Abstract
We measured interferon γ–induced protein 10 (IP-10) levels in 428 patients at baseline, week 1, and week 2 of all-oral treatment for hepatitis C virus (HCV) infection. An increased baseline IP-10 level was associated with a T allele in the IL28B gene, an increased alanine aminotransferase level in treatment-naive but not experienced patients, and an increased body mass index. At week 1, the mean decline in plasma IP-10 levels was the same in treatment-naive and treatment-experienced patients (−49%), whereas during week 2 the mean decline in IP-10 levels in treatment-naive patients (−14%) was significantly larger than in treatment-experienced patients (−2%; P = .0176). IP-10 thus may be a surrogate marker of the rate of intracellular viral replication complex decay.
Keywords: IP-10, hepatitis C, direct-acting antiviral therapy, innate immunity
Hepatitis C virus (HCV) establishes and maintains chronic infection in the liver by evading the host innate and adaptive immune responses. After viral infection of hepatocytes, pathogen-associated molecular patterns (PAMPs), including single-stranded or double-stranded RNA and polyuridine signatures, are recognized by Toll-like receptor 3 and retinoic acid–inducible gene 1 and trigger signaling pathways. This activates transcription factors, such as interferon regulatory transcription factor 3, that then mediate gene expression (early phase response), ultimately resulting in the production of interferon β (IFN-β) and activation of the Jak-STAT pathway, with transcription of IFN-stimulated genes (ISGs) during the second phase response [1]. ISGs limit HCV replication by disrupting viral RNA translation, inhibiting negative-strand RNA synthesis, and limiting cell-to-cell viral spread through paracrine effects. Many PAMP receptors are themselves ISGs, and levels increase markedly after IFN production, resulting in an autocrine and paracrine amplification loop that limits HCV replication [1].
IFN-γ–inducible protein 10 (IP-10; also known as CXCL-10) is a member of the CXC subfamily of chemokines induced in monocytes, fibroblasts, and endothelial cells by IFN-γ. IP-10 stimulates monocytes, natural killer cell, and T-cell migration and acts as a chemoattractant for CXCR3+ T cells. Baseline plasma IP-10 levels reflect intrahepatic levels and are a prognostic marker of treatment outcome for patients treated with pegylated interferon and ribavirin [2]. Higher baseline levels of IP-10 are associated with lower sustained virologic response (SVR) rates to interferon-based regimens [3–6]. The fold-increase in IP-10 after the first dose of pegylated interferon predicts SVR [6]. IP-10 concentrations decrease to lower than baseline levels in patients who clear infection but remain unchanged in nonresponders [7], and they similarly decrease to baseline in those who spontaneously clear infection but remain elevated in those who develop chronic infection [8], suggesting that HCV infection itself is driving the elevated IP-10 levels.
Interferon-free treatment regimens for HCV infection composed of directly acting antiviral agents (DAAs) provide a unique opportunity to elucidate mechanistic and kinetic intracellular relationships between HCV and the innate immune response, potentially providing insight into the rate of decay of the viral replication complex itself. Since exogenously administered interferons stimulate IP-10 production, these relationships are obscured in patients treated with interferon. The recent availability of interferon-free DAA regimens for the treatment of HCV infection has provided an unprecedented opportunity to better unravel the relationship between HCV and the innate immune response in the absence of perturbations caused by exogenous interferon. Accordingly, we have studied the effects of potent DAA anti-HCV regimens on plasma IP-10 levels in 428 subjects from 3 phase 2 clinical trials.
METHODS
We studied DAA therapy for chronic HCV genotype 1 infection in treatment-naive subjects (GS-US-248-0120), interferon-ineligible or -intolerant subjects (GS-US-248-132), and treatment-experienced subjects (GS-US-248-0131). Patients with chronic HCV genotype 1 infection were randomly assigned to receive a combination of 3–4 oral compounds: ledipasvir (LDV; NS5A inhibitor), GS-9451 (NS3/4A inhibitor), tegobuvir (TGV; NS5B inhibitor), and ribavirin (RBV). Study designs for these 3 phase 2 clinical trials are detailed online (http://www.clinicaltrials.gov; clinical trials registrations NCT01353248, NCT01435226, and NCT01434498) [9].
