Abstract
Background
Human immunodeficiency virus (HIV)–infected pregnant women are at increased risk of malaria and its complications. In vitro and in vivo data suggest that the HIV protease inhibitors lopinavir/ritonavir may have potent antimalarial activity. We sought to evaluate whether lopinavir/ritonavir-based antiretroviral therapy (ART) reduced the risk of placental malaria.
Methods
HIV-infected, ART-naive pregnant women were enrolled between gestational weeks 12 and 28 and randomly assigned to receive lopinavir/ritonavir-based or efavirenz-based ART. Women received daily trimethoprim-sulfamethoxazole prophylaxis and insecticide-treated bed nets at enrollment and were followed up to 1 year after delivery. The primary outcome was placental malaria, defined by the detection of malaria parasites, using microscopy or polymerase chain reaction (PCR) analysis of placental blood specimens. Secondary outcomes included placental malaria, defined by histopathologic results; adverse birth outcomes; incidence of malaria; and prevalence of asymptomatic parasitemia. Analyses were done using an intention-to-treat approach.
Results
Of 389 subjects randomly assigned to a treatment group, 377 were followed through to delivery. There was no significant difference in the risk of placental malaria, as defined by thick smear or PCR findings, between the lopinavir/ritonavir-based and efavirenz-based ART arms (7.4% vs 9.8%; P = .45). Similarly, there were no differences in secondary outcomes between the 2 treatment arms.
Conclusions
Lopinavir/ritonavir-based ART did not reduce the risk of placental or maternal malaria or improve birth outcomes, compared with efavirenz-based ART.
Clinical Trials Registration
Keywords: HIV, malaria, pregnancy, lopinavir/ritonavir, efavirenz
Malaria in pregnancy is associated with adverse maternal and neonatal outcomes, such as spontaneous abortions, stillbirth, intrauterine growth restriction, preterm delivery, low birth weight (LBW), maternal anemia, and neonatal death [1]. Human immunodeficiency virus (HIV)–infected pregnant women have an increased risk of parasitemia, clinical malaria, and placental malaria, compared with HIV-uninfected pregnant women [2]. In addition, coinfection with HIV and placental malaria parasites is associated with an increased risk of low birth weight and preterm delivery, compared with either infection alone [2]. The attributable risk for placental malaria due to HIV infection is more pronounced with higher parity, a phenomenon supported by laboratory studies indicating that HIV impairs parity-specific immunity [3, 4]. Current strategies for the prevention of malaria during pregnancy include the use of insecticide-treated bed nets (ITNs) and intermittent preventive treatment (IPTp) with sulfadoxine-pyrimethamine (SP). For HIV-infected pregnant women receiving daily trimethoprim-sulfamethoxazole (TMP-SMX) prophylaxis, the World Health Organization (WHO) recommends avoiding the use of IPTp with SP because of the risk of adverse drug reactions [5]. However, the spread of resistance to the pyrethroid class of insecticides used in ITNs and to the antifolate class of drugs used for ITPp and prophylaxis suggest the need for novel strategies for the prevention of malaria among HIV-infected and uninfected pregnant women [6, 7].
Combination antiretroviral therapy (ART) is now recommended for all HIV-infected pregnant and breast-feeding women per 2013 WHO consolidated guidelines [8]. In addition to benefits in improving women's health and reducing the risk of HIV transmission, the protease inhibitor class of antiretroviral agents may also provide protection against malaria. HIV protease inhibitors demonstrate in vitro activity against Plasmodium falciparum [9–11], and it is thought that this occurs through inhibition of plasmepsins, although the exact mechanism remains unclear [12]. Lopinavir is the most potent of these inhibitors and is active in vitro at levels commonly achieved with ritonavir boosting [10, 11]. In a recent randomized controlled trial of HIV-infected Ugandan children, coformulated lopinavir/ritonavir (LPV/r)–based ART was associated with a 41% reduction in the incidence of malaria, compared with nonnucleoside reverse-transcriptase inhibitor (NNRTI)–based ART, with the lower incidence attributable largely to a significant reduction in the recurrence of malaria after treatment with artemether-lumefantrine (AL) [13]. The efficacy of HIV protease inhibitors for the prevention of malaria and its complications among pregnant women has not been previously evaluated in clinical trials.
To test the hypothesis that HIV protease inhibitors are protective against malaria, HIV-infected, ART-naive pregnant women living in an area of Uganda where malaria is highly endemic were randomly assigned to receive LPV/r-based or efavirenz (EFV)–based ART and followed up to 1 year after delivery. Outcomes of interest included measures of placental malaria, adverse birth outcomes, incidence of malaria, and prevalence of asymptomatic parasitemia.
