Abstract
Background:
Tamoxifen has been US Food and Drug Administration–approved for primary prevention of breast cancer since 1998 but has not been widely adopted, in part because of increased risk of serious side effects. Little is known about the risk-benefit profiles of women who use chemoprevention outside of a clinical trial. We examined characteristics associated with initiation and discontinuation of tamoxifen for primary prevention of breast cancer within a large cohort of women with a first-degree family history of breast cancer.
Methods:
This research was conducted within The Sister Study, a cohort of 50884 US and Puerto Rican women age 35 to 74 years enrolled from 2003 to 2009. Eligible women were breast cancer–free at enrollment and had a sister who had been diagnosed with breast cancer. Participants reported tamoxifen use, ages started and stopped taking tamoxifen, and total duration of use at enrollment. We identified 788 tamoxifen users and 3131 nonusers matched on age and year of enrollment who had no history of contraindicating factors (stroke, transient ischemic attack, cataract, endometrial or uterine cancer). Characteristics associated with tamoxifen initiation were evaluated with multivariable conditional logistic regression. All statistical tests were two-sided.
Results:
Based on published risk-benefit indices, 20% of women who used tamoxifen had insufficient evidence that the benefits of tamoxifen outweigh the risk of serious side effects. After 4.5 years, 46% of women had discontinued tamoxifen.
Conclusions:
While the majority of women who used tamoxifen for primary prevention of breast cancer were likely to benefit, substantial discontinuation of tamoxifen before five years and use by women at risk of serious side effects may attenuate benefits for breast cancer prevention.
An estimated 15% of US women age 35 to 79 years are eligible for breast cancer chemoprevention; however, less than 5% may have favorable risk-benefit profiles (1). Tamoxifen can decrease risk of invasive breast cancer up to 48% and was US Food and Drug Administration (FDA)–approved for primary prevention in 1998 for women 35 years and older at high risk of breast cancer (typically determined by a five-year probability of developing invasive breast cancer greater than 1.67%) and low risk of serious side effects (including endometrial cancer, stroke, pulmonary embolism, deep vein thrombosis, and cataract) (2–6). It is the only approved pharmacologic agent for breast cancer prevention available to premenopausal women (7).
The US Preventive Services Task Force (USPSTF) recently published a recommendation statement for risk reduction of primary breast cancer that encourages clinicians to offer to prescribe tamoxifen or raloxifene to reduce breast cancer risk (8). National estimates indicate that less than 1% of eligible women use tamoxifen for prevention (9). It is unknown why few women use tamoxifen for breast cancer prevention, although vasomotor symptoms, increased risk of adverse health effects, and difficulties in estimating or communicating risk-benefit profiles can be deterrents.
Whether women who do use tamoxifen for breast cancer prevention are among those most likely to benefit is also unknown. In previous studies, willingness to use tamoxifen has not consistently been associated with invasive breast cancer risk (10–13). Further, substantial nonadherence to the recommended five-year course of tamoxifen has been reported in prevention trials. To date, there has been no large study of tamoxifen users outside of a trial setting to evaluate risk-benefit profiles or early discontinuation.
We examined risk-benefit profiles of women who used tamoxifen for primary breast cancer prevention and characteristics associated with initiation and discontinuation among a sample of adult US and Puerto Rican women with a first-degree family history of breast cancer.
Methods
The Sister Study prospective cohort was designed to address genetic and environmental risk factors for breast cancer. From 2003 to 2009, 50884 US and Puerto Rican women age 35 to 74 years were recruited through a national multimedia campaign and network of recruitment volunteers, breast cancer professionals, and advocates. Eligible women had a sister who had been diagnosed with breast cancer but did not have breast cancer themselves. Participants completed baseline computer-assisted telephone interviews on medical and family history, lifestyle factors, and demographics. All participants were asked, “Have you ever used tamoxifen or Nolvadex (these are taken to prevent breast cancer)?” at enrollment and reported ages they started and/or stopped using tamoxifen and their total duration of use. All participants provided informed consent. This research was approved by the Institutional Review Boards of the National Institute of Environmental Health Sciences, the National Institutes of Health, and the Copernicus Group.
Study Design
We identified 1046 women who reported ever using tamoxifen (“tamoxifen users”) at enrollment. Of these, 1038 women provided information on the age they started and stopped using tamoxifen. For comparison subjects, we randomly selected up to four women from the cohort who reported never using tamoxifen (“nonusers”) matched on age and year of enrollment to each tamoxifen user. An index age was defined as the age of tamoxifen initiation for each user and the corresponding age for her matched control patients. The index age allowed for comparisons between tamoxifen users and nonusers to be made relative to the time of tamoxifen initiation, rather than at enrollment when tamoxifen use could have influenced participant characteristics (eg, postmenopausal hormone use, hysterectomy status, etc.).
We excluded women with a history of contraindicating medical conditions as of the index age (n = 52, including 41 cataract, one stroke, five transient ischemic attack [TIA], four uterine cancer, one combination TIA, stroke, and cataract) or who participated in a clinical trial of tamoxifen (n = 173). Twenty-five women were excluded because of a diagnosis of lobular carcinoma in situ that preceded tamoxifen use or missing date of breast cancer diagnosis. In total, 788 women contributed information as tamoxifen users, of which 767 were matched with four nonusers and 21 matched with three (n = 3131) nonusers.
