Abstract
This review examines the evidence indicating a role for parasitic mites in the transmission and maintenance of Hantaan virus in nature. The available data, much of it from recent studies in China, indicate that both trombiculid and gamasid mites are naturally infected with Hantaan virus and that infected mites can transmit the virus by bite to laboratory mice and transovarially (vertically) through eggs to their offspring. Collectively, these findings challenge the current paradigm of hantavirus transmission, namely, that rodents serve as the reservoir of human pathogenic hantaviruses in nature and that humans are infected with these viruses by inhalation of aerosols of infectious rodent excreta. Further research is needed to confirm the mite-hantavirus association and to determine if parasitic mites are in fact the major source and principal vectors of human pathogenic hantaviruses, such as Hantaan. If the mite hypothesis is correct, then it will significantly alter current concepts about the epidemiology, prevention, and control of human hantavirus infection.
Keywords: Hantaan virus, hantavirus infection, hantavirus transmission, vector biology, arbovirus infection, mite-borne disease, hemorrhagic fever with renal syndrome
Viruses in the family Bunyaviridae constitute a large and very diverse group of animal and plant viruses that are currently assigned to 5 genera (Orthobunyavirus, Hantavirus, Nairovirus, Phlebovirus, and Tospovirus) [1]. With the exception of the hantaviruses, members of the other 4 genera are all biologically transmitted by arthropod vectors (mosquitoes, ticks, phlebotomine sandflies, culicoid midges, and thrips) [1]. It is generally believed that the hantaviruses do not have an arthropod transmission cycle and that they are transmitted to mammals (rodents, insectivores, bats, and humans) by inhalation of aerosols of infected animal excreta or by bite [1–4]. A large number of hantavirus species and serotypes have been described, and new ones are continually being discovered; most are associated with a specific animal reservoir, principally rodents of the families Murinae, Avicolinae, and Sigmodontinae [5]. The rodent-associated hantaviruses are a major public health problem and cause hemorrhagic fever with renal syndrome (HFRS) in Asia and Europe and hantavirus pulmonary syndrome (HPS) in the Americas [2, 3]. In China, for example, up to 10 000 HFRS cases are reported annually with a significant morbidity and mortality [2, 6].
Although HFRS had been studied by Japanese and Russian scientists earlier [7–9]; the disease first came to the attention of American and Western European scientists in 1951, during the Korean War. At that time, a severe hemorrhagic disease of unknown etiology, designated “Korean hemorrhagic fever,” was affecting United Nations troops involved in the conflict [9, 10]. In 1952, the Armed Forces Epidemiologic Board, Office of the US Army Surgeon General appointed a team consisting of physicians, epidemiologists, microbiologists, and entomologists to investigate the epidemiology and etiology of Korean hemorrhagic fever (HFRS). Although members of the team were unable to identify the etiologic agent, their conclusions, based on epidemiolgic features of the disease, were that the disease was probably transmitted by an arthropod and that the reservoir of the causative agent was not humans but some member of the local fauna [10]. Bloodsucking mites (laelaptids) and trombiculid mites (chiggers) were high on the list of suspects, because of their rural distribution, marked season peaks, patchy or focal distribution, and the nature of their bite (short with minimal host response at the bite site) [10]. These characteristics fit well with the observed epidemiology of the hemorrhagic disease (HFRS) in Korea. In fact, the investigators noted a similarity in the epidemiology of HFRS with scrub typhus, another mite-transmitted disease [10, 11].
Then in 1978, Lee et al [12] reported the isolation of Hantaan virus, the principal etiologic agent of HFRS in Asia, from the lungs of infected rodents (Apodemus agrarius). Subsequent laboratory studies demonstrated transmission of Hantaan virus among caged A. agrarius [13]. Later experimental and field studies confirmed that Hantaan and other hantaviruses were present in the blood, urine, feces, and throat of their natural rodent reservoirs for a short period during the acute infection and then persisted in the lungs of many infected individuals in a chronic form [2]. These observations combined with occasional reports of human infections in persons handling hantavirus-infected rodents or their nest material led to the current paradigm that hantaviruses are transmitted primarily among their mammalian hosts by inhalation of infectious aerosols or sometimes by bite during mating, fighting, or other close encounters between infected and noninfected individuals [2–4]. Most virologists, epidemiologists, and mammalogists in the West have now discarded the possibility of hantavirus transmission by arthropods.