METHODS
Study Site and Participants
The study was conducted from December 2009 to March 2013 in Tororo, Uganda, an area of high-intensity malaria transmission [14]. Women were recruited from the Tororo District Hospital (TDH) antenatal clinic, The AIDS Support Organization (TASO), and other health centers in the district. Eligible women were ≥16 years of age, infected with HIV-1 confirmed by two assays, lived within 30 km of the study site, and had a pregnancy between 12–28 weeks gestation by last menstrual period with confirmation by ultrasound. Women were eligible for enrollment at any CD4 cell count. Women were excluded if they had ever received highly active combination ART or single dose nevirapine or other abbreviated monotherapy or dual therapy in the last 24 months. Women were also excluded if they had prior dose-limited toxicity to TMP-SMX within 14 days, active tuberculosis or other WHO stage 4 diseases, cardiac disease, or abnormal screening laboratory values including, hemoglobin <7.5 g/dL, absolute neutrophil count <750/mm3, platelet count <50 000/mm3, ALT >225 U/L, AST >225 U/L, total bilirubin ≥2.5 times the upper limit of normal, and creatinine ≥1.8 times the upper limit of normal.
All participants provided written informed consent in their preferred language. The study protocol was approved by the Makerere University School of Medicine Research and Ethics Committee, the Uganda National Council for Science and Technology, and the University of California–San Francisco Committee on Human Research.
Study Design
This was an open-label, single-site, randomized controlled trial of LPV/r-based ART versus EFV-based ART among HIV-infected, ART-naive pregnant Ugandan women. Participants were randomized in a 1:1 ratio after stratification by gravidity (gravida 1 vs gravida ≥2) and gestational age at enrollment (<24 weeks vs ≥24 weeks). Randomization was performed in permuted blocks of 2 or 4. The dosing of study drugs was as follows: EFV 600 mg once daily; or LPV/r (Aluvia; AbbVie Pharmaceuticals, North Chicago, IL) 200 mg/50 mg, 2 tablets twice daily, with an increase to 3 tablets twice daily from gestation week 30 until delivery, after which 2 tablets were administered twice daily. Women in both arms received lamivudine/zidovudine 150 mg/300 mg twice daily. Tenofovir was used in cases of zidovudine intolerance.
Study Procedures
At enrollment, all women had their medical history recorded, underwent physical examination (including assessment of gestational age, calculated by last menstrual period and ultrasonographic biometry), and underwent baseline laboratory studies. All women received daily TMP-SMX prophylaxis (160 mg/800 mg), a long-lasting ITN, and a basic care package that included multivitamins and condoms. Throughout the study, women received standard antenatal care that accorded with Uganda Ministry of Health guidelines [15]. Women returned to the study clinic every 4 weeks for scheduled visits, including collection of blood specimens for routine blood smears, as well as for any health conditions requiring evaluation. Transportation costs were reimbursed for all clinic visits. Women who presented with a documented fever (tympanic temperature, ≥38.0°C) or history of fever in the previous 24 hours had a blood specimen obtained by finger prick for a thick blood smear. If the smear was positive for malaria parasites, malaria was diagnosed. Episodes of uncomplicated malaria were treated with AL, and complicated malaria was treated with quinine in accordance with Ugandan guidelines.
Women were encouraged to deliver at TDH. Women delivering at home were visited by study staff at the time of delivery or as soon as possible afterward. At delivery, a standardized assessment was completed, including assessment of gestational age and of birth weight, using an electronic scale. Following delivery, participants continued to receive ART and were followed up to 52 weeks after delivery or until study completion, in March 2013.
Laboratory Procedures
Thick and thin blood smears were stained with 2% Giemsa and read in duplicate by experienced microscopists who were blinded to study arm and to each other's readings. A smear was considered negative if no parasites were seen after review of 100 high-powered fields. Discrepant readings were settled by a third microscopist. Clinical laboratory tests, including complete blood count, determination of CD4+/CD8+ T-lymphocyte subsets, and measurement of HIV-1 RNA load by polymerase chain reaction (Cobas Amplicor, version 1.5, Roche Molecular Diagnostics, Pleasanton, CA) were performed at enrollment and during the follow-up period.