Participant Characteristics
Characteristics defined prior to the index age included smoking, hysterectomy, oophorectomy, menopausal status, postmenopausal hormone use, and raloxifene use. Characteristics at enrollment included demographics, general health, breast biopsy history, and BRCA1/2 testing. We calculated a five-year probability of developing invasive breast cancer with the SAS macro for the Breast Cancer Risk Assessment Tool (BCRAT) designed by the National Cancer Institute and the National Surgical Adjuvant Breast and Bowel Project (14–19), which uses information on age, age at menarche, nulliparity or age at first live birth, first-degree family history of breast cancer, breast biopsy history, and race/ethnicity (http://www.cancer.gov/bcrisktool/).
Risk-Benefit Estimates
We categorized women according to a risk-benefit index proposed by Gail et al. (16) and recently updated by Freedman et al. (20) that is recommended for patient counseling by the USPSTF (8) and the National Comprehensive Cancer Network (NCCN) (21). Briefly, the risk-benefit index classifies women according to the level of evidence (none, moderate, strong) for tamoxifen benefits (breast cancer and fracture prevention) to exceed the risk of serious side effects (endometrial cancer, stroke, pulmonary embolism, deep vein thrombosis, and cataract) using age, five-year projected risk of invasive breast cancer, hysterectomy, and race. Detailed information on the construction of the index has been published (16,20). In our analysis, we used index age, hysterectomy status at index age, and five-year probability of invasive breast cancer risk calculated using age at enrollment because of a lack of age-specific information on breast biopsy history.
Among the 788 tamoxifen users, we characterized the risk-benefit index for 742 (94%) women. In our sample, 131 women had a BCRAT score greater than or equal to 7.5% which exceeds the highest listed BCRAT category (7.0%). For analysis, these women were included in the 7.0% BCRAT category to preserve the maximum sample size; results were largely unchanged when they were alternatively excluded from analysis. Risk-benefit categories were not defined for 46 tamoxifen users for the following reasons: Categories have not been defined for Hispanic women younger than 50 years (n = 10), other race-ethnicities (n = 18), BCRAT scores of less than 1.5% (n = 12), or ages younger than 35 years (n = 1). Finally, scores could not be calculated for five women missing components of the index.
Statistical Analysis
Odds ratios and 95% confidence intervals for characteristics associated with tamoxifen use were calculated with multivariable conditional logistic regression to account for the matched design. In analyses of tamoxifen users only, unconditional logistic regression was applied. Two-sided P values of less than or equal to .05 were considered to be statistically significant. Kaplan–Meier curves were produced to display tamoxifen discontinuation over five years with Stata 12 software (StataCorp., College Station, TX) (22). All other statistical analyses were performed with SAS 9.3 (SAS Institute, Inc., Cary, NC).
Sensitivity Analyses
Sister Study eligibility precluded previous breast cancer diagnosis. Women with higher BCRAT scores and correspondingly favorable risk-benefit indices at the index age may have been disproportionately ineligible to participate if they developed breast cancer in the intervening years. Therefore, we performed sensitivity analyses to evaluate associations with the BCRAT score and risk-benefit index among tamoxifen users who began use within five years (n = 363) and two years (n = 120) of study enrollment and their matched nonusers (n = 1443 and n = 475, respectively).
BCRAT scores were calculated for age at study enrollment rather than tamoxifen initiation. To evaluate the sensitivity of results to possible changes in BCRAT score between these ages, we restricted the evaluation of the risk-benefit index to current tamoxifen users (n = 272) for whom the interval between tamoxifen initiation and study enrollment (mean = 2.6 years, SD = 1.9, IQR = 1–4) was shorter. Risk-benefit index categories were defined for 95% of this subgroup (n = 258).
Results
Tamoxifen Use for Breast Cancer Prevention
Among tamoxifen users, the mean age at tamoxifen initiation was 50.7 years (SD = 7.4). The median calendar year of tamoxifen initiation was 2001; the mode was 2000. Overall, 74% of classified tamoxifen users had a favorable (defined as moderate to strong evidence for benefits to exceed risks) risk-benefit profile and 20% had no evidence that the benefits exceeded risks (Figure 1); the risk-benefit index could not be calculated for 6%. The risk-benefit index distribution across calendar years is provided in Supplementary Table 1 (available online).
Figure 1.
