Abstract
Background. The household has traditionally been the site for studying acute respiratory illnesses (ARIs). Most studies were conducted many years ago, and more broadly sensitive laboratory methods to determine ARI etiology are now available.
Methods. We recruited and followed households with children over 3 annual surveillance periods and collected respiratory tract specimens from subjects with reported ARI. Virus etiology was determined by real-time reverse-transcription polymerase chain reaction (RT-PCR) analysis.
Results. Individuals in larger households (defined as households with >4 members) and those in households with children aged <5 years had significantly higher ARI frequencies than others. ARI frequency generally declined with increasing age. Virus etiology was most likely to be determined in young children, who were also most likely to have virus coinfection. Overall, 16% of ARIs with 1 virus identified had ≥1 coinfecting virus. Rhinoviruses and coronaviruses were the most frequently identified agents of ARI in all age categories. Influenza virus and adenovirus were less frequently identified but were most likely to cause ARI that required medical attention.
Conclusions. Longitudinal studies in families remain a valuable way to study respiratory infections. RT-PCR has increased the sensitivity of virus detection, including coinfecting viruses, and expanded our ability to detect viruses now known to cause ARI.
Keywords: acute respiratory illnesses, influenza, respiratory viruses, households with children, surveillance
Acute respiratory infections are the most common cause of symptomatic illness in the general population. As a result, they have been studied often over the past century, first in terms of their frequency and characteristics and then, in the last 50 years, their etiology, after laboratory techniques made it possible to identify the agents involved [1–3]. The household has traditionally been the site for studying these illnesses since it allowed identification of varying illness frequencies in different members of the family in a setting where significant transmission occurred [4]. While it is possible to draw general conclusions from the findings of these household studies, there were clear differences among them, based on the methods used and the populations investigated [5–9]. Differing were the number of households followed, the way they were identified for participation, the method and frequency of contact for reporting illness onset, whether specimens were collected on a regular basis or only at illness onset, and the case definition that triggered specimen collection.
Household studies have not been performed for many years, in large part because of expense and logistical challenges. In the intervening years, there have been changes in society, especially in family size and the roles of family members. In addition, newer, more broadly sensitive methods of identifying illness etiology have been developed [10–12], and vaccines (eg, influenza, Haemophilus influenzae type b, and pneumococcal vaccines) potentially affecting acute respiratory illness (ARI) frequencies have become part of standard childhood vaccination programs [13–15]. As a result, there has been interest in performing household studies in the current environment, especially since updated household transmission data could inform approaches to the control of influenza pandemics [16]. However, such studies are labor-intensive, and previously used methods, such as home visits, would be logistically difficult and prohibitively expensive.
We have developed an updated approach, modeled on the design of the Tecumseh Study of Respiratory Illnesses, which has allowed us to recruit and follow a similar number of households [6]. However, in contrast to the year-round surveillance performed in that study, surveillance activities were focused on the fall/winter months, when ARI events were most likely; in addition, rather than home visits, illness visits with specimen collection were performed at the research study site. We examined ARI frequency, virus seasonality, and agent-specific infection frequency over 3 respiratory illness seasons in this prospective cohort of households with children. Data from this study have been previously used to examine influenza vaccine effectiveness, influenza transmission parameters, and factors associated with vaccination decisions [17–19].
METHODS
Study Participants
Over 3 years, beginning in 2010, households (shared residence) with at least 4 members, at least 2 of whom were children aged <18 years, were enrolled and followed through annual respiratory illness surveillance periods. Each annual cohort of households was derived from persons who had selected a primary healthcare provider from within the University of Michigan Health System in Ann Arbor; interested households were permitted to participate during multiple years. At enrollment visits, adult household members provided written informed consent for participation for themselves and their children, and children aged 7–17 years provided their oral assent. Member demographic data were reported, and study access to health system medical records was granted. All study contacts with participants, including enrollment and illness visits, were performed at the research study site at the University of Michigan School of Public Health (UM-SPH). The study was approved by the institutional review board at the University of Michigan Medical School.
ARI Surveillance
During the first and third study years (the 2010–2011 and 2012–2013 seasons, respectively), households were enrolled during the summer months, and respiratory illness surveillance was performed from October through early May (2010–2011, 30 weeks; 2012–2013, 32 weeks); in the second study year (the 2011–2012 season), enrollment was delayed until the fall months, and surveillance was initiated in December and continued through early May (23 weeks). Households were instructed at enrollment and via weekly e-mail reminders to report all ARIs defined by ≥2 of the following symptoms: cough, fever or feverishness, nasal congestion, chills, headache, body aches, or sore throat. This case definition facilitated inclusion of even mild symptomatic illnesses. Subjects with eligible illnesses attended an illness visit within 7 days of illness onset and had a combined throat and nasal swab specimen (or nasal swab specimen only, for children aged <3 years) collected for identification of virus etiology. During the first, second, and third surveillance periods, 96%, 95%, and 92% of reported ARIs, respectively, had a specimen collected for virus identification; only ARIs with a specimen available for laboratory testing were included here for analysis. Illnesses were followed for collection of data on illness characteristics, including whether the subject sought medical care; documentation of healthcare contact for treatment of ARI was also noted based on medical record review.