Placental specimens were collected within 30 minutes of delivery in the hospital (or as early as possible, if delivery occurred at home) and included placental blood from an incision on the maternal surface and placental tissue. Placental blood was tested for malaria parasites, using a thick blood smear in accordance with the procedures described above, a rapid diagnostic test (RDT; Paracheck-Pf, Orchid, Goa, India) performed immediately after placental blood was collected, and PCR of dried blood spots. For PCR, aliquots of approximately 25 µL of placental blood were placed on filter paper, air dried, and stored in individual bags with desiccant. DNA was extracted from dried blood spots by use of Chelex and was amplified using a nested PCR reaction as previously described [16]. PCR reactions were performed at the University of California–San Francisco by experienced staff and included appropriate controls and quality assurance. For histopathologic analysis, a 2 × 2 full-thickness biopsy specimen was placed in 10% fresh buffered formalin, using a 1:10 ratio of tissue to formalin. After 24 hours in formalin, placental tissue was trimmed into a 1 × 1-cm block of tissue and placed into a fresh jar of 10% buffered formalin (1:10 ratio) and stored out of direct sunlight. Tissue specimens were fixed with ethanol and xylene, embedded in paraffin wax, sectioned into slides, and stained with hematoxylin-eosin and Giemsa. Slides for histopathologic analysis were read by a trained investigator (V. A.) and included examination for malaria parasites and hemozoin pigment in intervillous fibrin and macrophages, using standardized criteria as previously described [17]. Quality control of histopathologic findings was performed on a random sample of 25% of positive slides and 10% of negative slides by an experienced pathologist (Atis Muehlenbachs, Centers for Disease Control and Prevention).
Study End Points
The primary end point for this study was placental malaria, defined as the detection of P. falciparum in a thick blood smear or by PCR of placental blood. Secondary outcomes included (1) prevalence of placental malaria, as determined by RDT or histopathologic analysis; (2) adverse birth outcomes, including stillbirth (intrauterine fetal demise ≥20 weeks of gestation) or spontaneous abortion (miscarriage <20 weeks of gestation), preterm delivery (<37 weeks of gestation), low birth weight (<2500 g), neonatal death within 28 days of delivery, or a composite outcome, defined as any of the adverse birth outcomes listed above; and (3) measures of maternal malaria, including the incidence of symptomatic malaria and the prevalence of asymptomatic parasitemia at the time routine blood smears were performed, stratified by the periods during and after pregnancy. For women giving birth to twins, outcomes were classified according to whether either infant met criteria for the outcome of interest. HIV-related outcomes, including maternal and infant safety profile, as well as virologic and immunologic responses, are presented in a separate report [18].
Statistical Analysis
To test the hypothesis that use of LPV/r-based ART will reduce the prevalence of placental malaria, as defined by positive findings of a placental blood smear or PCR, we assumed a prevalence of 20% in the EFV-based ART arm, based on previous data from the study site [19]. We then calculated that a sample size of 500 was needed to show a 48.5% reduction in prevalence of placental malaria in the LPV/r-based arm (2-sided significance level, 0.05; power, 80%), assuming that 15% of women enrolled would not have evaluable results at delivery. Because of the slower than anticipated study accrual, 389 women were enrolled.
Data were double entered in Access (Microsoft, Redmond, WA), and analyses were performed using Stata, version 12 (StataCorp, College Station, TX). All analyses were done using an intention-to-treat approach. Comparisons of baseline characteristics and birth outcomes were made using a t test for continuous variables and a χ2 test for categorical variable. Comparisons of malaria incidence between study arms were made using a negative binomial regression model. Comparison of the risk of recurrent malaria with 42 days of treatment with AL was made using a Cox proportional hazard model. with adjustment for repeat observations in the same study participant. Comparison of the prevalence of asymptomatic parasitemia at the time routine blood smears were performed was made using generalized estimating equations, with adjustment for repeated measures in the same patient by using exchangeable correlation and robust standard errors. A P value of <.05 was considered statistically significant.