But the question remains, are hantaviruses the only members of the family Bunayviridae that are not vector-borne? Other bunyaviruses, such as Rift Valley fever virus (Phlebovirus) [14] and Crimean-Congo hemorrhagic virus (Nairovirus) [15], can also be aerosol transmitted, but their basic maintenance mechanism and usual route of infection involve the bite of an infected arthropod. A review of the Chinese scientific literature during the past 50 years suggests that this may also be the case with Hantaan virus and that the original hypothesis that biting arthropods are involved in the maintenance and transmission of HFRS may be correct. The purpose of this review is to reexamine the evidence that parasitic mites are involved in hantavirus transmission, including more recent research from China. In reviewing the Asian literature on human hantavirus infection, 2 points of possible confusion need to be clarified:
The names given to the human disease caused by hantavirus infection in Eastern Asia have varied over time and with different authors [4, 8]. At least 5 different names (Far Eastern hemorrhagic fever, hemorrhagic nephroso-nephritis, epidemic hemorrhagic fever, hemorrhagic fever with renal syndrome, and Korean hemorrhagic fever) have been commonly used in publications to describe the human disease in that region. In this review, we will consider them as synonymous and a single name, hemorrhagic fever with renal syndrome (HFRS), will generally be used.
Because of the various names used for the disease, different names have also been used in publications for the etiologic agent. In the initial report by Lee et al, [12] describing the isolation of the etiologic agent of Korean hemorrhagic fever (HFRS), the name “Hantaan virus” was proposed for the virus. A few years later, a second hantavirus, designated “Seoul virus,” was recovered from Rattus sp. in Korea [16]. Although both viruses can cause HFRS, they are ecologically and genetically different and are classified as distinct species within the genus Hantavirus [1]. Hantaan virus is largely restricted in its geographic distribution to rural areas of northeastern China, Korea, and Eastern Russia, where it is usually associated with the striped field mouse, Apodemus agrarius, and is the major cause of HFRS in that region [3]. In contrast, Seoul virus occurs worldwide and has a more urban distribution, is only an occasional cause of HFRS, and is associated with Rattus norvegicus and Rattus rattus [3, 4]. In addition, in some of the earlier Chinese reports, the viruses isolated from A. agrarius and from mites were identified as “hemorrhagic fever with renal syndrome virus (HFRSV)” or “epidemic hemorrhagic fever virus (EHFV)” [17–22]. Based on the association of HFRSV and EHFV with A. agrarius in rural areas and with mites collected directly from field mice or from their habitat (ie, tall grass and rodent nests), we feel that Hantaan, HFRSV, and EHFV are different names for the same virus; in this review, it will be referred to as Hantaan virus.
ECOLOGY AND SAMPLING OF GAMASID AND TROMBICULID MITES IN CHINA
Parasitic mites associated with Hantaan virus in China are assigned to 2 different taxonomic groups (gamasid and trombiculid), depending on their principal habitat and the life stages that are blood-feeding. Gamasid mites (Laelapinae) live in rodent nests and attach to the host only during feeding. They are fairly short-lived and all life stages feed on vertebrates [23]. These mites can be collected directly from rodent nests in the field [24]. Trombiculid mites or chiggers (Trombiculidae) live in the soil, and only the larval form feeds on vertebrates [10, 11, 25]. The life cycle of trobiculid mites is longer, and they live for up to a year. The host-questing larvae can be collected from grass where they rest awaiting a passing animal; and the larvae are common ectoparasites on rodents and insectivores during certain seasons of the year. The distribution of chigger mites is very focal and dependent on the home ranges of their small animal hosts, which do not usually overlap. Consequently, chigger mite colonies tend to be isolated from each other and to occur in “mite islands” [25]. Humans get bitten by chiggers when they intrude into a mite island. Larval forms of trombiculid mites (ie, Leptotrombidium scutellarae) can be collected by placing a black board (15 × 15 cm) on the ground and removing the larvae as they crawl onto it [17]. Adult L. scutellarae can be obtained by taking a block of soil 6–10 cm deep from the site where the larvae were observed and adding it to a container with water [26]. The adult mites float to the surface and can be retrieved. Adult L. scutellarae mites will lay eggs in the laboratory, which hatch to larvae, but it is difficult to maintain these mites in the laboratory. In contrast, gamasid mites collected from rodent nests can be reared in the laboratory and have been maintained for multiple generations in China [24].