Plasma from 428 subjects (obtained at baseline, week 1, and week 2) was analyzed for IP-10 levels, using the BMS 284INST Human IP-10 Instant enzyme-linked immunosorbent assay (eBioscience) as per the manufacturer's protocol, using a DTX 880 Multimode Detector (Beckman-Coulter). The assay has a sensitivity of 1 pg/mL.
Interim clinical data were available for association tests. To explore the association between baseline IP-10 level (<600 pg/mL or ≥600 pg/mL) and demographic and baseline disease characteristics, mean values (±standard deviation) and P values (calculated by the t test) were determined for continuous characteristics, and numbers and percentages of patients and P values (by χ2 tests) were determined for categorical characteristics. One-way analysis of variance was used to explore the association of baseline IP-10 (log10) levels with virologic outcomes. Piecewise regression analysis was performed to characterize the biphasic trend of IP-10 (log10) levels. The regression model included patient population (treatment-naive and interferon-ineligible or -intolerant subjects combined vs treatment-experienced subjects), time with breakpoint at week 1, and the interaction term between patient population and the breakpoint as predictors and the random intercept with subjects nested within patient population. Model estimates were then transformed back to the original scale.
RESULTS
The mean plasma IP-10 level at baseline was 575 pg/mL. We compared baseline demographic and disease characteristics, as well as treatment outcome, for 268 patients with an IP-10 level of <600 pg/mL to those for 159 patients with an IP-10 level of ≥600 pg/mL (Table 1). An IP-10 level of <600 has previously been shown to predict interferon responsiveness and SVR [4].
Table 1.
Baseline Demographic and Disease Characteristics, by Plasma Interferon γ–Inducible Protein 10 Level
| Characteristic | <600 pg/mL (n = 268) | >600 pg/mL (n = 159) | P Value |
|---|---|---|---|
| Demographic | |||
| Age, y | 51 ± 10.6 | 53 ± 9.0 | .0264 |
| Sex | |||
| Female | 113 (42.2) | 60 (37.7) | .3675 |
| Male | 155 (57.8) | 99 (62.3) | |
| Race | |||
| Black | 40 (14.9) | 17 (10.7) | .2137 |
| Not black | 228 (85.1) | 142 (89.3) | |
| Ethnicity | |||
| Hispanic | 32 (12.0) | 12 (7.6) | .1511 |
| Non-Hispanic | 235 (88.0) | 146 (92.4) | |
| Not provided | 1 | 1 | |
| BMIa | 27.5 ± 4.44 | 29.0 ± 5.20 | .0018 |
| Clinical | |||
| IL28B genotype | |||
| CC | 77 (28.7) | 29 (18.4) | .0167 |
| Non-CC | 191 (71.3) | 129 (81.7) | |
| Missing | 0 | 1 | |
| HCV RNA level, log10 IU/mL | 6.48 ± 0.704 | 6.57 ± 0.664 | .2155 |
| HCV genotype | |||
| 1a | 191 (71.3) | 109 (68.6) | .5529 |
| 1b | 77 (28.7) | 50 (31.5) | |
| ALT level, U/L | 70.5 (57.46) | 81.5 (63.09) | .0664 |
| vRVR | |||
| GS-US-248-0120 | |||
| Yes | 64 (78.1) | 29 (69.1) | .2733 |
| No | 18 (22.0) | 13 (31.0) | |
| GS-US-248-0131 | |||
| Yes | 47 (54.0) | 39 (52.0) | .7970 |
| No | 40 (46.0) | 36 (48.0) | |
| GS-US-248-0132 | |||
| Yes | 70 (70.7) | 28 (66.7) | .6337 |
| No | 29 (29.3) | 14 (33.3) | |
| SVR12 | |||
| GS-US-248-0120 | |||
| Yes | 46 (56.1) | 24 (57.1) | .9115 |
| No | 36 (43.9) | 18 (42.9) | |
| GS-US-248-0131 | |||
| Yes | 22 (25.3) | 12 (16.0) | .1478 |
| No | 65 (74.7) | 63 (84.0) | |
| GS-US-248-0132 | |||
| Yes | 44 (44.4) | 11 (26.2) | .0421 |
| No | 55 (55.6) | 31 (73.8) | |
Data are no. (%) of subjects or mean ± SD. P values ≤.05 were considered statistically significant.