RESULTS
Study Participants and Follow-up
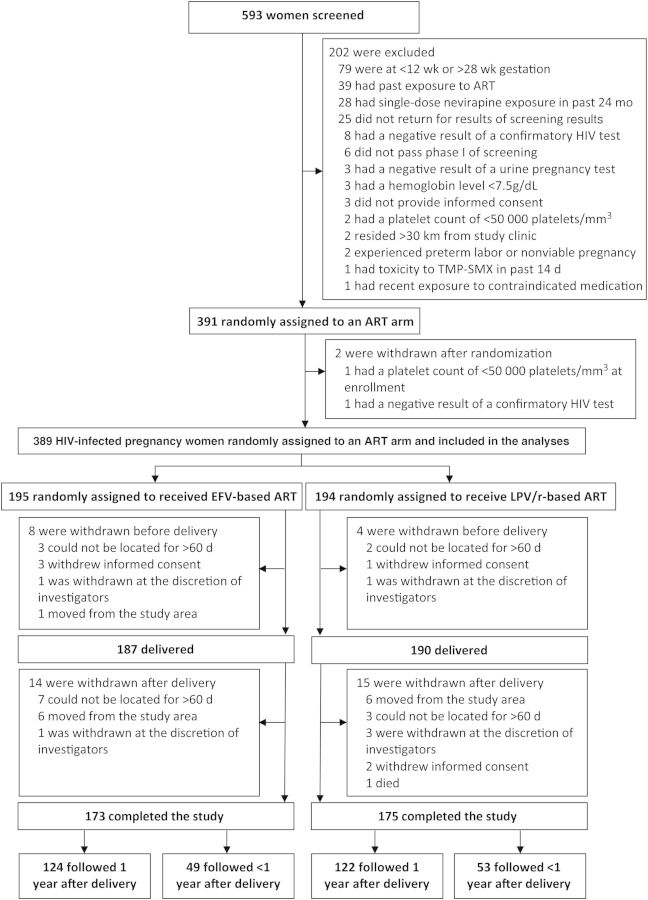
Of 593 women screened, 391 study participants were enrolled and randomly assigned to one of the ART groups (Figure 1). The majority of exclusions were due to gestational age outside the study range and prior ART exposure. After enrollment, 2 women were found to be ineligible, resulting in 389 eligible participants; 195 were assigned to receive EFV-based ART, and 194 were assigned to receive LPV/r-based ART. Baseline characteristics were similar between the 2 treatment arms (Table 1). The majority of study participants (82.2%) were multigravida (≥2 prior pregnancies). Prior to enrollment, 37.8% and 64.0% of women reported owning an ITN and were receiving TMP-SMX prophylaxis, respectively. The women enrolled in the study had a preserved immune status, with a median CD4+ T-cell count at randomization of 374 cells/mm3 (interquartile range [IQR], 270–485 cells/mm3) in the EFV arm and 368 cells/mm3 (IQR, 282–506 cells/mm3) in the LPV/r arm. A total of 93% of women in the EFV arm and 97% of women in the LPV/r arm had WHO stage 1 HIV disease. Women in both arms had high levels of viral suppression (89% and 87% for the EFV and LPV/r arms, respectively) 8 weeks after starting ART.
Figure 1.
Flow of participants through the study. Abbreviations: ART, antiretroviral therapy; EFV, efavirenz; HIV, human immunodeficiency virus; LPV/r, lopinavir/ritonavir; TMP-SMX, trimethoprim-sulfamethoxazole.
Table 1.
Baselines Characteristics of Pregnant Study Participants Who Were Randomly Assigned to Receive Efavirenz (EFV)–Based Antiretroviral Therapy (ART) or Lopinavir/Ritonavir (LPV/r)–Based ART
| Characteristic | Treatment Arm |
P Value | |
|---|---|---|---|
| EFV-Based ART (n = 195) | LPV/r-Based ART (n = 194) | ||
| Age, y | 29.5 ± 5.4 | 29.0 ± 5.4 | .31 |
| Gestational age, wk | 21.1 ± 4.1 | 21.2 ± 4.3 | .91 |
| Previous pregnancies | |||
| 0 | 16 (8.2) | 8 (4.1) | .20 |
| 1 | 20 (10.3) | 25 (12.9) | |
| ≥2 | 159 (81.5) | 161 (83.0) | |
| Bed net ownership | |||
| None | 85 (43.6) | 85 (44.3) | .84 |
| Untreated | 28 (14.4) | 33 (17.2) | |
| ITN | 77 (39.5) | 70 (36.4) | |
| Yes; unknown treatment status | 5 (2.5) | 4 (2.1) | |
| Receiving TMP-SMX prophylaxis | 125 (64.1) | 124 (63.9) | .97 |
| Hemoglobin level, g/dL | 10.9 ± 1.3 | 11.0 ± 1.2 | .82 |
| CD4+ T-cell count, cells/mm3 | 374 (270–485) | 368 (282–506) | .31 |
| HIV RNA load, log10 copies/mL | 4.3 (3.5–4.8) | 4.1 (3.3–4.7) | .35 |
| WHO stage HIV disease | |||
| 1 | 181 (92.8) | 189 (97.4) | .09 |
| 2 | 13 (6.7) | 5 (2.6) | |
| 3 | 1 (0.5) | 0 | |
Data are no. (%) of participants, mean ± SD, or median (interquartile range).
Abbreviations: HIV, human immunodeficiency virus; TMP-SMX, trimethoprim-sulfamethoxazole; WHO, World Health Organization.
Twelve subjects withdrew from the study prior to delivery, resulting in 377 women (96.9%) followed through birth, which including 10 sets of twins, 5 of which had 2 placentas. A total of 285 women (75.6%) gave birth at TDH, 74 (19.6%) gave birth at home, and 18 (4.8%) gave birth at another health facility. Missing data at the time of delivery included birth weight (n = 18), placental blood smear findings (n = 61), placental RDT results (n = 65), placental PCR results (n = 75), and placental histopathologic findings (n = 50). Of 327 women with placentas collected, 45 (13.8%) gave birth at home. An additional 29 women (7.7% of those who gave birth) were withdrawn after delivery, 246 (65.3%) were followed to year 1 after delivery, and 102 (27.1%) were followed for <1 year after delivery, up to March 2013, when the study ended (Figure 1). ART adherence based on self-reported recall was 97% in the EFV arm and 99% in the LPV/r arm. TMP-SMX adherence was reported as 98% in the EFV arm and 99% in the LPV/r arm.