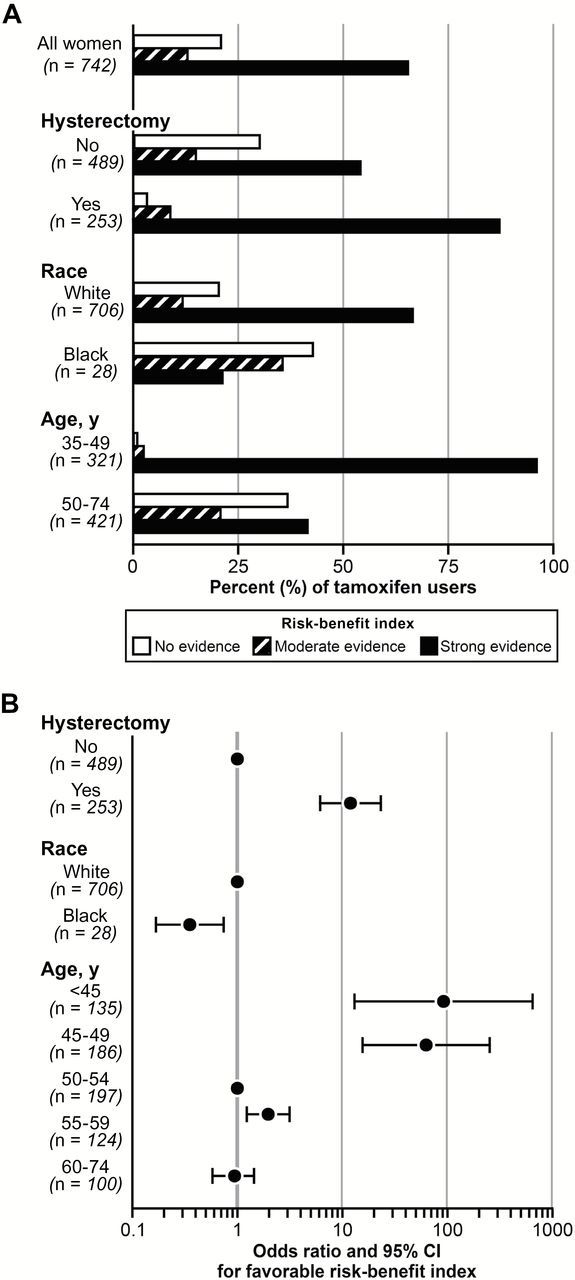
A) Percent of tamoxifen users with a risk-benefit index (16,20) indicating no, moderate, or strong evidence that the benefits of tamoxifen for the primary prevention of breast cancer and fracture exceed the risk of serious side effects (ie, endometrial cancer, stroke, pulmonary embolism, deep vein thrombosis, and cataract). Percentages are shown overall and according to hysterectomy status, race, and age at tamoxifen initiation. B) Odds ratios (95% confidence intervals) for a favorable risk-benefit profile (moderate or strong evidence that benefits of tamoxifen outweigh the risks) according to hysterectomy status, race, and age at tamoxifen initiation. CI = confidence interval.
When stratified by factors contributing to the risk-benefit score (eg, hysterectomy, race, age), the proportion with insufficient evidence of net benefit varied between groups. Women who had hysterectomies prior to starting tamoxifen were 11 times more likely to have a favorable risk-benefit profile compared with women with an intact uterus (OR = 11.87, 95% CI = 5.94 to 23.73). Younger women were likely to have a favorable risk-benefit index; only three of the 321 women who started tamoxifen before age 50 years had no evidence of benefit. Compared with non-Hispanic whites, the 28 African American women were 65% less likely to have a favorable risk-benefit profile (OR = 0.35, 95% CI = 0.16 to 0.75) (Figure 1). These patterns were also apparent in sensitivity analyses restricted to current users (Supplementary Figure 1, available online).
More than 90% of tamoxifen users were non-Hispanic white. African American and Hispanic women were 48% to 59% less likely compared with Non-Hispanic white women to report use (OR = 0.41, 95% CI = 0.26 to 0.63 and OR = 0.52, 95% CI = 0.30 to 0.90, respectively) (Table 1). Tamoxifen users were 50% more likely to have had a hysterectomy prior to tamoxifen use (OR = 1.56, 95% CI = 1.21 to 2.01); however, the majority of users (66%) had an intact uterus at tamoxifen initiation. After accounting for hysterectomy status, bilateral oophorectomy did not contribute to odds of tamoxifen use, though women who reported using unopposed estrogens (the recommended formulation for women without a uterus) were less likely to use tamoxifen compared with never-users of postmenopausal hormones (OR = 0.70, 95% CI = 0.51 to 0.95). More extensive family histories of breast cancer and higher BCRAT scores were strongly related to odds of tamoxifen initiation. Five- and six-fold increases in tamoxifen use were observed among women with BCRAT scores of 3% to 6% (OR = 4.97, 95% CI = 3.57 to 6.24) or with more than one sister with breast cancer (OR = 6.53, 95% CI = 4.66 to 9.12). Tamoxifen users were also more likely to have moderate (OR = 1.64, 95% CI = 1.22 to 2.19) or strong (OR = 4.33, 95% CI = 3.27 to 5.74) evidence of benefit compared with nonusers. Women who reported having a breast biopsy were twice as likely to use tamoxifen compared with those with no biopsy history (OR = 2.06, 95% CI = 1.65 to 2.56). Tamoxifen users were more likely to report worse general health, but were not more likely to smoke (Table 1).
Table 1.