Abbreviations: ALT, alanine aminotransferase; HCV, hepatitis C virus.
a Body mass index (BMI) is defined as the weight in kilograms divided by the height in square meters.
Higher baseline plasma IP-10 level was associated with older age (mean age, 53 vs 51 years; P = .0264), IL28B rs12979860 genotype CT or TT (81.7% vs 71.3%; P = .0167), and increased body mass index (defined as the weight in kilograms divided by the height in square meters; mean, 29.0 vs 27.5; P = .0018), all factors associated with impaired interferon response. Baseline IP-10 levels were not associated with sex (P = .3675), ethnicity (P = .1511), African American race (P = .2137), HCV RNA level (P = .2155), or HCV subtype (P = .5529). Although the baseline IP-10 level was not associated with alanine aminotransferase (ALT) level in treatment-experienced patients (P = .5357), in treatment-naive patients a higher ALT level was associated with higher baseline IP-10 levels (P = .0032; data not shown).
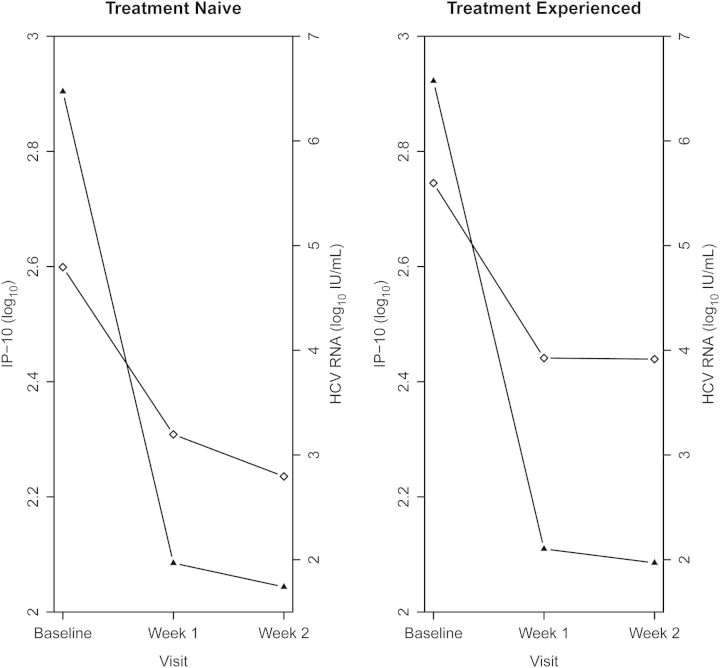
Over the first 2 weeks of treatment, a biphasic pattern of decline in plasma IP-10 levels was observed in each patient population (P <.0001). From baseline to week 1, the mean IP-10 level declined by 49% in treatment-naive patients and by 49% in treatment-experienced patients. From week 1 to week 2, the mean IP-10 level declined by an additional 14% in treatment-naive and interferon-ineligible or -intolerant subjects but by only 2% in treatment-experienced subjects, which was statistically significant (P = .0176). Plasma HCV RNA levels also decayed rapidly, with a biphasic pattern over this interval (Figure 1).
Figure 1.
Plasma interferon γ–inducible protein 10 (IP-10) levels (squares) and mean log10 hepatitis C virus (HCV) RNA levels (triangles) in treatment-naive patients (left panel) and treatment-experienced patients (right panel) prior to initiation of therapy and following weeks 1 and 2 of therapy.
No significant associations between baseline plasma IP-10 levels or change in IP-10 levels and vRVR (defined as an HCV RNA level less than the lower limit of quantification from treatment weeks 2–10) or SVR12 (defined by the absence of detectable HCV RNA 12 weeks after cessation of therapy) results were observed (Table 1). Plasma IP-10 kinetics did not differ between those on three- or four-drug regimens.