Measures of Placental Malaria
Four methods were used to diagnose placental malaria. Considering the 285 outcomes with results for all 4 methods, the prevalence of placental malaria was 3.5%, using placental blood smear findings; 4.6%, using placental blood RDT results; 8.4%, using placental blood PCR results; and 33.0%, using any histopathologic evidence of placental malaria. All samples that were positive by placental blood smear were also positive by the other 3 methods. The much higher sensitivity of histopathologic analysis could be explained by the fact that 77.1% of samples positive by histopathologic analysis were based on the presence of malaria pigment without asexual parasites, an indication of past infection. The prevalence of placental malaria determined on the basis of blood smear, RDT, and PCR findings was similar between the 2 ART arms (Table 2). There was a trend toward an increased risk of placental malaria in the LPV/r-based ART arm, based on any histopathologic evidence, but this difference did not reach statistical significance (38.3% vs 28.5%; relative risk, 1.34 [95% confidence interval, .98–1.83]; P = .06; Table 2).
Table 2.
Outcomes Assessed at the Time of Delivery Among Pregnant Study Participants Who Were Randomly Assigned to Receive Efavirenz (EFV)–Based Antiretroviral Therapy (ART) or Lopinavir/Ritonavir (LPV/r)–Based ART
| Outcome | Treatment Arm |
RR (95% CI) | P Value | |
|---|---|---|---|---|
| EFV-based ART | LPV/r-based ART | |||
| Malaria positivity, by test | ||||
| Placental blood smear | 6/158 (3.8) | 5/158 (3.2) | 0.83 (.26–2.67) | .76 |
| Placental blood RDT | 7/155 (4.5) | 6/157 (3.8) | 0.85 (.29–2.46) | .76 |
| Placental blood PCR | 15/153 (9.8) | 11/149 (7.4) | 0.75 (.36–1.59) | .45 |
| Histopathologic analysis | 47/165 (28.5) | 62/162 (38.3) | 1.34 (.98–1.83) | .06 |
| Birth outcome | ||||
| Spontaneous abortion or stillbirth | 7/187 (3.7) | 5/190 (2.6) | 0.70 (.23–2.18) | .54 |
| Neonatal deatha | 4/180 (2.2) | 8/185 (4.3) | 1.95 (.60–6.35) | .26 |
| Preterm delivery | 34/187 (18.2) | 39/190 (20.5) | 1.13 (.75–1.71) | .56 |
| Low birth weight | 33/178 (18.5) | 39/181 (21.5) | 1.16 (.77–1.76) | .48 |
| Composite adverse birth outcome | 50/180 (27.8) | 63/186 (33.9) | 1.22 (.89–1.66) | .21 |
Data are no. of participants with the outcome/no. evaluated (%).
Abbreviations: CI, confidence interval; PCR, polymerase chain reaction; RDT, rapid diagnostic test; RR, relative risk.
a Only including live births.
Birth Outcomes
Among 377 pregnancies followed through delivery, 1 resulted in a spontaneous abortion at 17 weeks of gestation, 11 resulted in stillbirth between 22 and 37 weeks of gestation, and 12 resulted in neonatal death within 28 days of delivery. Overall, the prevalence of preterm delivery and low birth weight were 19.4% and 20.1%, respectively. Among 10 sets of twins (4 in the EFV arm and 6 in the LPV/r arm), 8 sets had preterm delivery, and 17 of 20 neonates in these sets had low birth weight. There were no significant differences in the risk of any of the individual adverse birth outcomes or a composite adverse birth outcome between the 2 ART arms (Table 2).
Maternal Malaria Outcomes
A total of 68 episodes of symptomatic malaria in 51 different women were diagnosed, of which 67 were uncomplicated (treated with AL) and 1 was complicated (treated with quinine). The incidence of malaria was 0.27 episodes/person-year at risk during pregnancy and 0.10 episodes/person-year at risk after delivery. There were no differences in the incidence of malaria between the 2 ART arms both during and after pregnancy (Table 3). Considering all episodes of malaria treated with AL, there were no differences in the risk of recurrent malaria within 42 days between the LPV/r-based and EFV-based ART arms (8.1% vs 10.3%; P = .74). Considering routine blood smears performed at monthly intervals, the prevalence of asymptomatic parasitemia was 2.3% and did not differ between the periods during pregnancy and after delivery (Table 3). There were no significant differences in the risk of asymptomatic parasitemia between the 2 ART arms both during and after pregnancy (Table 3).
Table 3.