Odds ratios and 95% confidence intervals for tamoxifen use for breast cancer chemoprevention according to Sister Study participant characteristics
| Participant characteristics | Tamoxifen user | Nonuser | OR (95% CI)* | OR (95% CI)† |
|---|---|---|---|---|
| No. (%) | No. (%) | |||
| Total | 788 (100.0) | 3131 (100.0) | N/A | N/A |
| Education | ||||
| High school diploma or equivalent | 101 (12.8) | 430 (13.7) | 0.93 (0.73 to 1.18) | 0.72 (0.55 to 0.95) |
| Some college | 262 (33.2) | 1009 (32.2) | 1.02 (0.85 to 1.22) | 0.91 (0.51 to 1.12) |
| Four-year college degree or higher | 420 (53.3) | 1655 (52.9) | 1.0 (reference) | 1.0 (reference) |
| Race | ||||
| Non-Hispanic white | 720 (91.4) | 2717 (86.8) | 1.0 (reference) | 1.0 (reference) |
| Black | 32 (4.1) | 215 (6.9) | 0.54 (0.36 to 0.79) | 0.41 (0.26 to 0.63) |
| Hispanic | 18 (2.3) | 118 (3.8) | 0.55 (0.33 to 0.92) | 0.52 (0.30 to 0.90) |
| Smoking before index age | ||||
| Never | 412 (52.3) | 1688 (53.9) | 1.0 (reference) | 1.0 (reference) |
| Former | 306 (38.8) | 1130 (36.1) | 1.12 (0.94 to 1.32) | 0.98 (0.81 to 1.18) |
| Current | 67 (8.5) | 304 (9.7) | 0.90 (0.67 to 1.19) | 0.81 (0.59 to 1.12) |
| Hysterectomy before index age | ||||
| No | 520 (66.0) | 2302 (73.5) | 1.0 (reference) | 1.0 (reference) |
| Yes | 267 (33.9) | 824 (26.3) | 1.46 (1.23 to 1.73) | 1.56 (1.21 to 2.01) |
| Bilateral oophorectomy before index age | ||||
| No | 662 (84.0) | 2746 (87.7) | 1.0 (reference) | 1.0 (reference) |
| Yes | 125 (15.9) | 383 (12.2) | 1.38 (1.10 to 1.73) | 1.18 (0.86 to 1.62) |
| Postmenopausal hormone use before index age | ||||
| Never | 482 (61.2) | 1911 (61.0) | 1.0 (reference) | 1.0 (reference) |
| Estrogen only (E) | 142 (18.0) | 544 (17.4) | 1.02 (0.81 to 1.28) | 0.70 (0.51 to 0.95) |
| Both E and E+P | 25 (3.2) | 97 (3.1) | 1.01 (0.64 to 1.60) | 0.71 (0.42 to 1.31) |
| Estrogen and progestin only (E+P) | 120 (15.2) | 523 (16.7) | 0.92 (0.71 to 1.16) | 1.00 (0.77 to 1.31) |
| Sister diagnosed with breast cancer before index age | ||||
| None | 263 (33.4) | 1594 (50.9) | 1.0 (reference) | 1.0 (reference) |
| One sister | 419 (53.2) | 1420 (45.4) | 1.94 (1.62 to 2.32) | 1.89 (1.56 to 2.29) |
| More than one sister | 106 (13.5) | 117 (3.7) | 6.00 (4.43 to 8.14) | 6.52 (4.66 to 9.12) |
| BCRAT score‡ | ||||
| <1.67 | 18 (2.3) | 259 (8.3) | 0.32 (0.17 to 0.62) | 0.31 (0.16 to 0.60) |
| 1.67–2.99 | 132 (16.8) | 1429 (45.6) | 1.0 (reference) | 1.0 (reference) |
| 3.00–5.99 | 406 (51.5) | 1138 (36.3) | 5.04 (4.01 to 6.33) | 4.97 (3.95 to 6.24) |
| ≥6.00 | 228 (28.9) | 302 (9.6) | 13.27 (9.96 to 17.68) | 13.37 (9.99 to 17.89) |
| Mean score (SD), continuous trend | 5.1 (2.4) | 3.4 (1.9) | 1.58 (1.50 to 1.65) | 1.60 (1.53 to 1.68) |
| Risk-benefit index§ | ||||
| No evidence of benefit | 158 (20.1) | 1012 (32.3) | 1.0 (reference) | 1.0 (reference) |
| Moderate evidence of benefit | 98 (12.4) | 428 (13.7) | 1.51 (1.14 to 2.00) | 1.64 (1.22 to 2.19) |
| Strong evidence of benefit | 486 (61.7) | 1406 (44.9) | 3.70 (2.86 to 4.78) | 4.33 (3.27 to 5.74) |
| Not calculated | 46 (5.8) | 285 (9.1) | ||
| Breast biopsy history | ||||
| None | 290 (36.8) | 1843 (58.9) | 1.0 (reference) | 1.0 (reference) |
| 1 | 185 (23.5) | 518 (16.5) | 2.22 (1.80 to 2.75) | 2.06 (1.65 to 2.56) |
| >1 | 270 (34.3) | 392 (12.5) | 4.46 (3.62 to 5.49) | 4.27 (3.44 to 5.29) |
| General health last 12 months | ||||
| Excellent | 269 (34.1) | 1247 (39.8) | 1.0 (reference) | 1.0 (reference) |
| Very good | 311 (39.5) | 1161 (37.1) | 1.25 (1.04 to 1.50) | 1.27 (1.04 to 1.55) |
| Good | 154 (19.5) | 557 (17.8) | 1.29 (1.04 to 1.62) | 1.18 (0.92 to 1.51) |
| Fair | 44 (5.6) | 144 (4.6) | 1.43 (1.00 to 2.06) | 1.37 (0.91 to 2.06) |
| Poor | 10 (1.3) | 20 (0.6) | 2.37 (1.08 to 5.21) | 2.77 (1.18 to 6.48) |
* Adjusted for age and study enrollment year through matching. BCRAT = Breast Cancer Risk Assessment Tool; CI = confidence interval; N/A = not applicable; OR = odds ratio.
† Mutually adjusted for other variables in the table except BCRAT score or the risk-benefit index (composite variables).
‡ Multivariable model does not adjust for family history of breast cancer, race, or breast biopsy history (components of the BCRAT score). Continuous trend is the odds ratio for BCRAT score as a numeric parameter in multivariable models.
§ Multivariable model does not adjust for family history of breast cancer, race, hysterectomy status, or breast biopsy history (components of the BCRAT score or risk-benefit index).
Patterns with BCRAT scores persisted in analyses restricted to women with tamoxifen initiation within five or two years of enrollment (for whom risk scores calculated at enrollment were a better reflection of breast cancer risk at the time of tamoxifen initiation) (Supplementary Figure 2, available online). In sensitivity analyses restricted to women with an index age within five years of study enrollment, tamoxifen associations with risk-benefit indices were attenuated but still apparent for moderate and strong evidence categories (OR = 1.41, 95% CI = 0.93 to 2.14 and OR = 3.21, 95% CI = 2.10 to 4.89, respectively) (data not shown).