DISCUSSION
DAA therapeutics provide a unique opportunity to study the bidirectional relationship between HCV and the innate immune response. In our studies, lower baseline IP-10 levels are associated with IL28B CC genotype but not with treatment outcome, as defined by vRVR or SVR, when using DAAs as opposed to interferon. Although IL28B predicts responsiveness to pegylated interferon/ribavirin regimens, it may have less relevance in predicting the responsiveness of DAA-based therapies because of the increased potency of DAAs. As in other studies, higher baseline IP-10 levels are also associated with more hepatic inflammation and higher ALT levels [10–13].
Potent DAA therapy is associated with a rapid reduction in plasma IP-10 level that parallels the reduction in HCV RNA level in a biphasic pattern, both in treatment-naive patients and treatment-experienced patients. However, the decline in IP-10 level is significantly more pronounced during the second week of the biphasic decay pattern in treatment-naive patients, compared with treatment-experienced patients. Thus, IP-10 may be a surrogate marker of a slower decay of intracellular viral replication complexes in treatment-experienced patients.
The rapid decline in IP-10 levels and HCV RNA levels in both treatment-naive and treatment-experienced patients during all-oral therapy for HCV infection supports prior viral kinetics data indicating superior potency of these regimens, compared with pegylated interferon/ribavirin therapy [14]. Since plasma IP-10 levels are in the range of 75–100 pg/mL in those without HCV infection or following clearance of the virus [10], one might consider plasma IP-10 levels in those who are HCV infected to reflect both HCV-induced and HCV-independent contributions. Thus, in this study, since we calculated relative declines from baseline levels in terms of total IP-10 levels, our calculations underestimate the decline in HCV-induced IP-10 levels. This further emphasizes the rate and magnitude of the early phase innate immune response kinetics induced by these potent new direct-acting agents.
Finally, in addition to serving as a biomarker of the collapse of the HCV population within the liver, it is possible that the brisk decline in IP-10 levels is an indicator of disruption of an intrahepatic virus-host equilibrium that enables chronic productive viral infection in an immunologically precarious niche [15]. Retroviruses take advantage of an immunologically silent integrated proviral DNA intermediary stage to maintain chronic infection for the life of the host. Lacking such an immunologically silent intermediary, HCV has developed a number of immune evasion strategies that allow individual hepatocytes to produce virus for weeks without immune elimination. Rapidly evolving innate and adaptive immune responses result in viral clearance during 15%–40% of acute HCV infections [S1–S3]. If the virus is able to evade immune elimination during the initial 4–6 months of infection, equipoise between the virus and its host is established, and viral replication persists at levels 10–100 times those of human immunodeficiency virus (HIV) for the life of the host despite these potentially effective innate and adaptive responses [14, S4, S6].
The ability of the virus to persist in the presence of adaptive immune mechanisms may be explained, at least in part, by the replicative infidelity of its RNA-dependent RNA polymerase that enables the development of an extremely diverse viral population early in the acute phase of infection [S2–S5]. Intrahepatic downregulation of virus-specific cytotoxic T lymphocyte activity may also contribute to evasion of cellular immunity [S6]. Viral diversity would, however, not explain the ability of HCV to evade the innate immune response. Indeed, the virus has developed strategies to modulate several innate immune response mechanisms that are triggered by detection of HCV RNA and protein within the hepatocyte [1, S7, S8]. Viral products that interfere with innate immunity include the NS3-4a protease, which may disable RIG-I and NS5a, and the E2 core protein, which may interfere with protein kinase R signaling [S9]. Establishment of these innate immune evasion mechanisms during acute infection and their maintenance during chronic infection are likely to be the key determinants of viral persistence.