Laboratory Testing
Collected specimens were tested for influenza virus and 11 other common respiratory viruses by means of real-time reverse-transcription polymerase chain reaction (RT-PCR) on an ABI 7500 RT-PCR system platform (Life Technologies). The SuperScript III Platinum One-Step Quantitative RT-PCR system, plus primers and probes developed by the Centers for Disease Control and Prevention (CDC) Influenza Division, were used for detection of influenza viruses [20]. The AgPath-ID One-Step RT-PCR system, plus primers and probes developed by the CDC Division of Viral Diseases, Gastroenteritis, and Respiratory Viruses, were used for detection of respiratory syncytial viruses (RSVs), human metapneumoviruses (HMPVs), parainfluenza viruses 1–3, rhinoviruses, adenoviruses, and 4 coronaviruses (229E, OC43, HKU1, and NL63) [10]. RNA was extracted from specimens for influenza virus testing by means of the Qiagen QIAamp Viral RNA Mini Kit, and DNA/RNA was extracted for additional respiratory virus testing by means of the Qiagen QIAamp MinElute Virus Vacuum Kit, using the accompanying vacuum manifold protocols. Laboratory testing was performed in the investigators' respiratory virus laboratory at the UM-SPH.
Statistical Analyses
Each study year, households were characterized by size (number of members) and composition (presence of members <5 years of age), and subjects were characterized by sex, age category, and high-risk health status. High-risk health conditions, including cardiac and pulmonary disorders, were documented on the basis of medical record review. For each surveillance period, the mean number of reported ARIs with specimen collection per individual was examined and compared by household and subject characteristics, using 2-sample or pairwise t tests with corrections for multiple testing, where appropriate. Frequency distributions of household and household member experiences with ARI events were examined, and the mean number of ARIs per household and per individual, by age category, were estimated and compared across study years. The seasonality of virus-specific circulation was examined, and annual epidemic curves were compared. Agent-specific infection frequencies were examined and compared by age category, likelihood of medical attention, and presence of coinfecting viruses; frequencies were pooled across study years. Statistical analyses were conducted using R (version 3.0.2) statistical software.
RESULTS
Population Characteristics
In the first (2010-2011) and third (2012-2013) study years, 328 and 321 households, respectively, were enrolled and followed, with >1400 subjects participating each year (Table 1). As a result of the delayed and shorter enrollment period, fewer households (213 households and 943 subjects) were enrolled and followed in the second study year (2011–2012). In all 3 study years, mean household size was approximately 4.5 persons; 58% of subjects were children <18 years of age, and 4% were older adults (age, >49 years). Each study year, approximately 11% of subjects had medical record documentation of 1 or more high-risk health conditions, and approximately 60% had received an annual influenza vaccination.
Table 1.
Characteristics of Enrolled Households and Study Participants and Number of Acute Respiratory Illness (ARI) Cases Per Individual—Ann Arbor, Michigan, 2010–2013
| Characteristic | 2010-2011 Season, 30 wks Follow-up |
2011-2012 Season, 23 wks Follow-up |
2012-2013 Season, 32 wks Follow-up |
|||
|---|---|---|---|---|---|---|
| Households or Participants, Proportion (%) | ARI Cases, No., Mean (SD) | Households or Participants, Proportion (%) | ARI Cases, No., Mean (SD) | Households or Participants, Proportion (%) | ARI Cases, No., Mean (SD) | |
| Households | ||||||
| Overall | 328/328 (100) | 3.0 (3.7) | 213/213 (100) | 1.9 (3.0) | 321/321 (100) | 3.5 (4.3) |
| Sizea | ||||||
| >4 members | 505/1441 (35.0) | 0.8 (1.1)b | 359/943 (38.1) | 0.6 (0.9)b | 542/1426 (38.0) | 0.8 (1.2) |
| 4 members | 936/1441 (65.0) | 0.6 (0.9) | 584/943 (61.9) | 0.3 (0.7) | 884/1426 (62.0) | 0.8 (1.1) |
| Children aged <5 ya | ||||||