Tamoxifen Discontinuation
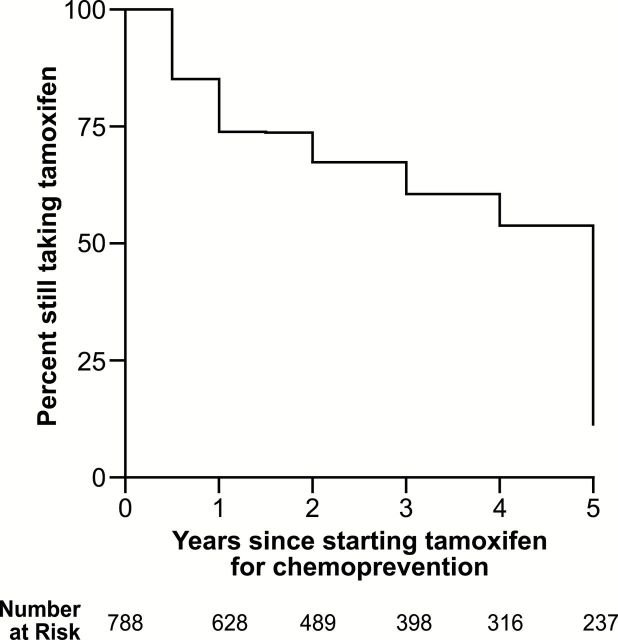
At 4.5 years, 46% of tamoxifen users had discontinued use (Figure 2). Among former users, the median duration of tamoxifen use was 3.0 years. Women who reported using raloxifene after tamoxifen were 55% less likely to complete five years (OR = 0.45, 95% CI = 0.27 to 0.75) (Table 2). Women who had a sister with breast cancer before the index age or reported BRCA testing appeared 29% to 39% less likely to complete five years of tamoxifen, although these estimates did not meet the threshold for statistical significance. For every percentage point increase in BCRAT score, the odds of completing five years of tamoxifen increased by 7% (OR = 1.07, 95% CI = 1.00 to 1.15). Age at tamoxifen initiation, race, smoking, and menopausal status were not statistically significantly associated with discontinuation (Table 2). Five-year discontinuation rates across calendar years are provided in Supplementary Table 1 (available online).
Figure 2.
Kaplan–Meier survival curve of discontinuation before five years among women taking tamoxifen for primary prevention of breast cancer.
Table 2.
Odds ratios and 95% confidence intervals for completion of 5 years or more of tamoxifen for chemoprevention according to Sister Study participant characteristics
| Participant characteristics | Tamoxifen ≥5 y | Tamoxifen <5 y | OR (95% CI)* | OR (95% CI)† |
|---|---|---|---|---|
| No. (%) | No. (%) | |||
| Total | 237 (100.0) | 318 (100.0) | N/A | N/A |
| Age at tamoxifen initiation, y | ||||
| 26–44 | 50 (21.1) | 72 (22.6) | 0.84 (0.51 to 1.37) | 0.75 (0.44 to 1.28) |
| 45–49 | 62 (26.2) | 74 (23.3) | 1.01 (0.63 to 1.63) | 0.98 (0.59 to 1.60) |
| 50–54 | 63 (26.6) | 76 (23.9) | 1.0 (reference) | 1.0 (reference) |
| 55–59 | 34 (14.3) | 47 (14.8) | 0.87 (0.50 to 1.52) | 0.93 (0.52 to 1.66) |
| 60–73 | 28 (11.8) | 49 (15.4) | 0.69 (0.39 to 1.22) | 0.79 (0.43 to 1.46) |
| Race | ||||
| Non-Hispanic white | 222 (93.7) | 279 (87.7) | 1.0 (reference) | 1.0 (reference) |
| Black | 9 (3.8) | 20 (6.3) | 0.57 (0.25 to 1.27) | 0.61 (0.27 to 1.40) |
| Hispanic | 3 (1.3) | 10 (3.1) | 0.38 (0.10 to 1.39) | 0.33 (0.09 to 1.24) |
| Smoking status at tamoxifen initiation | ||||
| Never | 121 (51.1) | 151 (47.5) | 1.0 (reference) | 1.0 (reference) |
| Former | 94 (39.7) | 129 (40.6) | 0.91 (0.64 to 1.30) | 0.90 (0.62 to 1.30) |
| Current | 21 (8.9) | 37 (11.6) | 0.71 (0.39 to 1.27) | 0.75 (0.41 to 1.39) |
| Menopausal status at tamoxifen initiation | ||||
| Premenopausal | 109 (46.0) | 125 (39.3) | 1.0 (reference) | 1.0 (reference) |
| Postmenopausal | 76 (32.1) | 118 (37.1) | 0.74 (0.50 to 1.09) | 0.77 (0.48 to 1.23) |
| BCRAT score at enrollment‡ | ||||
| <1.67 | 1 (0.4) | 15 (4.7) | 0.10 (0.01 to 0.81) | 0.08 (0.01 to 0.67) |
| 1.67–2.99 | 35 (14.8) | 53 (16.7) | 1.0 (reference) | 1.0 (reference) |
| 3.00–5.99 | 115 (48.5) | 156 (49.1) | 1.13 (0.69 to 1.83) | 1.19 (0.72 to 1.95) |
| ≥6.00 | 85 (35.9) | 92 (28.9) | 1.41 (0.85 to 2.36) | 1.60 (0.94 to 2.72) |
| Mean score (SD), continuous trend | 5.4 (2.3) | 5.1 (2.7) | 1.04 (0.97 to 1.11) | 1.07 (1.00 to 1.15) |
| Raloxifene | ||||
| Never | 197 (83.1) | 236 (74.2) | 1.0 (reference) | 1.0 (reference) |
| Yes, prior to tamoxifen start age | 14 (5.9) | 15 (4.7) | 1.12 (0.53 to 2.37) | 1.19 (0.55 to 2.60) |
| Yes, after tamoxifen start age | 26 (11.0) | 67 (21.1) | 0.47 (0.29 to 0.76) | 0.45 (0.27 to 0.75) |
| Sister(s) diagnosed with breast cancer before starting tamoxifen | ||||
| No | 87 (36.7) | 90 (28.3) | 1.0 (reference) | 1.0 (reference) |
| Yes, one sister | 117 (49.4) | 179 (56.3) | 0.67 (0.46 to 0.98) | 0.71 (0.48 to 1.04) |
| Yes, more than one sister | 33 (13.9) | 49 (15.4) | 0.70 (0.41 to 1.18) | 0.76 (0.44 to 1.32) |
| BRCA1/2 testing | ||||
| No | 208 (87.8) | 262 (82.4) | 1.0 (reference) | 1.0 (reference) |
| Yes | 27 (11.4) | 55 (17.3) | 0.62 (0.38 to 1.02) | 0.61 (0.36 to 1.02) |
| BRCA1/2 mutation carrier§ | ||||
| No | 25 (10.5) | 31 (9.7) | 1.0 (reference) | 1.0 (reference) |
| Yes | 2 (0.8) | 19 (6.0) | 0.13 (0.03 to 0.62) | N/A |
* Odds ratio and 95% confidence interval among 555 women who reported formerly using tamoxifen (n = 515) or currently using tamoxifen and starting five or more years before study enrollment (n = 40). BCRAT = Breast Cancer Risk Assessment Tool; CI = confidence interval; N/A = not applicable; OR = odds ratio.
† Mutually adjusted for other variables in the table except BCRAT score (a composite variable) or BRCA1/2 mutation testing or carrier status (because of small sample sizes).