CORRELATION OF THE PEAK SEASONS OF TROMBICULID AND HFRS
Following the earlier observations by the US Army Commission, Chinese scientists in the 1970s began to study the association of mite abundance with HFRS. They initially observed that the field mouse Apodemus agrarius, the rodent reservoir of Hantaan virus, was heavily infested with Leptotrombidium scutellare in Shaanxi Province in October and November, which was the peak season for HFRS in that region of China [24, 27, 28]. They also observed that a reduction of mite density, resulting from the application of insecticides to fields in October, appeared to decrease the incidence of HFRS [27]. In one study, [24] a total of 38 940 trombiculid mites were collected from rodents during various months of the year. The overall species composition of the mites was 54.4% L. scutellare, 38.3% L. palpis, 5.7% Gahrliepia fragilis, and 1.6% other species. But during October and November, 90% of the mites found on the rodents were L. scutellare [24]. The monthly mite index (average number of mites per rodent) for L. scutellare was also highest (up to 100 mites per rodent) in October and November, demonstrating a close correlation between mite density and the seasonal occurrence of reported human cases of HFRS in Shaanxi Province [27, 28].
In another study of mite abundance on rodents carried out in the towns of Linyi and Jinan in Shandong Province from May 1995 to April 1996, it was again observed that the peak months of chigger infestations on rodents were October and November, when the monthly mite indexes were 26.7 and 36.6 per mouse, respectively [29]. These results closely correlated with the observed peak of HFRS cases in the 2 Shandong communities during the study period.
ISOLATION OF HANTAAN VIRUS FROM TROMBICULID MITES
Several publications in the Chinese scientific literature [17–19, 22, 24, 26, 30] have described the isolation of Hantaan virus (HFRSV) in Vero cell cultures inoculated with pools of L. scutellare larvae collected in grassy habitats or hatched in the laboratory from eggs laid by field-collected adult chiggers. These studies indicate that L. scutellare are naturally infected with Hantaan virus and also that the virus can be vertically (transovarially) transmitted by infected adult female mites to their progeny. Zhang et al [18] also reported the isolation of Hantaan virus from newborn mice inoculated with pools of L. scutellare nymphs that molted in the laboratory from larvae taken off wild rodents. In addition, Hantaan virus has been detected in L. scutellare larvae and nymphs by reverse-transcription polymerase chain reaction (RT-PCR), immunohistochemical staining using Hantaan monoclonal antibodies, and by DNA-RNA hybridization [31]. The detection of Hantaan virus in L. scutellare nymphs, which do not feed on animals, is evidence for transstadial transmission of the virus in these mites.
ISOLATION OF HANTAAN VIRUS FROM GAMASID MITES
There are recent reports [19, 20] in the Chinese literature of the isolation of Hantaan virus from pools of 3 different species of gamasid mites (Eulaelaps stabularis, Haemolaelaps glasgowi, and Laelaps cynognathus) collected from the nests of field mice (A. agrarius) and from laboratory-reared offspring of these mites. These studies indicate that several species of gamasid mites are naturally infected with Hantaan virus, as well as demonstrating transovarial and transstadial transmission of the virus in these arachnids.
BITE TRANSMISSION OF HANTAAN VIRUS BY MITES
Bite transmission of Hantaan virus by trombicuid mites has been demonstrated by allowing field-collected L. scutellare larvae to feed on newborn mice in the laboratory and then subsequently isolating the virus from brain and lung samples of the mice inoculated into Vero cell cultures [17, 30]. Bite transmission of Hantaan virus by gamasid mites has also been shown by allowing laboratory-reared E. stabularis and H. glasgowi nymphs and adults to feed on baby mice and then isolating the virus in Vero cells from tissue samples of the mice [21, 24]. These studies strongly suggest that both trombiculid (L. scutellare) and gamasid mites can transmit Hantaan virus by bite to susceptible mammals.
In addition, Zhuge et al [32] reported experimental bite transmission of Seoul virus to Wistar rats by the tropical rat mite Omithonyssus bacoti (Macronyssidae) and suggested that this mite might play a role as both a vector and reservoir host of Seoul virus. This cosmopolitan mite is a parasite of rats and other rodents in both tropical and temperate regions, so its geographic distribution fits the worldwide distribution of Seoul virus [23].
DURATION OF HANTAAN VIRUS INFECTION IN MITES
The duration of Hantaan virus infection in mites was also investigated. Suspensions of L. scutellare larvae and nymphs were tested by culture in Vero cells at 20, 80, 100, and 115 days after hatching or molting. Hantaan virus was isolated from pools of the immature L. scutellare at each time period [21]. Similar results were obtained with E. stabularis and H. glasgowi, indicating that Hantaan virus persisted in the mites for at least 168 days [24]. These experiments confirm persistence of the virus and suggest that Hantaan virus infection in mites probably persists for the life of the arthropod. These results are compatible with the aforementioned studies demonstrating transstadial and transovarial transmission of the virus in mites.