HIV therapeutics was initially complicated by the rapid development of drug-resistant variants under the selective pressure of incompletely suppressive therapy. Durable viral suppression was dependent on the development of several classes of potent antiretroviral agents with orthogonal resistance mechanisms that made it possible to overcome the dual challenges of high viral replication rates and high degrees of viral diversity [S10, S11]. In the case of HIV, clinical investigation validated mathematical models that predicted that durable viral suppression would require combinations of ≥3 drugs that would require at least 5 different point mutations for the virus to develop resistance to the entire regimen [S12]. Despite replication rates 10–100 times those of HIV and degrees of viral diversity in the range of 10 times that of HIV, it is striking that SVR rates in HCV therapy of 90%–95% are being achieved with as few as 2 DAAs [S13, S14]. Although there are a number of potential explanations for these perhaps better than expected response rates, it is possible that a rapid decay of viral components within hepatocytes responsible for undermining the innate immune response (including the NS3-4a, NS5a, and E2 proteins) results in a reciprocal restoration of critical elements of innate immunity that augment the effectiveness of antiviral therapy. With the loss of protection from these immune effector mechanisms, HCV clearance is being achieved despite drug regimens that might have been predicted to be incompletely effective on the basis of viral diversity and replication kinetics. More direct measurements of the relationships of intrahepatic events during the early DAA therapy will be required to more thoroughly investigate this hypothesis.
Supplementary Material
Notes
Financial support. This work was supported by the National Institutes of Health grants RO1A1076558 [to R. S. and D. W.] and 2T32AI007036 [to J. L.] and Gilead Sciences.
Potential conflicts of interest. N. A. has received research support from Merck, GlaxoSmithKline, Vertex, Gilead, Abbott, and BMS; is a consultant/advisory board member for Merck, Gilead, Echosens, GlaxoSmithKline, Vertex, Novartis, Boehringer Ingelheim, Ligand, Springbank, Medgenics, and Kadmon; is a stockholder of Springbank and Medgenics; and is the editor of the Journal of Viral Hepatitis. F. H. has received lecture fees, travel grants, and investigator fees from Gilead Sciences, BMS, and Transgene; lecture and investigator fees from Roche; investigator fees from Boerhinger Ingelheim; and lecture fees from Merck. E. J. L. has received research/grant support from AbbVie, Achillion Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Idenix Pharmaceuticals, Intercept Pharmaceuticals, Janssen, Medtronic, Merck, Novartis, Presidio, Roche, Santaris Pharmaceuticals, and Vertex Pharmaceuticals; is a speaker for Gilead, GlaxoSmithKline, Kadmon, Merck, and Vertex; and is on the advisory board for AbbVie, Achillion Pharmaceuticals, BioCryst, Biotica, Enanta, Idenix Pharmaceuticals, Janssen, Merck, Novartis, Santaris Pharmaceuticals, Theravance, and Vertex Pharmaceuticals. M. R.-T. has received grant/research support from Abbott, Anadys Pharmaceuticals, Akros, Boehringer Ingelheim, BMS, Gilead, GSK, Idenix Pharmaceuticals, Idera Pharmaceuticals, Inhibitex Pharmaceuticals, Medtronic, Merck, Novartis, Pharmasset, Roche, Santaris Pharmaceuticals, Scynexis Pharmaceuticals, Tibotec, Vertex Pharmaceuticals, ViroChem Pharma, and ZymoGenetics and has served as a consultant/advisor for Anadys Pharmaceuticals, BMS, Genentech, Gilead, Inhibitex Pharmaceuticals, Merck, Pharmasset, Santaris Pharmaceuticals, Tibotec, and Vertex. R. S. has received research support from Gilead Sciences, serves on the scientific advisory board for Gilead Sciences, and has consulted for Merck and Santaris. M. S. has received research grants paid to Johns Hopkins University; is on the scientific advisory board and serves as a consultant for Gilead, AbbVie, BIPI, BMS, Janssen, Merck, and Vertex; and is on the steering committee for Pfizer. D. L. W. has received research support paid to the University of California–San Diego from Gilead, AbbVie, Vertex, BMS, and Merck and is a consultant/advisor for Gilead, Janssen, and AbbVie. M. P., Y. Z., M. S., and J. M. are employees and stockholders of Gilead Sciences. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Gale M, Jr, Foy EM. Evasion of intracellular host defence by hepatitis C virus. Nature. 2005;436:939–45. doi: 10.1038/nature04078. [DOI] [PubMed] [Google Scholar]