| Present | 706/1441 (49.0) | 0.9 (1.15)b | 359/943 (38.1) | 0.6 (0.9)b | 678/1426 (47.5) | 1.0 (1.3)b |
| Absent | 735/1441 (51.0) | 0.5 (0.8) | 584/943 (61.9) | 0.3 (0.7) | 748/1426 (52.5) | 0.6 (0.9) |
| Participants | ||||||
| Overall | 1441/1441 (100) | 0.7 (1.0) | 943/943 (100) | 0.4 (0.8) | 1426/1426 (100) | 0.8 (1.2) |
| Age categoryc | ||||||
| <5 y (reference) | 233/1441 (16.2) | 1.1 (1.2) | 112/943 (11.9) | 0.7 (1.1) | 217/1426 (15.2) | 1.4 (1.6) |
| 5–11 y | 392/1441 (27.2) | 0.7 (1.0)b | 289/943 (30.7) | 0.4 (0.8)d | 404/1426 (28.3) | 0.8 (1.1)b |
| 12–17 y | 214/1441 (14.9) | 0.5 (0.9)b | 149/943 (15.8) | 0.2 (0.5)b | 212/1426 (14.9) | 0.5 (0.7)b |
| 18–49 y | 544/1441 (37.8) | 0.6 (1.0)b | 352/943 (37.3) | 0.4 (0.8)d | 536/1426 (37.6) | 0.8 (1.1)b |
| ≥50 y | 58/1441 (4.0) | 0.4 (0.7)b | 41/943 (4.4) | 0.2 (0.5)d | 57/1426 (4.0) | 0.4 (0.6)b |
| Sexa | ||||||
| Female | 728/1441 (50.5) | 0.7 (1.1) | 463/943 (49.1) | 0.4 (0.8) | 712/1426 (49.9) | 0.9 (1.2)b |
| Male | 713/1441 (49.5) | 0.6 (0.9) | 480/943 (50.9) | 0.4 (0.7) | 714/1426 (50.1) | 0.7 (1.0) |
| High-risk health conditiona | ||||||
| Any | 162/1441 (11.2) | 0.7 (1.0) | 109/943 (11.6) | 0.4 (0.7) | 136/1426 (9.5) | 0.7 (1.1) |
| None | 1279/1441 (88.8) | 0.7 (1.0) | 834/943 (88.4) | 0.4 (0.80) | 1290/1426 (90.5) | 0.8 (1.2) |
Data are from the Household Influenza Vaccine Effectiveness (HIVE) study.
a Comparisons were made using the 2-sample t test.
b P < .001.
c The pairwise t test with correction for multiple tests was used to assess differences in mean number of ARI cases between each group and the reference.
d P < .05 for within-category differences.
ARI Frequencies
Surveillance periods covered much of each respiratory season, lasting 30 and 32 weeks in the first and third years, respectively, and 23 weeks in the second year. Since it would not be possible to estimate the illnesses that would have occurred in the remaining weeks, annual rates could not be calculated. In all surveillance periods, the mean number of reported ARI episodes with specimen collection was <1 per individual (Table 1). The highest mean frequency during the respective surveillance period was observed in years 1 (0.7) and 3 (0.8); in year 2 there were 0.4 ARIs per individual. Individuals in households with >4 members experienced significantly higher mean values than those in households with only 4 members in periods 1 and 2, as did those in households with young children (age, <5 years) during all 3 surveillance periods. Young children had the highest mean ARI frequency of any age group during each period. The average number of ARIs generally declined with increasing age, but adults aged 18–49 years had more-frequent ARIs than older children (age, 12–17 years) in all study years, and estimates were similar to those seen in children 5–11 years of age. Female subjects experienced more ARIs than male subjects, but the difference was significant only in study year 3. Presence of any high-risk health conditions was not associated with a higher mean ARI frequency.
The distributions of household and household member experiences with ARI episodes over the 3 seasons are presented in Tables 2 and 3. The number of ARI events reported by individual households each season ranged from 0 to ≥10; the average number of ARIs per household was 3.0 and 3.5 in study years 1 and 3, respectively, and 1.9 in year 2. Each season, the number of ARI events experienced by individual subjects ranged from 0 to 6.
Table 2.
The Distribution of Cases of Acute Respiratory Illness (ARI) per Household Over 3 Surveillance Seasons—Ann Arbor, Michigan, 2010–2013
| Cases/Household, No. | Households, No., by Surveillance Year |
||
|---|---|---|---|
| 2010–2011 (n = 328) | 2011–2012 (n = 213) | 2012–2013 (n = 321) | |
| 0 | 95 | 106 | 91 |
| 1 | 59 | 31 | 34 |
| 2 | 42 | 15 | 52 |
| 3 | 26 | 24 | 26 |
| 4 | 28 | 11 | 32 |
| 5 | 18 | 7 | 16 |
| 6 | 15 | 3 | 10 |
| 7 | 11 | 2 | 11 |
| 8 | 8 | 5 | 12 |
| 9 | 3 | 1 | 6 |
| 10 | 5 | 1 | 8 |
| >10 | 18 | 7 | 23 |
| Total cases | 984 | 398 | 1133 |
| Mean cases | 3.0 | 1.9 | 3.5 |
Data are from the Household Influenza Vaccine Effectiveness (HIVE) study.
Table 3.