CONCLUSION AND INTERPRETATION
A review of the recent Chinese literature on the association of Hantaan virus with mites clearly indicates that several species of trombiculid and gamasid mites are naturally infected with Hantaan virus. Furthermore, convincing evidence has been presented of both transovarial and transstadial transmission of the virus by L. scutellare and by several species of gamasid mites. The Chinese studies also demonstrated bite transmission of Hantaan virus to laboratory mice by these same mite species. In summary, these reports imply that certain parasitic mites can maintain Hantaan virus in nature, and that they can transmit the virus by bite to susceptible mammals. This is not a new concept, as discussed in the introduction; but it has never gained wide support, because of the widely accepted current paradigm that rodents are the sole reservoir of Hantaan virus and that the virus is transmitted principally by inhalation of infectious aerosols of rodent excreta.
Although largely forgotten or ignored, these were several previous reports in the world scientific literature of the association of hantaviruses with mites. Chumakov [8] reported that pools of gamasid mites (Haemolaelaps glasgowi, H. nidi, and Histionyssus isabellinus) inoculated into human subjects produced signs and symptoms of HFRS. Similarly, the US Army Hemorrhagic Fever Commission, sent in 1952 to investigate outbreaks of HFRS among UN troops during the Korean War, also concluded that trombiculid mites (chiggers) should be considered a potential vector, because their abundance on the rodent reservoir (A. agrarius) was closely correlated with the seasonal incidence of the disease [10]. Traub and Wisseman [10] noted the similar ecology of Korean hemorrhagic fever (HFRS) and scrub typhus. Because trombiculid mites generally live in the superficial layers of soil, they have a focal distribution and very limited home range, often spending their entire life within a few feet of their birth place [10]. This ecologic characteristic of trombiculid mites led to the concept of “infectious mite islands” in explaining the epidemiology of scrub typhus [11]. The same concept fits the observed focal pattern of hantavirus infection among wild rodents [33–35].
Despite the clear association of hantaviruses with rodents, there is remarkably little information available about the duration, amount, or periodicity of virus present in the blood or shed in the excreta of infected rodents [2]. The paucity of information about this essential part of the transmission cycle is largely due to the difficulty of assaying hantaviruses in excreta [12, 36] and to biosafety constrains imposed on work with these agents in their natural rodent hosts [37]. It is generally believed that Hantavirus infection is lifelong and that it has little or no deleterious effects on survival or reproduction of the rodent host [38, 39]. Experimental studies with pregnant field mice (A. agrarius) and rats infected with Hantaan and Seoul viruses, respectively, showed that these hantaviruses were not transmitted vertically to the rodents' offspring [13, 39]. Furthermore, female rodents transfer maternal antibodies against hantaviruses to their offspring, providing protection against horizontal infection during the first 6–8 weeks of life [39]. Knowledge about the shedding of hantaviruses in rodent excreta comes from studies that used only a few hantavirus-rodent pairs and did not include measurement of virus shedding or pathological examinations [38]. Most experimental studies of hantavirus excretion and transmission by rodents have been performed by caging infected and noninfected rodents together and then observing that the controls become infected [13, 40–43]. Results of such experiments have been interpreted as demonstrating virus excretion and aerosol transmission; but because of their design, the possibility of other modes of transmission (ie, bite, sexual contact, ingestion, and/or tiny ectoparasites) cannot be excluded. The ecology of hantaviruses in their natural rodent hosts is still poorly understood.
Retrospective epidemiologic studies of patients with HPS and HFRS have revealed 3 common risk factors: (1) direct handling of hantavirus-infected rodents, [43–45] (2) cleaning rodent-infested rooms or buildings, especially within a closed space [3], and (3) sleeping outdoors on the ground [46–49]. All of these activities have been interpreted as exposing people to aerosols of infected rodent excreta [9, 43, 45, 49, 50]. But in view of the evidence suggesting mite involvement, an alternative explanation is that these activities expose people to mites that are present on rodents, in their nests, or on the ground. Interestingly, a recent case-control study of risk factors for HFRS in soldiers of the South Korean Army found that the 2 most protective factors against contacting the disease were sleeping in barracks rather than outside on the ground and the use of insecticides or insect repellent [47].
