicts all-cause mortality in men: results from a 9-year follow-up of the MEMO Study. Age (Dordr). 2011;33:209–217 doi:10.1007/s11357-010-9165-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Salanitro AH, Ritchie CS, Hovater M, et al. Inflammatory biomarkers as predictors of hospitalization and death in community-dwelling older adults. Arch Gerontol Geriatr. 2012;54:e387–e391 doi:10.1016/j.archger.2012.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nafziger AN, Bowlin SJ, Jenkins PL, Pearson TA. Longitudinal changes in dehydroepiandrosterone concentrations in men and women. J Lab Clin Med. 1998;131:316–323. [DOI] [PubMed] [Google Scholar]
- 38. Cappola AR, Xue QL, Fried LP. Multiple hormonal deficiencies in anabolic hormones are found in frail older women: the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2009;64:243–248 doi:10.1093/gerona/gln026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cappola AR, O’Meara ES, Guo W, Bartz TM, Fried LP, Newman AB. Trajectories of dehydroepiandrosterone sulfate predict mortality in older adults: the cardiovascular health study. J Gerontol A Biol Sci Med Sci. 2009;64:1268–1274 doi:10.1093/gerona/glp129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vanfleteren LE, Spruit MA, Groenen M, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:728–735 doi:10.1164/rccm.201209-1665OC [DOI] [PubMed] [Google Scholar]
- 41. Akbaraly TN, Hamer M, Ferrie JE, et al. Chronic inflammation as a determinant of future aging phenotypes. CMAJ. 2013;185:E763–E770 doi:10.1503/cmaj.122072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weiss CO, Boyd CM, Yu Q, Wolff JL, Leff B. Patterns of prevalent major chronic disease among older adults in the United States. JAMA. 2007;298:1160–1162. [DOI] [PubMed] [Google Scholar]
- 43. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43 doi:10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.