The Distribution of Cases of Acute Respiratory Illness (ARI) per Household Member Over 3 Surveillance Seasons—Ann Arbor, Michigan, 2010–2013
| Members, No., by Surveillance Year and Age |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2010–2011 (N = 1441) |
2011–2012 (N = 943) |
2012–2013 (N = 1426) |
|||||||
| Cases/Member, No. | <5 y (n = 232) | 5–17 y (n = 607) | ≥18 y (n = 602) | <5 y (n = 112) | 5–17 y (n = 438) | ≥18 y (n = 393) | <5 y (n = 217) | 5–17 y (n = 616) | ≥18 y (n = 593) |
| 0 | 97 | 363 | 378 | 67 | 320 | 281 | 88 | 355 | 325 |
| 1 | 71 | 145 | 149 | 24 | 91 | 77 | 53 | 169 | 173 |
| 2 | 31 | 69 | 44 | 12 | 19 | 24 | 26 | 57 | 58 |
| 3 | 22 | 21 | 19 | 7 | 6 | 9 | 25 | 22 | 18 |
| 4 | 7 | 5 | 7 | 1 | 0 | 1 | 15 | 8 | 14 |
| 5 | 2 | 2 | 5 | 0 | 2 | 1 | 6 | 3 | 3 |
| 6 | 2 | 2 | 0 | 1 | 0 | 0 | 4 | 2 | 2 |
| Total cases | 249 | 388 | 347 | 80 | 157 | 161 | 298 | 409 | 426 |
| Mean cases | 1.07 | 0.64 | 0.58 | 0.71 | 0.36 | 0.41 | 1.37 | 0.66 | 0.72 |
Data are from the Household Influenza Vaccine Effectiveness (HIVE) study.
Virus Seasonality
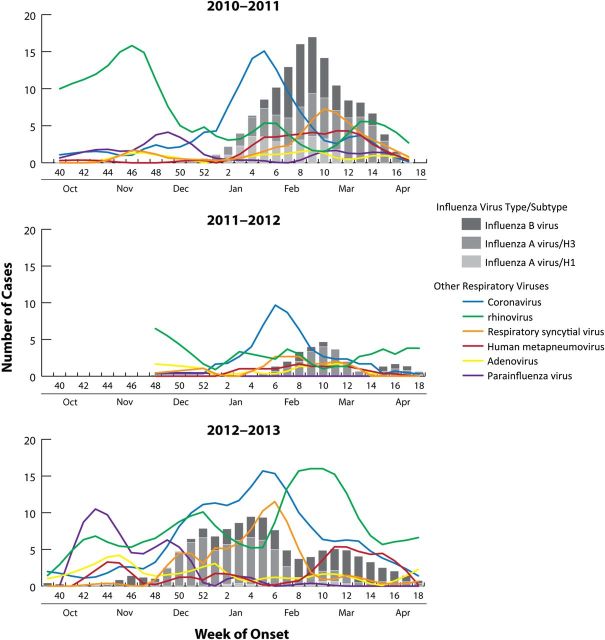
The agent-specific seasonality of etiologically defined respiratory virus circulation over the 3 surveillance periods is presented in Figure 1. The ARI outbreaks were similarly intense in the approximately 30-week surveillance periods in study years 1 and 3 and considerably milder in the shorter (23 week) period in year 2. Influenza virus circulated intensively from January through April 2011 (year 1), sporadically from February through April 2012 (year 2), and with moderate intensity but far longer duration from November 2012 through April 2013 (year 3). Influenza A(H3N2) virus, 2009 pandemic influenza A(H1N1) virus, and influenza B virus cocirculated in year 1, influenza A(H3N2) virus predominated in year 2, and influenza A(H3N2) virus and influenza B virus cocirculated in year 3. Each year, rhinoviruses and coronaviruses were both frequently identified throughout surveillance periods. Peak circulation of rhinoviruses occurred in October–November in year 1 and February–March in year 3; peak circulation of coronaviruses was during the winter months either immediately prior to or consistent with peak influenza virus circulation. In study years 1 and 3, coronavirus types NL63 and OC43 predominated throughout, with types HKU1 and 229E occurring less frequently and later in the season; in year 2, all 4 types circulated with similar frequencies. Parainfluenza viruses and adenoviruses circulated at relatively low levels in study years 1 and 2, with modest increases in activity for both in year 3; parainfluenza virus infections (42% due to type 2 and 51% due to type 3) were primarily identified from October through December, and adenovirus infections were identified at low frequency throughout the surveillance periods. RSVs circulated for extended periods in study years 1 and 3, and the timing of peak activity was similar to peak influenza activity in those years; RSV circulation was less intense and of shorter duration in year 2. In all 3 study years, the timing of peak HMPV circulation was consistent with the timing of influenza virus circulation.
Figure 1.