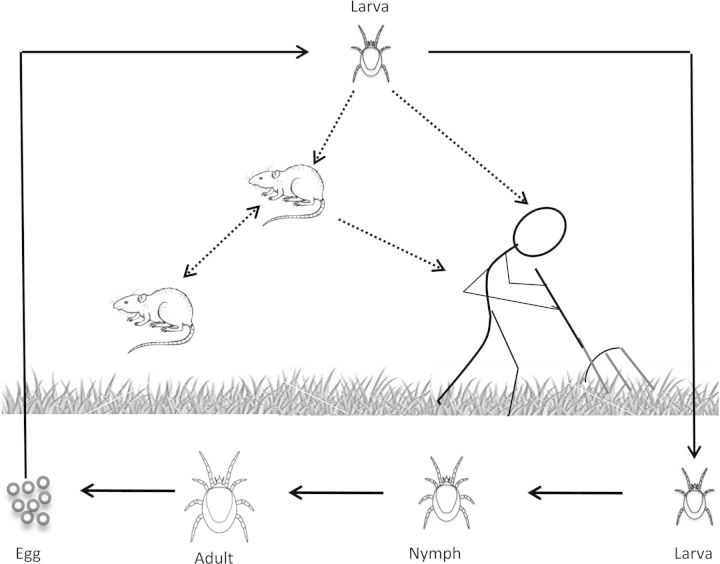
In conclusion, the results of these recent Chinese reports, as well as the earlier Russian [8] and American [10] studies, strongly suggest that mites play a role in the maintenance and transmission of Hantaan virus and possibly other hantaviruses in nature. These findings do not eliminate the possible role of aerosol or bite transmission of hantaviruses by infected rodents; but in view of the present data, mite transmission would appear to be the primary maintenance mechanism for the virus. The attached graphic (Figure 1) shows proposed maintenance and transmission cycles for Hantaan virus, involving trombiculid mites (L. scutellare), field mice (A. agrarius), and humans, based on the aforementioned publications. The data suggest that there are 2 distinct cycles: a basic maintenance cycle that maintains the virus from generation to generation within the mites, and a more complex transmission cycle that involves infected mite larvae, field mice, and people. In the maintenance cycle, the virus is passed from generation to generation within the mite population by transovarial and trasstadial transmission. After hatching from eggs deposited by an infected adult female mite, the infected larvae seek a vertebrate host. After feeding, the larva leaves the host and returns to the soil where it molts into a nymph and the mite life cycle continues. In the vertebrate transmission cycle, field mice (Apodemus) acquire the virus from the bite of transovarially infected mite larvae, or horizontally by bite, or inhalation of aerosols from other infected mice. A noninfected mite feeding upon a viremic field mouse also could theoretically be infected with virus, but it seems unlikely given the transient nature of Hantaan virus viremia in the rodent host and the minute amount of blood that these tiny (0.25 mm) larvae must ingest during the several days they are attached to the host [25]. Trombiculid mite larvae feed on host tissues, sucking up liquefied tissue digested by their saliva. Unlike mosquitoes or ticks, blood is not directly ingested in this process, although a few blood cells can sometimes be found in the mite's stomach [25]. Humans can be infected by the bite of infected mite larvae or by inhalation of aerosols of infected rodent excreta, but people are probably dead-end hosts for the virus. Studies indicate that Hantaan virus RNA can only be detected in plasma of HFRS patients during the febrile/hypotensive and oliguric stages of the disease (days 3–12 of the illness) [50], and it seems unlikely that such patients would be exposed to mite larvae at that time.
Figure 1.
Complete life cycle (egg, larva, nymph, and adult) of the trombiculid mite, Leptotrombium scutellarae. Only the larval stage feeds on vertebrates; the nymphs and adults live in superficial layers of the soil, feeding on detritus. This also illustrates proposed maintenance and transmission cycles for Hantaan virus in this mite species. The basic maintenance cycle of the virus (solid line) involves transovarial and transstadial passage of the virus in L. scutellarae. The proposed vertebrate transmission cycles involve (hatched lines) infected mites, field mice (Apodemus agrarius), and humans.
The efficiency of the proposed transmission scenarios are unknown, so it is not yet possible to compare their importance in the epidemiology of Hantaan virus infection. For mathematical model and control efforts, the filial infection rates among L. scutellare adults, the bite transmission rate of infected mite larvae, the amount and duration of virus in rodent excreta, and the threshold of human infection by aerosol or by mite bite are all essential information in attempting to evaluate the relative importance of the rodent and mite transmission cycles. Obviously, additional research in this area is needed to confirm the mite hypothesis; but if correct, it will significantly change current concepts about the transmission and control of hantavirus infection in humans.
Notes
Acknowledgments. The authors wish to thank Dr Frederick A. Murphy for valuable discussions and suggestions during the preparation of this review and Hilda Guzman for preparing Figure 1.