- 2.Askarieh G, Alsio A, Pugnale P, et al. Systemic and intrahepatic interferon-gamma-inducible protein 10 kDa predicts the first-phase decline in hepatitis C virus RNA and overall viral response to therapy in chronic hepatitis C. Hepatology. 2010;51:1523–30. doi: 10.1002/hep.23509. [DOI] [PubMed] [Google Scholar]
- 3.Butera D, Marukian S, Iwamaye AE, et al. Plasma chemokine levels correlate with the outcome of antiviral therapy in patients with hepatitis C. Blood. 2005;106:1175–82. doi: 10.1182/blood-2005-01-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darling JM, Aerssens J, Fanning G, et al. Quantitation of pretreatment serum interferon-gamma-inducible protein-10 improves the predictive value of an IL28B gene polymorphism for hepatitis C treatment response. Hepatology. 2011;53:14–22. doi: 10.1002/hep.24056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lagging M, Askarieh G, Negro F, et al. Response prediction in chronic hepatitis C by assessment of IP-10 and IL28B-related single nucleotide polymorphisms. PLoS One. 2011;6:e17232. doi: 10.1371/journal.pone.0017232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeremski M, Markatou M, Brown QB, Dorante G, Cunningham-Rundles S, Talal AH. Interferon gamma-inducible protein 10: a predictive marker of successful treatment response in hepatitis C virus/HIV-coinfected patients. J Acquir Immune Defic Syndr. 2007;45:262–8. doi: 10.1097/QAI.0b013e3180559219. [DOI] [PubMed] [Google Scholar]
- 7.Diago M, Castellano G, Garcia-Samaniego J, et al. Association of pretreatment serum interferon gamma inducible protein 10 levels with sustained virological response to peginterferon plus ribavirin therapy in genotype 1 infected patients with chronic hepatitis C. Gut. 2006;55:374–9. doi: 10.1136/gut.2005.074062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chattergoon MA, Levine JS, Latanich R, Osburn WO, Thomas DL, Cox AL. High plasma interleukin-18 levels mark the acute phase of hepatitis C virus infection. J Infect Dis. 2011;204:1730–40. doi: 10.1093/infdis/jir642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wyles D, Rodriguez-Torres M, Lawitz E, et al. All-oral combination therapy with ledipasvir, GS-9451, tegobuvir, and ribavirin in treatment-naive genotype 1 HCV. Hepatology. 2014;60:56–64. doi: 10.1002/hep.27053. [DOI] [PubMed] [Google Scholar]
- 10.Reiberger T, Aberle JH, Kundi M, et al. IP-10 correlates with hepatitis C viral load, hepatic inflammation and fibrosis and predicts hepatitis C virus relapse or non-response in HIV-HCV coinfection. Antivir Ther. 2008;13:969–76. [PubMed] [Google Scholar]
- 11.Roe B, Coughlan S, Hassan J, et al. Elevated serum levels of interferon- gamma -inducible protein-10 in patients coinfected with hepatitis C virus and HIV. J Infect Dis. 2007;196:1053–7. doi: 10.1086/520935. [DOI] [PubMed] [Google Scholar]
- 12.Zeremski M, Petrovic LM, Chiriboga L, et al. Intrahepatic levels of CXCR3-associated chemokines correlate with liver inflammation and fibrosis in chronic hepatitis C. Hepatology. 2008;48:1440–50. doi: 10.1002/hep.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeremski M, Dimova R, Brown Q, Jacobson IM, Markatou M, Talal AH. Peripheral CXCR3-associated chemokines as biomarkers of fibrosis in chronic hepatitis C virus infection. J Infect Dis. 2009;200:1774–80. doi: 10.1086/646614. [DOI] [PubMed] [Google Scholar]
- 14.Chatterjee A, Guedj J, Perelson AS. Mathematical modelling of HCV infection: what can it teach us in the era of direct-acting antiviral agents? Antivir Ther. 2012;17:1171–82. doi: 10.3851/IMP2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kandathil AJ, Graw F, Quinn J, et al. Use of laser capture microdissection to map hepatitis C virus-positive hepatocytes in the human liver. Gastroenterology. 2013;145:1401–14. doi: 10.1053/j.gastro.2013.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.