Circulation of influenza and non-influenza respiratory viruses over 3 surveillance seasons. Weekly cases are presented using LOWESS smoothing and 3-week moving averages
Agent-Specific Frequencies
Virus etiology was determined in 57% of ARIs in study year 1, 47% in year 2, and 63% in year 3. The estimated contribution of tested respiratory viruses in producing ARIs each season was examined and compared by age-category and likelihood of seeking medical attention; frequencies were pooled across surveillance periods and are presented in Table 4. The specific viruses causing ARIs were most likely to be identified in children aged <5 years (77% of ARIs were etiologically confirmed) and least likely in adults aged 18–49 years (46% were etiologically confirmed). Rhinoviruses and coronaviruses were the most frequently identified viruses in all age categories (21% and 16% overall, respectively), with coronaviruses more frequent than rhinoviruses in adults; ARIs caused by these viruses and ARIs with no agent identified were least likely to be medically attended. Conversely, ARIs caused by influenza B virus and adenoviruses were least frequently identified overall but were most likely to be medically attended. Among older children (age, 12–17 years) and adult subjects, other than ARIs caused by rhinoviruses and coronaviruses, influenza A virus and RSV were the viruses most frequently identified. ARIs caused by HMPV and parainfluenza viruses were similarly prevalent, most frequently identified in young children and intermediate in terms of being medically attended. Overall, 10% of ARIs had ≥2 coinfecting viruses identified; 67% of infections involving adenoviruses, 41% involving HMPVs, and 32% involving RSVs had other coinfecting viruses identified. Among ARIs with at least 1 virus identified, 16% had 1 or more coinfecting viruses identified; coinfection was most likely to occur in young children (23%).
Table 4.
Estimated Contribution of Tested Respiratory Viruses to Cases of Acute Respiratory Illness (ARI), by Age Category and Likelihood of Medical Attention—Ann Arbor, Michigan, 2010–2013
| Characteristic | ARI Cases | ARI Cases, Proportion (%), by PCR-Confirmed Etiologic Agent |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RV | CoVa | RSV | HMPV | HPIVb | ADV | FLUAV | FLUBV | Unknown Agent | Coinfection | ||
| Age category | |||||||||||
| <5 y | 627/2515 (25) | 206/627 (33) | 102/627 (16) | 70/627 (11) | 58/627 (9) | 54/627 (9) | 51/627 (8) | 34/627 (5) | 23/627 (4) | 147/627 (23) | 109/480 (23) |
| 5–11 y | 696/2515 (28) | 127/696 (18) | 117/696 (17) | 46/696 (7) | 35/696 (5) | 32/696 (5) | 29/696 (4) | 59/696 (8) | 48/696 (7) | 279/696 (40) | 78/417 (19) |
| 12–17 y | 258/2515 (10) | 56/258 (22) | 29/258 (11) | 12/258 (5) | 4/258 (2) | 3/258 (1) | 3/258 (1) | 19/258 (7) | 9/258 (3) | 127/258 (49) | 6/131 (5) |
| 18–49 y | 879/2515 (35) | 119/879 (14) | 152/879 (17) | 33/879 (4) | 20/879 (2) | 30/879 (3) | 11/879 (1) | 58/879 (7) | 18/879 (2) | 479/879 (54) | 45/400 (11) |
| ≥50 y | 55/2515 (2) | 8/55 (15) | 12/55 (22) | 3/55 (5) | 2/55 (4) | 1/55 (2) | 0/55 (0) | 6/55 (11) | 0/55 (0) | 25/55 (45) | 2/30 (7) |
| Total | 2515/2515 (100) | 516/2515 (21) | 412/2515 (16) | 164/2515 (7) | 119/2515 (5) | 120/2515 (5) | 94/2515 (4) | 176/2515 (7) | 98/2515 (4) | 1057/2515 (42) | 240/1458 (16) |
| Medically attended ARI | 527/2515 (21) | 86/516 (17) | 69/412 (17) | 37/164 (23) | 33/119 (28) | 35/120 (29) | 33/94 (35) | 53/176 (30) | 42/98 (43) | 200/1057 (19) | … |
| Coinfection | 240/2515 (10) | 119/516 (23) | 101/412 (25) | 53/164 (32) | 49/119 (41) | 33/120 (28) | 63/94 (67) | 42/176 (24) | 19/98 (19) | … | … |
Data are from the Household Influenza Vaccine Effectiveness (HIVE) study and pooled across seasonal surveillance activities conducted during 2010-2011, 2011-2012, and 2012-2013.
Abbreviations: ADV, adenovirus; CoV, coronavirus; FLUAV, influenza A virus; FLUBV, influenza B virus; HMPV, human metapneumovirus; HPIV, parainfluenza virus; PCR, polymerase chain reaction; RSV, respiratory syncytial virus; RV, rhinovirus.
a Among 120 ARIs with ≥1 HPIV identified, 10 involved HPIV-1, 51 involved HPIV-2, and 61 involved HPIV-3.
b Among 412 ARIs with ≥1 CoVs identified, 35 involved CoV-229E, 182 involved CoV-OC43, 152 involved CoV-NL63, and 64 involved CoV-HKU1.
DISCUSSION
Multiyear observational studies of respiratory illnesses performed many years ago formed the basis of current knowledge of the behavior of respiratory viruses in the general population [1–9]. While all of the studies were longitudinal in nature, they involved varied population groups and differed in other characteristics, such as case definitions, ways of ascertaining that an illness had occurred, and how etiologic agents were identified [6–8]. Despite this variation, certain concepts emerged involving both how illnesses in general varied in the population by age, sex, and family composition and how the individual viruses varied by frequency of identification and by season [21].