Financial support. This work was supported in part by National Institutes of Health [contract HHSN272201000040I/HHSN2720004/D04].
Potential conflicts of interest. Both authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Nichol ST, Beaty BJ, Elliott RM, et al. Family bunyaviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus taxonomy. 8th ed. Amsterdam: Elsevier Academic Press; 2005. pp. 695–716. [Google Scholar]
- 2.Schmaljohn C, Hjelle B. Hantaviruses: a global disease problem. Emerg Infect Dis. 1997;3:95–104. doi: 10.3201/eid0302.970202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fulhorst CF, Koster FT, Enria DA, Peters CJ. Hantavirus infections. In: Guerrant RL, Walker DH, Weller PF, editors. Tropical infectious diseases: principals, pathogens and practice. 3rd ed. Philadelphia: Elsevier; 2011. pp. 470–80. [Google Scholar]
- 4.LeDuc JW. Hantavirus infections. In: Porterfield JS, editor. Exotic viral infections. London: Chapman and Hall Medical; 1995. pp. 261–84. [Google Scholar]
- 5.Yanagihara R, Gu SH, Arai S, Kang HJ, Song JW. Hantaviruses: rediscovery and new beginnings. Virus Res. 2014;187:6–14. doi: 10.1016/j.virusres.2013.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guang MY, Liu GZ, Cosgriff TM. Hemorrhage in hemorrhagic fever with renal syndrome in China. Rev Infect Dis. 1989;2(suppl 4):884–90. doi: 10.1093/clinids/11.supplement_4.s844. [DOI] [PubMed] [Google Scholar]
- 7.Mayer CF. Epidemic hemorrhagic fever of the Far East or endemic hemorrhagic nephrose-nephritis. Mil Surg. 1952;110:276–84. [PubMed] [Google Scholar]
- 8.Chumakov MP. Etiology, epidemiology and prophyloxis of hemorrhagic fever. Pub Health Monographs. 1957;50(suppl 1):19–25. [Google Scholar]
- 9.Yanagihara R, Gajdusek DC. Hemorragic fever with renal syndrome, a historical prospective and review of recent advances. In: Gear JHS, editor. CRC Handbook of viral and rickettsial hemorrhagic fevers. Boca Raton: CRC Press; 1988. pp. 155–81. [Google Scholar]
- 10.Traub R, Hertig M, Lawrence WH, Harriss TT. Potential vectors and reservoirs of hemorrhagic fever in Korea. Am J Hyg. 1954;59:291–305. doi: 10.1093/oxfordjournals.aje.a119642. [DOI] [PubMed] [Google Scholar]
- 11.Traub R, Wisseman CL., Jr The ecology of chigger-borne rickettsiosis (scrub typhus) J Med Entomol. 1974;11:237–303. doi: 10.1093/jmedent/11.3.237. [DOI] [PubMed] [Google Scholar]
- 12.Lee HW, Lee PW, Johnson KM. Isolation of the etiologic agent of Korean hemorrhagic fever. J Infect Dis. 1978;137:298–308. doi: 10.1093/infdis/137.3.298. [DOI] [PubMed] [Google Scholar]
- 13.Lee HW, Lee PW, Baek LJ, Song CK, Seong IW. Intraspecific transmission of Hantaan virus, etiologic agent of Korean hemorrhagic fever, in the rodent Apodemus agrarius. Am J Trop Med Hyg. 1981;30:1106–12. doi: 10.4269/ajtmh.1981.30.1106. [DOI] [PubMed] [Google Scholar]
- 14.Peters CJ, Makino S, Morrill JC. Rift valley fever. In: Guerrant RL, Walker DH, Weller PF, editors. Tropical infectious diseases: principals, pathogens and practice. 3rd ed. Philadelphia: Elsevier; 2011. pp. 462–5. [Google Scholar]
- 15.Tarantola A, Egonul O, Tattevin P. Estimates and prevention of Crimean-Congo hemorrhagic fever risk for health care workers. In: Ergonul O, Whitehouse CA, editors. Crimean-Congo hemorrhagic fever. Dordrecht: Springer; 2007. pp. 281–94. [Google Scholar]
- 16.Lee HW, Baek LJ, Johnson KM. Isolation of Hantaan virus, the etiologic agent of Korean hemorrhagic fever, from wild urban rats. J Infect Dis. 1982;146:638–44. doi: 10.1093/infdis/146.5.638. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Hu YL, Zhao XZ, Wu GH, Jiang KJ. Study on natural infection of EHFV in Leptrotrombiduim scutellare (in Chinese) Virologica Sinica. 1995;10:94–6. [Google Scholar]