Incidence of Malaria and Parasite Prevalence During and After Pregnancy Among Pregnant Study Participants Who Were Randomly Assigned to Receive Efavirenz (EFV)–Based Antiretroviral Therapy (ART) or Lopinavir/Ritonavir (LPV/r)–Based ART
| Treatment Arm | During Pregnancy |
After Pregnancy |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants, No. | Malaria Episodes, No. | PYAR, No. | Malaria Incidence, Episodes/PYAR | IRR (95% CI) | P Value | Participants, No. | Malaria Episodes, No. | PYAR, No. | Malaria Incidence, Episodes/PYAR | IRR (95% CI) | P Value | |
| EFV-based ART | 195 | 17 | 63.5 | 0.27 | 1.0 (reference) | … | 187 | 13 | 159.9 | 0.08 | 1.0 (reference) | … |
| LPV/r-based ART | 194 | 17 | 62.3 | 0.27 | 1.01 (.49–2.09) | .97 | 190 | 21 | 164.9 | 0.13 | 1.55 (.66–3.63) | .31 |
| Asymptomatic Parasitemia, Prevalencea |
Prevalence Ratio (95% CI) |
Asymptomatic Parasitemia, Prevalencea |
Prevalence Ratio (95% CI) |
|||||||||
| EFV-based ART | 22/795 (2.8) | 1.0 (reference) | … | 43/1659 (2.6) | 1.0 (reference) | … | ||||||
| LPV/r-based ART | 13/786 (1.7) | 0.59 (.28–1.24) | .17 | 36/1753 (2.1) | 0.78 (.38–1.62) | .51 | ||||||
Abbreviations: CI, confidence interval; IRR, incidence rate ratio; PYAR, person-years at risk.
a Data are routine blood smears with positive results/routine blood smears evaluated (%).
DISCUSSION
WHO guidelines now recommend combination ART for all pregnant and breast-feeding women, with EFV-based ART as a first-line regimen and LPV/r-based ART as an alternative regimen [8]. In this study, pregnant women were randomly assigned to receive LPV/r-based or EFV-based ART to test the hypothesis that a protease inhibitor–based ART regimen would be associated with a lower risk of malaria. We found there was no difference between the 2 ART regimens in the risk of placental malaria, the incidence of malaria, the prevalence of asymptomatic parasitemia, and the risk of adverse birth outcomes.
The rationale behind this study was evidence from laboratory studies showing that HIV protease inhibitors have moderate activity against P. falciparum in vitro [9–11] and against rodent malaria parasites in vivo [20]. In addition, HIV protease inhibitors have been shown to impair CD36-mediated cytoadherence and nonopsonic phagocytosis of parasitized erythrocytes by human macrophages in vitro [21]. Despite these encouraging laboratory findings, there are no data that HIV protease inhibitors can directly provide clinically relevant protection against malaria in human populations. In a retrospective study among 444 HIV-infected women randomly assigned to receive LPV/r-based or NNRTI-based ART, the protease inhibitor–containing regimen was not associated with protection against malaria [22]. These data come from 6 sites in Africa and relied on laboratory-confirmed malaria parasite infection, based on blood smear results, RDT results, or histidine-rich protein 2 antigenemia, using stored specimens. Limitations of this study included a semi-immune population of adults at relatively low risk for malaria; a high rate of TMP-SMX prophylaxis, which has been shown to provide protection against malaria [23]; and a lower than expected incidence of malaria, resulting in the possibility that the study was underpowered and only able to detect a relatively large difference between the arms.
In contrast, in a recent randomized controlled trial of young HIV-infected Ugandan children conducted at the same study site as this report, LPV/r-based ART was associated with a 41% reduction in the incidence of malaria, compared with NNRTI-based ART [13]. Interestingly, in this study the primary benefit in the LPV/r-based ART arm was not protection against a first episode of malaria prior to treatment with AL; rather, the benefit involved a dramatic reduction in the risk of recurrent malaria after treatment with AL, owing to increased lumefantrine levels that led to a prolonged posttreatment prophylactic effect. Increased lumefantrine exposure with concomitant LPV/r therapy has been observed in healthy adults and is thought to be the result of inhibition of cytochrome P450 3A4 metabolism by ritonavir [24].
The key question in this study was why LPV/r failed to reduce the risk of malaria in pregnant women after a similar study in young children showed that LPV/r was associated with a significant reduction in the incidence of malaria. One explanation is that symptomatic malaria requiring treatment with AL was relatively uncommon in our population of semi-immune pregnant women, resulting in few opportunities for pharmacologic enhancement of lumefantrine, as seen in children, among whom the risk of recurrent malaria shortly after treatment was high [13]. In addition, pharmacologic interactions between LPV/r and lumefantrine may differ between pregnant women and young children, as evidenced by a similar risk of recurrent malaria following treatment with AL between the 2 ART arms in this study. Indeed, pregnancy has been shown to induce metabolism of AL, possibly offsetting any protective effects that may be provided from an interaction between LPV/r and lumefantrine [25, 26].