There was a perception that these studies could not again be performed, for reasons of logistics and expense. This was despite the concern that social changes might have altered the frequency of illnesses and transmission characteristics; previous observations had formed the basis of strategies for control of an influenza pandemic, which would make initiation of such studies desirable. There have also been recent major improvements in the determination of illness etiology, driven by the development of the RT-PCR assay [10–12]. This technique has increased the sensitivity of virus detection and expanded the ability to detect viruses now known to cause ARIs (eg, HMPVs and new coronaviruses) [22–26]. RT-PCR is also more easily and reliably performed than isolation of virus in cell culture or serology, both of which were used extensively in past studies to determine etiology [21, 27–29].
We began the current study of respiratory tract infection in households as a way to supply updated information on influenza frequency and transmission, using a design that would manage costs and overcome some of the logistical challenges. We concentrated surveillance on the period when we expected influenza virus to be circulating, but we also extended this period so that occurrence of other viruses, when influenza virus was not circulating, could be determined. In fact, the period covered would include most of the annual time of increased transmission of respiratory viruses in general. We used an ARI case definition more rigorous than that used in the Tecumseh study [6] and required that ill individuals come to the research study site to have specimens collected. This could partially explain the lower frequency of illnesses currently identified. However, the overall patterns of illness frequency observed here were similar to those observed previously. Specifically, ARI frequency declined with increasing age in childhood, and increased among young adults with exposure to children [21, 27, 29]. However, even if it were possible to annualize the total age-specific frequencies of illnesses seen, they would be lower than those seen in the older studies [5, 21, 27, 29]. There could be many explanations for this, including changes in living conditions and implementation of vaccination programs, in addition to differences in study design. Indeed, such differences related to design were demonstrated within the Seattle Virus Watch, a classic household study [8]. The trade-off between intensively following a small number of households and less intensive surveillance of larger, more diverse households has been previously recognized [30].
In other ways, the current results confirm observations of the original household studies. Rhinoviruses were the most commonly identified viruses in the original and current studies, even though the surveillance periods in the current study did not include the period immediately following opening of schools, when that virus predominates [31–33]. Coronavirus infections were documented in the Tecumseh study only by means of serology; at that time, only 229E and OC43 were recognized, with the latter being more common [28, 34]. Now, NL63 is a commonly identified coronavirus, with HKU1 similar in frequency to 229E [22–24]. It had been suspected that the role of coronaviruses in common respiratory infections had been underestimated, and our findings support that hypothesis. RSVs, HMPVs, and parainfluenza viruses are mainly thought of as childhood viruses [26, 35–38]; while they were more common in young children, they were found in all age groups and were intermediate in requiring medical attention. Too few parainfluenza viruses were detected to note the alternative patterns of serotype circulation seen previously [36, 39]. During the 3 seasons, 274 influenza viruses were identified, mainly in years 1 and 3, despite vaccination coverage of approximately 60% [17]. Medical consultation was higher for influenza B virus than for influenza A virus; this may be a reflection of the higher frequency of type B seen in children.
Previously, using cell culture, isolation of >1 virus from the same illness was rare. Now, with RT-PCR, detection of >1 virus has become common. This was particularly the case in younger children and especially with adenoviruses, but we also observed substantial coinfection with HMPV and RSV. It will be challenging to separate long-term shedding, which is probably the case with adenovirus, and potentially incidental coinfection, which may be the case with some other viruses (eg, rhinoviruses), from actual relation to the current illness [40]. Frequent identification of coinfecting viruses has also been demonstrated in studies involving medically attended ARIs [41]. Other studies, involving day care attendees, have found no relation between the number of infectious agents identified and illness severity [42], an observation in line with our finding of coinfection even in those with nonmedically attended illnesses.
Overall, we have demonstrated that it is possible to perform longitudinal studies of respiratory illnesses in US households. Comparison with the older studies shows more similarities than differences in terms of patterns of illness occurrence. While the absolute frequency of illnesses may have decreased, young children are still the individuals most frequently infected. Coronaviruses have now joined the rhinoviruses in causing most illnesses, and medical consultation remains a good indicator of illness severity [27]. The PCR technique has detected a new issue, virus coinfection. The etiological role of each virus identified will need to be examined, perhaps by specimens collected early and late in the illness. This type of household study is logistically challenging but permits not only the observations reported here, but also, for example, longitudinal analyses of interactions between viruses that could affect subsequent infection risks. As is illustrated by the year-to-year variation observed [29], evaluations need to be performed over time, allowing the maximum use of the data collected.
Notes
Acknowledgments. We thank laboratory and study staff, including Barbara Aaron, Casey Martens, Anne Kaniclides, Emileigh Johnson, Rachel Cross, and Elizabeth Vickers, for their ongoing contributions to the success of the HIVE study; Dean Erdman (CDC), for sharing his primers/probes and laboratory protocols for RT-PCR identification of non–influenza respiratory viruses; and the Influenza Division, CDC, for sharing similar materials for influenza virus identification.