- 18.Zhang Y, Li XF, Tao KH, et al. Preliminary observation on EHFV's transstadial transmission and proliferation in Leptrotrombiduim scutellare infected naturally with EHFV (in Chinese) Chinese J Pub Health. 1995;14:176–8. [Google Scholar]
- 19.Zhang Y, Zhu J, Deng XZ, Wu GH, Zhang JJ, Zhou YP. Experimental study on the roles of gasmid mite and chigger mite in the transmission of hemorrhagic fever with renal syndrome virus (in Chinese) Chinese J Epidemiol. 2001;22:352–4. [PubMed] [Google Scholar]
- 20.Zhang Y, Zhu J, Deng XZ, Wu GH, Zhang J, Zhou YP. Distribution of hemorrhagic fever with renal syndrome virus in gamasid mites and chigger mites (in Chinese) Chinese J Preventive Med. 2002;36:232–4. [PubMed] [Google Scholar]
- 21.Zhang Y, Zhu J, Dneg XZ, et al. Study on distribution and life span of hemorrhagic fever with renal syndrome virus in gasmid mite and chigger mite (in Chinese) Chinese J Zoonoses. 2000;16:5–7. [Google Scholar]
- 22.Wu GH, Zhang Y, Zhao XZ, et al. Transmission of HFRSV by Leptotrombidium scutellare (in Chinese) Chinese Med J. 1992;72:481–3. [Google Scholar]
- 23.Mullen GR, Oconnor BM. Mites (Acari) In: Mullen G, Durden L, editors. Medical and veterinary entomology. San Diego: Academic Press/Elsevier Science; 2002. pp. 449–516. [Google Scholar]
- 24.Zhang Y, Tao KH, Zhu J, et al. Investigation of the relationship between gamasid mites, chigger mites and hemorrhagic fever with renal syndrome (in Chinese) China Pub Health. 2000;16:525–6. [Google Scholar]
- 25.Varma MGR. Ticks and mites (Acari) In: Lane RP, Crosskey RW, editors. Medical insects and arachnids. London: Chapman; 1993. pp. 597–658. [Google Scholar]
- 26.Qian JY, Xing AH, Wang L. The role of Leptotrombidium scutellare in transmission of HFRS. Chin J Epidemiol. 2008;29:425. [Google Scholar]
- 27.Zhang Y, Wu GH. Study on mite transmission of HFRSV (in Chinese) Chinese J Zoonoses. 2001;17:87–8. [Google Scholar]
- 28.Chen HX, Qiu FX, Zhao XQ, Luo CW, Li XQ. Characteristics of the distribution of epidemic season of hemorrhagic fever with renal syndrome in different reasons and different years in China (in Chinese) . Chinese J Exp Clin Virol. 1994;8:197–201. [Google Scholar]
- 29.Yang ZQ, Liu YX, Wang HW, Wang M, Rui QGZY. Studies on natural infection of HFRSV and R. tsutsugamushi of L. scutellare (in Chinese) Lit Inf Prev Med. 2002;8:6–7. [Google Scholar]
- 30.Yu J, Deng XZ, Yang ZQ, et al. Study on the bite transmission of Hantaan virus and Orientia tsutsugamushi by naturally dual infected Leptotrombidium scutellare (in Chinese) Chinese J Prev Med. 2010;44:324–8. [PubMed] [Google Scholar]
- 31.Tao KH, Zhang Y, Zhu J, et al. Detection of HFRSV structural protein genes in trombiculid mites and gamasid mites. China Public Health. 2000;16:17–8. [Google Scholar]
- 32.Zhuge HX, Meng YC, Wu JW, Zhu ZY, Liang WF, Yao PP. Studies on the experimental transmission of Rattus-borne hantaviruses by Omithonyssus bacoti (in Chinese) Clin J Parasitol Dis. 1998;16:445–8. [PubMed] [Google Scholar]
- 33.Mills JN, Ksiazek TG, Ellis BA, et al. Patterns of association with host and habitat: antibody reactive with Sin Nombre virus in small mammals in the major biotic communities of the southwestern United States. Am J Trop Med Hyg. 1997;56:273–84. doi: 10.4269/ajtmh.1997.56.273. [DOI] [PubMed] [Google Scholar]
- 34.Otteson EW, Riolo J, Rowe JE, et al. Occurrence of Hantavirus within the rodent population of northeastern California and Nevada. Am J Trop Med Hyg. 1996;54:127–33. doi: 10.4269/ajtmh.1996.54.127. [DOI] [PubMed] [Google Scholar]