Strengths of this study include a randomized controlled study design, a study site known to have high malaria transmission intensity, excellent retention of the women in this study, and the use of multiple outcome measures, including histopathologic analysis, the most sensitive method for the diagnosis of placental malaria [27]. However, the use of formalin fixation can lead to artifacts that mimic hemozoin pigment, resulting in misdiagnosis of past infection. Limitations of the study include failure to achieve our targeted sample size, a higher proportion of missing laboratory data for our primary outcome than planned, and a lower observed prevalence of our primary outcome in the control arm than predicted (10% vs 20%), all of which resulted in a reduction in statistical power. In addition, caution should be taken in generalizing the results of this study to other settings. The lower than expected risk of placental malaria was likely due to the high proportion of multigravida women enrolled and to a better than expected protective efficacy of TMP-SMX prophylaxis against placental malaria. It is interesting to note the surprisingly low risk of placental malaria in this study, despite the high prevalence (>90%) of the dhfr/dhps quintuple mutant associated with antifolate resistance in our area [23]. In another study from Malawi, daily TMP-SMX therapy was associated with a reduced risk of malaria parasitemia, compared with ITPp with SP, among HIV-infected pregnant women, despite a similar high prevalence of the dhfr/dhps quintuple mutant [28].
New WHO guidelines recommending EFV-based combination ART for all HIV-infected and breast-feeding women offer the potential of immense benefits in preservation of women's health and reducing transmission. The main conclusion of this study is that LPV/r did not have sufficient direct antimalarial activity to translate into a clinically meaningful benefit that would support using it in place of EFV in regions where malaria is highly endemic.
Notes
Acknowledgments. We thank the women who participated in the study, the dedicated study staff, practitioners at Tororo District Hospital, and members of the Infectious Diseases Research Collaboration.
Disclaimer. AbbVie had no role in study design, data accrual and analysis, or manuscript preparation.
Financial support. This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (grant P01 HD059454 to D. V. H.); and AbbVie Pharmaceuticals (grant on behalf of D. V. H. to the University of California–San Francisco).
Potential conflicts of interest. AbbVie provided lopinavir/ritonavir for this study through a grant on behalf of D. V. H. to the University of California–San Francisco. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Desai M, ter Kuile FO, Nosten F, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 2.Flateau C, Le Loup G, Pialoux G. Consequences of HIV infection on malaria and therapeutic implications: a systematic review. Lancet Infect Dis. 2011;11:541–56. doi: 10.1016/S1473-3099(11)70031-7. [DOI] [PubMed] [Google Scholar]
- 3.Ned RM, Moore JM, Chaisavaneeyakorn S, Udhayakumar V. Modulation of immune responses during HIV-malaria co-infection in pregnancy. Trends Parasitol. 2005;21:284–91. doi: 10.1016/j.pt.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 4.ter Kuile FO, Parise ME, Verhoeff FH, et al. The burden of co-infection with human immunodeficiency virus type 1 and malaria in pregnant women in sub-saharan Africa. Am J Trop Med Hyg. 2004;71:41–54. [PubMed] [Google Scholar]
- 5.World Health Organization. Guidelines on co-trimoxazole prophylaxis for HIV-related infections among children, adolescents and adults. Recommendatinos for a public health approach. http://www.who.int/hiv/pub/guidelines/ctxguidelines.pdf . Accessed May 2014.
- 6.Iriemenam NC, Shah M, Gatei W, et al. Temporal trends of sulphadoxine-pyrimethamine (SP) drug-resistance molecular markers in Plasmodium falciparum parasites from pregnant women in western Kenya. Malaria J. 2012;11:134. doi: 10.1186/1475-2875-11-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ranson H, N'Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 2011;27:91–8. doi: 10.1016/j.pt.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva, Switzerland: WHO; 2013. [PubMed] [Google Scholar]
- 9.Nsanzabana C, Rosenthal PJ. In vitro activity of antiretroviral drugs against Plasmodium falciparum. Antimicrobial Agents Chemother. 2011;55:5073–7. doi: 10.1128/AAC.05130-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parikh S, Gut J, Istvan E, Goldberg DE, Havlir DV, Rosenthal PJ. Antimalarial activity of human immunodeficiency virus type 1 protease inhibitors. Antimicrob Agents Chemother. 2005;49:2983–5. doi: 10.1128/AAC.49.7.2983-2985.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skinner-Adams TS, McCarthy JS, Gardiner DL, Hilton PM, Andrews KT. Antiretrovirals as antimalarial agents. J Infect Dis. 2004;190:1998–2000. doi: 10.1086/425584. [DOI] [PubMed] [Google Scholar]
- 12.Parikh S, Liu J, Sijwali P, Gut J, Goldberg DE, Rosenthal PJ. Antimalarial effects of human immunodeficiency virus type 1 protease inhibitors differ from those of the aspartic protease inhibitor pepstatin. Antimicrob Agents Chemother. 2006;50:2207–9. doi: 10.1128/AAC.00022-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Achan J, Kakuru A, Ikilezi G, et al. Antiretroviral agents and prevention of malaria in HIV-infected Ugandan children. N Engl J Med. 2012;367:2110–8. doi: 10.1056/NEJMoa1200501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okello PE, Van Bortel W, Byaruhanga AM, et al. Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg. 2006;75:219–25. [PubMed] [Google Scholar]
- 15.Uganda Ministry of Health. Uganda clinical guidelines. http://www.health.go.ug/docs/ucg_2010.pdf . Accessed May 2014.