Disclaimers. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (grant R01 AI097150) and the Centers for Disease Control and Prevention (grant U01 IP000474).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Sydenstricker E. A study of illness in a general population group: Hagerstown morbidity studies no. I: the method of study and general results. Public Health Rep. 1926;41:2069–88. [Google Scholar]
- 2.Frost WH, Gover M. The incidence and time distribution of common colds in several groups kept under continuous observation. Public Health Rep. 1932;47:1815–41. [Google Scholar]
- 3.Dingle JH. Illness in the home: a study of 25,000 illnesses in a group of Cleveland families. Cleveland: Press of Western Reserve University; 1964. p. 398. [Google Scholar]
- 4.Buck C. Acute upper respiratory infections in families. Am J Epidemiol. 1956;63:1–12. doi: 10.1093/oxfordjournals.aje.a119786. [DOI] [PubMed] [Google Scholar]
- 5.Dingle JH, Badger GF, Feller AE, et al. A study of illness in a group of Cleveland families: I. Plan of study and certain general observations. Am J Epidemiol. 1953;58:16–30. doi: 10.1093/oxfordjournals.aje.a119587. [DOI] [PubMed] [Google Scholar]
- 6.Monto AS, Napier JA, Metzner HL. The Tecumseh study of respiratory illness. I. Plan of study and observations on syndromes of acute respiratory disease. Am J Epidemiol. 1971;94:269–79. doi: 10.1093/oxfordjournals.aje.a121320. [DOI] [PubMed] [Google Scholar]
- 7.Fox JP, Elverback LR, Spigland I, et al. The Virus Watch program: a continuing surveillance of viral infections in metropolitan New York families: I. Overall plan, methods of collecting and handling information and a summary report of specimens collected and illnesses observed. Am J Epidemiol. 1966;83:389–412. [Google Scholar]
- 8.Fox JP, Hall CE, Cooney MK, Luce RE, Kronmal RA. The Seattle Virus Watch. II. Objectives, study population and its observation, data processing and summary of illnesses. Am J Epidemiol. 1972;96:270–85. doi: 10.1093/oxfordjournals.aje.a121458. [DOI] [PubMed] [Google Scholar]
- 9.Hope-Simpson RE, Higgins PG. A respiratory virus study in Great Britain: review and evaluation. Prog Med Virol. 1969;11:354–407. [PubMed] [Google Scholar]
- 10.Sakthivel SK, Whitaker B, Lu X, et al. Comparison of fast-track diagnostic respiratory pathogens multiplex real-time RT-PCR assay with in-house singleplex assays for comprehensive detection of human respiratory viruses. J Virol Meth. 2012;185:259–66. doi: 10.1016/j.jviromet.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brittain-Long R, Nord S, Olofsson S, Westin J, Anderson LM, Lindh M. Multiplex real-time PCR for detection of respiratory tract infections. J Clin Virology. 2008;41:53–6. doi: 10.1016/j.jcv.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim C, Ahmed JA, Eidex RB, et al. Comparison of nasopharyngeal and oropharyngeal swabs for the diagnosis of eight respiratory viruses by real-time reverse transcription-PCR assays. PLoS One. 2011;6:e21610. doi: 10.1371/journal.pone.0021610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Prevention and control of influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morbid Mortal Wkly Rep. 2010;59:1–62. [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Prevention and control of haemophilus influenzae type B disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2014. MMWR Morbid Mortal Wkly Rep. 2014;63:1–14. [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Prevention of pneumococcal disease among infants and children – use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morbid Mortal Wkly Rep. 2010;59:1–18. [Google Scholar]
- 16.Committee on Modeling Community Containment for Pandemic Influenza and Institute of Medicine. Modeling community containment for pandemic influenza: A letter report. 2007. http://books.nap.edu/catalog/11800.html#orgs. Accessed 25 January 2014.