- 35.Yanagihara R. Hantavirus infection in the United States: epizootiology and epidemiology. Rev Infect Dis. 1990;12:449–57. doi: 10.1093/clinids/12.3.449. [DOI] [PubMed] [Google Scholar]
- 36.Elliott LH, Ksiazek TG, Rollin PE, et al. Isolation of the causative agent of hantavirus pulmonary syndrome. Am J Trop Med Hyg. 1994;51:102–8. doi: 10.4269/ajtmh.1994.51.102. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention and National Institutes of Health. Biosafety in microbiological and biomedical laboratories. 5th ed. Washington: US Government Printing Office; 2007. [Google Scholar]
- 38.Meyer BJ, Schmaljohn CS. Persistent hantavirus infections: characteristics and mechanisms. Trends Microbiol. 2000;8:61–7. doi: 10.1016/s0966-842x(99)01658-3. [DOI] [PubMed] [Google Scholar]
- 39.Klein SL, Calisher CH. Emergence and persistence of Hantaviruses. CTMI. 2007;315:217–52. doi: 10.1007/978-3-540-70962-6_10. [DOI] [PubMed] [Google Scholar]
- 40.Bogdanova SB, Gavrilovkaya IN, Boyko NA. Persistent infection caused by hemorrhagic fever with renal syndrome in red mice (Clethrionomys glareolus), natural hosts of the virus (In Russian) Mikrobiol Z. 1987;49:106. [PubMed] [Google Scholar]
- 41.Gavrilovskaya IN, Apekina NS, Bernshtein AD, et al. Pathogenesis of hemorrhagic fever with renal syndrome virus infection and mode of horizontal transmission of hantavirus in bank voles. Arch Virol Suppl. 1990;1:57–62. [Google Scholar]
- 42.Yanagihara R, Amyx HL, Gajdusek DC. Experimental infection with Puumala virus, the etiologic agent of nephropathia epidemica, in bank voles (Clethrionomys glareolus) J Virol. 1985;55:34–8. doi: 10.1128/jvi.55.1.34-38.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brummer-Korvenkontio M, Henttonen H, Vaheri A. Hemorrhagic fever with renal syndrome in Finland: ecology and virology of nephropathia epidemica. Scand J Infect Dis Suppl. 1982;36:88–91. [PubMed] [Google Scholar]
- 44.Desmyter J, LeDuc JW, Johnson KM, Brasseur F, Deckers C, de van Ypersele SC. Laboratory rat associated outbreak of haemorrhagic fever with renal syndrome due to Hantaan-like virus in Belgium. Lancet. 1983;2:1445–8. doi: 10.1016/s0140-6736(83)90797-3. [DOI] [PubMed] [Google Scholar]
- 45.Vapalahti O, Plyusnin A, Vaheri A, Henttonen H. Hantavirus antibodies in European mammalogists. Lancet. 1995;345:1569. doi: 10.1016/s0140-6736(95)91114-6. [DOI] [PubMed] [Google Scholar]
- 46.Clement J, Underwood P, Ward D, Pilaski J, LeDuc J. Hantavirus outbreak during military manoeuvres in Germany. Lancet. 1996;347:336. doi: 10.1016/s0140-6736(96)90519-x. [DOI] [PubMed] [Google Scholar]
- 47.Dixon KE, Nang RN, Kim DH, et al. A hospital-based, case-control study of risk factors for hemorrhagic fever with renal syndrome in soldiers of the armed forces of the Republic of Korea. Am J Trop Med Hyg. 1996;54:284–8. doi: 10.4269/ajtmh.1996.54.284. [DOI] [PubMed] [Google Scholar]
- 48.Trencseni T. Clinical aspects and epidemiology of hemorrhagic fever with renal syndrome. Budapest: Akademiai Kiado; 1971. [Google Scholar]
- 49.Xu ZY, Guo CS, Wu YL, Zhang XW, Liu K. Epidemiological studies of hemorrhagic fever with renal syndrome: analysis of risk factors and mode of transmission. J Infect Dis. 1985;152:137–44. doi: 10.1093/infdis/152.1.137. [DOI] [PubMed] [Google Scholar]
- 50.Yi J, Xu Z, Zhuang R, et al. Hantaan virus RNA load in patients having hemorrhagic fever with renal syndrome: correlation with disease severity. J Infect Dis. 2013;207:1457–61. doi: 10.1093/infdis/jis475. [DOI] [PubMed] [Google Scholar]