- 16.Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown KN. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol Biochem Parasitol. 1993;58:283–92. doi: 10.1016/0166-6851(93)90050-8. [DOI] [PubMed] [Google Scholar]
- 17.Rogerson SJ, Pollina E, Getachew A, Tadesse E, Lema VM, Molyneux ME. Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am J Trop Med Hyg. 2003;68:115–9. [PubMed] [Google Scholar]
- 18.Cohan D, Natureeba P, Plenty A, et al. Efficacy and safety of LPV/r versus EFV in HIV+ pregnant and breast-feeding Ugandan women. Presented at: Conference on Retroviruses and Opportunistic Infections; Boston, Massachusetts. 2014. [Google Scholar]
- 19.Newman PM, Wanzira H, Tumwine G, et al. Placental malaria among HIV-infected and uninfected women receiving anti-folates in a high transmission area of Uganda. Malaria J. 2009;8:254. doi: 10.1186/1475-2875-8-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrews KT, Fairlie DP, Madala PK, et al. Potencies of human immunodeficiency virus protease inhibitors in vitro against Plasmodium falciparum and in vivo against murine malaria. Antimicrob Agents Chemother. 2006;50:639–48. doi: 10.1128/AAC.50.2.639-648.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nathoo S, Serghides L, Kain KC. Effect of HIV-1 antiretroviral drugs on cytoadherence and phagocytic clearance of Plasmodium falciparum-parasitised erythrocytes. Lancet. 2003;362:1039–41. doi: 10.1016/S0140-6736(03)14414-5. [DOI] [PubMed] [Google Scholar]
- 22.Skinner-Adams TS, Butterworth AS, Porter KA, et al. The frequency of malaria is similar among women receiving either lopinavir/ritonavir or nevirapine-based antiretroviral treatment. PLoS One. 2012;7:e34399. doi: 10.1371/journal.pone.0034399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandison TG, Homsy J, Arinaitwe E, et al. Protective efficacy of co-trimoxazole prophylaxis against malaria in HIV exposed children in rural Uganda: a randomised clinical trial. BMJ. 2011;342:d1617. doi: 10.1136/bmj.d1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.German P, Parikh S, Lawrence J, et al. Lopinavir/ritonavir affects pharmacokinetic exposure of artemether/lumefantrine in HIV-uninfected healthy volunteers. J Acquir Immune Defic Syndr. 2009;51:424–9. doi: 10.1097/QAI.0b013e3181acb4ff. [DOI] [PubMed] [Google Scholar]
- 25.McGready R, Stepniewska K, Lindegardh N, et al. The pharmacokinetics of artemether and lumefantrine in pregnant women with uncomplicated falciparum malaria. Eur J Clin Pharmacol. 2006;62:1021–31. doi: 10.1007/s00228-006-0199-7. [DOI] [PubMed] [Google Scholar]
- 26.Tarning J, McGready R, Lindegardh N, et al. Population pharmacokinetics of lumefantrine in pregnant women treated with artemether-lumefantrine for uncomplicated Plasmodium falciparum malaria. Antimicrob Agents Chemother. 2009;53:3837–46. doi: 10.1128/AAC.00195-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fried M, Muehlenbachs A, Duffy PE. Diagnosing malaria in pregnancy: an update. Expert Rev Anti Infect Ther. 2012;10:1177–87. doi: 10.1586/eri.12.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapito-Tembo A, Meshnick SR, van Hensbroek MB, Phiri K, Fitzgerald M, Mwapasa V. Marked reduction in prevalence of malaria parasitemia and anemia in HIV-infected pregnant women taking cotrimoxazole with or without sulfadoxine-pyrimethamine intermittent preventive therapy during pregnancy in Malawi. J Infect Dis. 2011;203:464–72. doi: 10.1093/infdis/jiq072. [DOI] [PMC free article] [PubMed] [Google Scholar]