- 17.Ohmit SE, Petrie JG, Malosh RE, et al. Influenza vaccine effectiveness in the community, and the household. Clin Infect Dis. 2013;56:1363–9. doi: 10.1093/cid/cit060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrie JG, Ohmit SE, Cowling BJ, et al. Influenza transmission in a cohort of households with children: 2010-2011. PLoS ONE. 2013;8:e75339. doi: 10.1371/journal.pone.0075339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malosh RE, Ohmit SE, Petrie JG, Thompson MG, Aiello AE, Monto AS. Factors associated with influenza vaccine receipt in community dwelling adults and their children. Vaccine. 2014;32:1841–7. doi: 10.1016/j.vaccine.2014.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohmit SE, Thompson MG, Petrie JG, et al. Influenza vaccine effectiveness in the 2011-2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis. 2014;58:319–27. doi: 10.1093/cid/cit736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev. 1994;16:351–73. doi: 10.1093/oxfordjournals.epirev.a036158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Hoek L, Pyrc K, Jebbink MF, et al. Identification of a new human coronavirus. Nat Med. 2004;10:368–73. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woo PC, Lau SK, Chu CM, et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005;79:884–95. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuypers J, Martin ET, Heugel J, Wright N, Morrow R, Englund J. Clinical disease in children associated with newly described coronavirus subtypes. Pediatrics. 2006;119:e70–6. doi: 10.1542/peds.2006-1406. [DOI] [PubMed] [Google Scholar]
- 25.Van den Hoogen BG, de Jong JC, Groen J, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–24. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamelin ME, Abed Y, Boivin G. Human metapneumovirus: a new player among respiratory viruses. Clin Infect Dis. 2004;38:983–90. doi: 10.1086/382536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monto AS, Sullivan KM. Acute respiratory illness in the community. Frequency of illness and the agents involved. Epidemiol Infect. 1993;110:145–60. doi: 10.1017/s0950268800050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavallaro JJ, Monto AS. Community-wide outbreak of infection with a 229E-like coronavirus in Tecumseh, Michigan. J Infect Dis. 1970;122:272–9. doi: 10.1093/infdis/122.4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monto AS. Occurrence of respiratory virus: time, place and person. Pediatr Infect Dis J. 2004;23:S58–64. doi: 10.1097/01.inf.0000108193.91607.34. [DOI] [PubMed] [Google Scholar]
- 30.Fox JP, Cooney MK, Hall CE. The Seattle virus watch. V. Epidemiologic observations of rhinovirus infections, 1965-1969, in families with young children. Am J Epidemiol. 1975;101:122–43. doi: 10.1093/oxfordjournals.aje.a112078. [DOI] [PubMed] [Google Scholar]
- 31.Hendley JO, Gwaltney JM, Jordan WS. Rhinovirus infections in an industrial population. IV. Infections within families of employees during two fall peaks of respiratory illness. Am J Epidemiol. 1969;89:184–96. doi: 10.1093/oxfordjournals.aje.a120928. [DOI] [PubMed] [Google Scholar]
- 32.Gwaltney JM, Jr, Hendley JO, Simon G, Jordan WS., Jr Rhinovirus infections in an industrial population. I. The occurrence of illness. N Engl J Med. 1966;275:1261–8. doi: 10.1056/NEJM196612082752301. [DOI] [PubMed] [Google Scholar]
- 33.Monto AS, Bryan ER, Ohmit S. Rhinovirus infections in Tecumseh, Michigan: frequency of illness and number of serotypes. J Infect Dis. 1987;156:43–9. doi: 10.1093/infdis/156.1.43. [DOI] [PubMed] [Google Scholar]
- 34.Monto AS, Lim SK. The Tecumseh study of respiratory illness. VI. Frequency of and relationship between outbreaks of coronavirus infection. J Infect Dis. 1974;129:271–6. doi: 10.1093/infdis/129.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fairchok MP, Martin ET, Chambers S, et al. Epidemiology of viral respiratory tract infections in a prospective cohort of infants and toddlers attending daycare. J Clin Virol. 2010;49:16–20. doi: 10.1016/j.jcv.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fry AM, Curns AT, Harbour K, Hutwagner L, Holman RC, Anderson LJ. Seasonal trends of human parainfluenza virual infections: United States, 1990-2004. Clin Infect Dis. 2006;43:1016–22. doi: 10.1086/507638. [DOI] [PubMed] [Google Scholar]
- 37.Reed G, Jewett PH, Thompson J, Tollefson S, Wright PF. Epidemiology and clinical impact of parainfluenza virus infections in otherwise healthy infants and young children <5 years old. J Infect Dis. 1997;175:807–13. doi: 10.1086/513975. [DOI] [PubMed] [Google Scholar]
- 38.Papenburg J, Boivin G. The distinguishing features of human metapneumovirus and respiratory syncytial virus. Rev Med Virol. 2010;20:245–60. doi: 10.1002/rmv.651. [DOI] [PubMed] [Google Scholar]
- 39.Monto AS. The Tecumseh study of respiratory illness. V. Patterns of infection with the parainfluenzaviruses. Am J Epidemiol. 1973;97:338–48. doi: 10.1093/oxfordjournals.aje.a121514. [DOI] [PubMed] [Google Scholar]
- 40.Fry AM, Lu X, Olsen SJ, et al. Human rhinovirus infections in rural Thailand: epidemiological evidence for rhinovirus as both pathogen and bystander. PLoS One. 2011;6:e17780. doi: 10.1371/journal.pone.0017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fowlkes A, Giorgi A, Erdman D, et al. Etiology of acute respiratory infections among outpatients from the Influenza Incidence Surveillance Project, 2010–2011. J Infect Dis. 2014;209:1715–25. doi: 10.1093/infdis/jit806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin ET, Fairchok MP, Stednick ZJ, Kuypers J, Englund JA. Epidemiology of multiple respiratory viruses in childcare attendees. J Infect Dis. 2013;207:982–9. doi: 10.1093/infdis/jis934. [DOI] [PMC free article] [PubMed] [Google Scholar]