‡ Adjusted for smoking status, menopausal status, and raloxifene use.
§ Among women who reported undergoing BRCA1/2 testing.
Few women reported stroke (three tamoxifen users, four nonusers), TIA (eight tamoxifen users, 22 nonusers), or uterine/endometrial cancer (two tamoxifen users, three nonusers) after the index age. Tamoxifen users were 49% more likely (95% CI = 1.05 to 2.11) to report cataract (55 tamoxifen users, 179 nonusers) compared with nonusers; cataract diagnosis was not statistically significantly related to discontinuation before five years (data not shown).
Discussion
In our sample of 788 women who used tamoxifen for breast cancer prevention outside of a clinical trial from 1986 to 2009, approximately one in five tamoxifen users had no evidence that the benefits were likely to exceed the risks. Absence of expected benefit was most pronounced for older women, African Americans, and those with an intact uterus. A 2011 update to the risk-benefit tables (20) has recently been included in the USPSTF (8), American Society of Clinical Oncology (ASCO) (7), and the NCCN (21) Breast Cancer Risk Reduction guidelines. Dissemination of the risk-benefit tables will expand the tools available to providers for counseling women about the potential value of breast cancer chemoprevention.
In our analysis, 50% of tamoxifen users were premenopausal. The risk-benefit index is more uniformly favorable for women younger than 50 years (16), and tamoxifen remains the only FDA-approved chemoprevention agent for premenopausal women. Recent trials of aromatase inhibitors (23,24) show important benefits for breast cancer prevention; however, their use is limited to postmenopausal women. Until alternative therapies for premenopausal women become available, tamoxifen will continue to have an important role in breast cancer primary prevention.
A recent systematic review of studies examining decision-making surrounding tamoxifen use concluded that higher perceived breast cancer risk increased willingness to consider tamoxifen for prevention, but few other characteristics have been identified (25). Factors associated with actual tamoxifen use may differ from those related to hypothetical decision-making. In our data, tamoxifen users were more likely than nonusers to report non-Hispanic white race, never using unopposed estrogens, stronger family histories of breast cancer, higher BCRAT scores, and worse general health status. We observed no statistically significant association with education, smoking status, or bilateral oophorectomy. These findings differ from studies that reported greater willingness to use tamoxifen among women who were less educated (26), used postmenopausal hormones (10), smoked (10), or had intact ovaries (27) and no association with general health or race (10,11,28). Three studies reported no association between BCRAT scores greater than or equal to vs less than 1.7 and tamoxifen-use (10,11,13), while our analysis and one other (12) observed positive associations using continuous BCRAT scores.
Discontinuation of tamoxifen before the recommended five years (46%) was somewhat greater than the 24% to 36% nonadherence reported in prevention trials (29). However, it is closely aligned with a recent metaregression estimate of 47.2% five-year discontinuation of tamoxifen as adjuvant endocrine therapy ([30], reviewed in [31]). Women who reported a family history of breast cancer, BRCA1/2 testing, or positive mutation status appeared less likely to complete five years of tamoxifen. Information on the efficacy of tamoxifen for chemoprevention among BRCA1/2 carriers is still limited based on small numbers of carriers in the NSABP P-1 trial (32). Race and smoking status were not statistically significantly associated with tamoxifen discontinuation in our analysis. However, the direction of the estimates was consistent with lower completion rates reported among minority race women and current smokers in the prevention trials (33,34). In our sample, age was also not statistically significantly associated with tamoxifen discontinuation, although there was a suggested U-shaped association with the youngest and oldest women less likely to complete five years. Age older or younger than 48 years was not associated with compliance in the IBIS-1 prevention trial (33); however, women age 60 years and older had lower compliance in the NSABP P-1 trial (34).
Limitations of this analysis include not having information on pulmonary embolism and deep vein thrombosis for subject selection; however, we excluded women who had other conditions that could contraindicate tamoxifen use. Some characteristics, such as BRCA1/2 testing and biopsy history, were assessed at enrollment rather than index age. However, the matched design ensured that equal time had elapsed since the index age and enrollment. Matching on age prohibited evaluation of the association between age and tamoxifen use. In our sample, 5% of tamoxifen users were excluded for medical history of cataract, stroke, TIA, uterine or endometrial cancer as of the age tamoxifen use was initiated. Our a priori restriction to women who had no known contraindicating medical conditions prior to the index age precluded us from evaluating associations between these conditions and tamoxifen initiation.
We relied on self-reported information on tamoxifen use for breast cancer prevention and clinical trial participation, which has potential to introduce misclassification if women do not provide accurate reports. Although validation data are unavailable for the current study, previous reports have demonstrated 90% to 94% agreement between self-reported tamoxifen use and medical records in the treatment context (35,36). Validation data for trial participation and information on the trial outcomes (ie, breast cancer prevention) were not available.
Tamoxifen also has other indications, including ovulation induction (37), retroperitoneal fibrosis (38), bipolar disorder (39), and osteoporosis prevention and treatment. In our sample, osteoporosis diagnosis and raloxifene use were not associated with tamoxifen initiation, although tamoxifen users were more likely to have had a bone density scan within the last 24 months. Raloxifene was approved for primary prevention of breast cancer in 2007 (40); the majority of study subjects were enrolled prior to this date. Raloxifene use after tamoxifen was associated with early discontinuation, potentially because of the availability of an alternative preventive therapy. Ninety-seven women reported starting tamoxifen prior to FDA approval; however, based on the highly favorable risk-benefit profiles of these women (Supplementary Table 1, available online) and overlap with the recruitment years for the prevention trials (initiated in 1990 [4–6]), we included them as off-label use. Lastly, our analysis was nested within a family-based cohort study and may not represent women at high risk of breast cancer because of other factors.
The Sister Study, a large cohort of women with a family history of breast cancer, provided a unique opportunity to evaluate tamoxifen use for chemoprevention outside of a clinical trial. Our findings suggest that although the majority of women who use tamoxifen for breast cancer prevention are likely to benefit, care should be directed toward evaluating risk-benefit profiles among women who are older, have an intact uterus, and are African American.
Funding
This research was supported in part by the Intramural Research Program of the National Institutes of Health, the National Institute of Environmental Health Sciences (Z01-ES044005), and the National Center for Advancing Translational Sciences (KL2-TR001109).
Supplementary Material
The authors have no conflicts of interest to report.
References
- 1. Freedman AN, Graubard BI, Rao SR, et al. Estimates of the number of US women who could benefit from tamoxifen for breast cancer chemoprevention. J Natl Cancer Inst. 2003;95(7):526–532. [DOI] [PubMed] [Google Scholar]
- 2. Cuzick J, Sestak I, Bonanni B, et al. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381(9880):1827–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cuzick J, Forbes J, Edwards R, et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet. 2002;360(9336):817–824. [DOI] [PubMed] [Google Scholar]
- 4. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371–1388. [DOI] [PubMed] [Google Scholar]
- 5. Powles T, Eeles R, Ashley S, et al. Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomised chemoprevention trial. Lancet. 1998;352(9122):98–101. [DOI] [PubMed] [Google Scholar]
- 6. Veronesi U, Maisonneuve P, Costa A, et al. Prevention of breast cancer with tamoxifen: preliminary findings from the Italian randomised trial among hysterectomised women. Italian Tamoxifen Prevention Study. Lancet. 1998;352(9122):93–97. [DOI] [PubMed] [Google Scholar]
- 7. Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31(23):2942–2962. [DOI] [PubMed] [Google Scholar]
- 8. Moyer VA. Medications for Risk Reduction of Primary Breast Cancer in Women: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2013;159(10):698–708. [DOI] [PubMed] [Google Scholar]
- 9. Waters EA, McNeel TS, Stevens WM, et al. Use of tamoxifen and raloxifene for breast cancer chemoprevention in 2010. Breast Cancer Res Treat. 2012;134(2):875–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bastian LA, Lipkus IM, Kuchibhatla MN, et al. Women’s interest in chemoprevention for breast cancer. Arch Intern Med. 2001;161(13):1639–1644. [DOI] [PubMed] [Google Scholar]
- 11. Tjia J, Micco E, Armstrong K. Interest in breast cancer chemoprevention among older women. Breast Cancer Res Treat. 2008;108(3):435–453. [DOI] [PubMed] [Google Scholar]
- 12. McKay A, Martin W, Latosinsky S. How should we inform women at higher risk of breast cancer about tamoxifen? An approach with a decision guide. Breast Cancer Res Treat. 2005;94(2):153–159. [DOI] [PubMed] [Google Scholar]
- 13. Tchou J, Hou N, Rademaker A, et al. Acceptance of tamoxifen chemoprevention by physicians and women at risk. Cancer. 2004;100(9):1800–1806. [DOI] [PubMed] [Google Scholar]
- 14. Costantino JP, Gail MH, Pee D, et al. Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst. 1999;91(18):1541–1548. [DOI] [PubMed] [Google Scholar]
- 15. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–1886. [DOI] [PubMed] [Google Scholar]
- 16. Gail MH, Costantino JP, Bryant J, et al. Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. J Natl Cancer Inst. 1999;91(21):1829–1846. [DOI] [PubMed] [Google Scholar]
- 17. Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women. J Natl Cancer Inst. 2007;99(23):1782–1792. [DOI] [PubMed] [Google Scholar]
- 18. Matsuno RK, Costantino JP, Ziegler RG, et al. Projecting individualized absolute invasive breast cancer risk in Asian and Pacific Islander American women. J Natl Cancer Inst. 2011;103(12):951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rockhill B, Spiegelman D, Byrne C, et al. Validation of the Gail et al. model of breast cancer risk prediction and implications for chemoprevention. J Natl Cancer Inst. 2001;93(5):358–366. [DOI] [PubMed] [Google Scholar]
- 20. Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29(17):2327–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Breast Cancer Risk Reduction Guidelines Version 1.2012 National Comprehensive Cancer Center Network. Available at: www.nccn.org. Accessed July 24, 2014.
- 22. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Am Stat Assoc J. 1958;53:457–480. [Google Scholar]
- 23. Goss PE, Ingle JN, Ales-Martinez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364(25):2381–2391. [DOI] [PubMed] [Google Scholar]
- 24. Cuzick J, Sestak I, Forbes JF, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2013;383(9922):1041–1048. [DOI] [PubMed] [Google Scholar]
- 25. Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol. 2010;28(18):3090–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fasching PA, von Minckwitz G, Fischer T, et al. The impact of breast cancer awareness and socioeconomic status on willingness to receive breast cancer prevention drugs. Breast Cancer Res Treat. 2007;101(1):95–104. [DOI] [PubMed] [Google Scholar]
- 27. Julian-Reynier CM, Bouchard LJ, Evans DG, et al. Women’s attitudes toward preventive strategies for hereditary breast or ovarian carcinoma differ from one country to another: differences among English, French, and Canadian women. Cancer. 2001;92(4):959–968. [DOI] [PubMed] [Google Scholar]
- 28. Melnikow J, Paterniti D, Azari R, et al. Preferences of Women Evaluating Risks of Tamoxifen (POWER) study of preferences for tamoxifen for breast cancer risk reduction. Cancer. 2005;103(10):1996–2005. [DOI] [PubMed] [Google Scholar]
- 29. Lin JH, Zhang SM, Manson JE. Predicting adherence to tamoxifen for breast cancer adjuvant therapy and prevention. Cancer Prev Res. 2011;4(9):1360–1365. [DOI] [PubMed] [Google Scholar]
- 30. Huiart L, Ferdynus C, Giorgi R. A meta-regression analysis of the available data on adherence to adjuvant hormonal therapy in breast cancer: summarizing the data for clinicians. Breast Cancer Res Treat. 2013;138(1):325–328. [DOI] [PubMed] [Google Scholar]
- 31. Chlebowski RT, Kim J, Haque R. Adherence to endocrine therapy in breast cancer adjuvant and prevention settings. Cancer Prev Res (Phila). 2014;7(4):378–387. [DOI] [PubMed] [Google Scholar]
- 32. King MC, Wieand S, Hale K, et al. Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2: National Surgical Adjuvant Breast and Bowel Project (NSABP-P1) Breast Cancer Prevention Trial. JAMA. 2001;286(18):2251–2256. [DOI] [PubMed] [Google Scholar]
- 33. Maurice A, Howell A, Evans DG, et al. Predicting compliance in a breast cancer prevention trial. Breast J. 2006;12(5):446–450. [DOI] [PubMed] [Google Scholar]
- 34. Land SR, Cronin WM, Wickerham DL, et al. Cigarette smoking, obesity, physical activity, and alcohol use as predictors of chemoprevention adherence in the National Surgical Adjuvant Breast and Bowel Project P-1 Breast Cancer Prevention Trial. Cancer Prev Res. 2011;4(9):1393–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barisic A, Glendon G, Weerasooriya N, et al. Accuracy of Self-Reported Breast Cancer Information among Women from the Ontario Site of the Breast Cancer Family Registry. J Cancer Epidemiol. 2012;2012:310804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Phillips KA, Milne RL, Buys S, et al. Agreement between self-reported breast cancer treatment and medical records in a population-based Breast Cancer Family Registry. J Clin Oncol. 2005;23(21):4679–4686. [DOI] [PubMed] [Google Scholar]
- 37. Steiner AZ, Terplan M, Paulson RJ. Comparison of tamoxifen and clomiphene citrate for ovulation induction: a meta-analysis. Hum Reprod. 2005;20(6):1511–1515. [DOI] [PubMed] [Google Scholar]
- 38. Vaglio A, Palmisano A, Alberici F, et al. Prednisone versus tamoxifen in patients with idiopathic retroperitoneal fibrosis: an open-label randomised controlled trial. Lancet. 2011;378(9788):338–346. [DOI] [PubMed] [Google Scholar]
- 39. Amrollahi Z, Rezaei F, Salehi B, et al. Double-blind, randomized, placebo-controlled 6-week study on the efficacy and safety of the tamoxifen adjunctive to lithium in acute bipolar mania. J Affect Dis. 2011;129(1–3):327–331. [DOI] [PubMed] [Google Scholar]
- 40. Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. New Engl J Med. 2006;355(2):125–137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.