Abstract
Background:
Laparoscopic colectomy has been shown to have equivalent oncologic outcomes to open colectomy for the management of colon cancer, but its adoption nationally has been slow. This study investigates the prevalence and factors associated with laparoscopic colorectal resection at National Comprehensive Cancer Network (NCCN) centers.
Methods:
Data on patients undergoing surgery for colon and rectal cancer at NCCN centers from 2005 to 2010 were obtained from chart review of medical records for the NCCN Outcomes Project and included information on socioeconomic status, insurance coverage, comorbidity, and physician-reported Eastern Cooperative Oncology Group (ECOG) performance status. Associations between receipt of minimally invasive surgery and patient and clinical variables were analyzed with univariate and multivariable logistic regression. All statistical tests were two-sided.
Results:
A total of 4032 patients, diagnosed between September 2005 and December 2010, underwent elective colon or rectal resection for cancer at NCCN centers. Median age of colon cancer patients was 62.6 years, and 49% were men. The percent of colon cancer patients treated with minimally invasive surgery (MIS) increased from 35% in 2006 to 51% in 2010 across all centers but varied statistically significantly between centers. On multivariable analysis, factors associated with minimally invasive surgery for colon cancer patients who had surgery at an NCCN institution were older age (P = .02), male sex (P = .006), fewer comorbidities (P ≤ .001), lower final T-stage (P < .001), median household income greater than or equal to $80000 (P < .001), ECOG performance status = 0 (P = .02), and NCCN institution (P ≤ .001).
Conclusions:
The use of MIS increased at NCCN centers. However, there was statistically significant variation in adoption of MIS technique among centers.
Although minimally invasive surgical (MIS) approaches have been widely adopted for treatment of many diseases, use of MIS in cancer care has lagged, pending studies demonstrating oncologic equivalence. Over the last decade, many reports have addressed this issue for colorectal cancer (CRC). In 2004, the Clinical Outcomes of Surgical Therapy (COST) Study Group published the results of a phase III randomized trial showing no statistically significant differences in three-year recurrence or survival for patients undergoing open vs laparoscopic colectomy for cancers of the left, right, or sigmoid colon (1). Other studies demonstrate quicker recovery with laparoscopic CRC surgery, including earlier resolution of postoperative ileus, less discomfort, and earlier discharge from hospital (2–4). A systematic review of short-term outcomes from randomized trials found that margin-negative resection rates and nodal evaluation were similar, with lower perioperative mortality following laparoscopy (4). In 2007, the COST group published their benchmark study of five-year results supporting equivalent long-term outcomes of MIS for CRC (5). Since then, other studies have affirmed that laparoscopy is not inferior to open colectomy and in many instances may be superior (6–10). Data from the Colon Cancer Laparoscopic or Open Resection (COLOR) (11) II trial show that, in skilled hands, MIS can provide similar short-term oncologic outcomes and briefer hospital stays. The long-term effects of MIS are less clear in rectal cancer and are still being investigated (12–14).
Despite the growing body of evidence supporting laparoscopic colectomy, adoption of MIS for CRC has been slow (2,15). Whether this is because of lack of surgeon experience, patient contraindications, inaccurate coding, or other factors, is not known. Current National Comprehensive Cancer Network (NCCN) guidelines (updated September 2013) state only that laparoscopy may be considered, citing rectal cancer or local involvement as contraindications.
This study aims to examine preferred surgical approaches to colon and rectal cancers at NCCN Centers over time, and the factors associated with use of MIS in treating colon cancer.
Methods
The study cohort consisted of patients with stage I–IV colon or rectal cancer, diagnosed between September 1, 2005 and December 31, 2010, who received primary surgical care at one of eight institutions participating in the NCCN Colorectal Cancer Outcomes Project. These institutions were selected for participation in the colorectal database project, based on participation in prior database collection efforts. They include the two largest specialty cancer centers in the United States, are geographically diverse, and represent the composition of patients seen at all 21 NCCN institutions. They are: The Ohio State University Comprehensive Cancer Center–James Cancer Hospital and Solove Research Institute, Columbus, OH; Memorial Sloan-Kettering Cancer Center, New York, NY; The University of Texas M.D. Anderson Cancer Center, Houston, TX; Dana-Farber/Brigham and Women’s Cancer Center, Boston, MA; Fox Chase Cancer Center, Philadelphia, PA; Roswell Park Cancer Institute, Buffalo, NY; City of Hope Comprehensive Cancer Center, Duarte, CA; Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago, IL.
Patients were excluded from the study if their primary operation was a Hartmann’s procedure, total or partial pelvic exenteration, exploratory laparoscopy, appendectomy, proctectomy, or transanal excision. Additional exclusion criteria included incomplete information, synchronous colon and rectal cancer, or incomplete staging data.
The data collection process, data transmission methods, and data storage protocols were approved by the participating institutions’ respective Institutional Review Boards. Rigorous data quality assurance processes were in place, as described in prior studies (16–18).
Data Variables
Data from patients’ medical records for the NCCN Outcomes Project were collected longitudinally from diagnosis, based on chart review. At baseline—corresponding to each patient’s date of presentation to the institution—information on socioeconomic status, insurance coverage, comorbidity, and physician-reported Eastern Cooperative Oncology Group (ECOG) performance status were abstracted. Comorbidity at presentation was assigned using the Charlson Index, based on chart review. The zip code of each patient’s residence is collected at first visit to an NCCN institution; this was used to estimate median household income by linking it to the 2000 Census data. Patients’ medical records are systematically reviewed at four, eight, and 12 months after presentation, and annually thereafter, to capture treatment and recurrence data. Starting at four months from baseline, data on preoperative clinical and pathologic TNM staging based on the American Joint Committee on Cancer Staging Manual (6th edition for patients presenting before January 1, 2010; 7th edition for patients presenting on or after January 1, 2010), all surgical treatment, neoadjuvant and/or adjuvant chemotherapy or radiation are abstracted into a database by NCCN-trained Clinical Research Assistants at each institution. Rigorous data quality assurance processes are maintained for the NCCN Outcomes Database Project. This includes initial and follow-up data management training for study personnel, programmed logic checks against the pooled data repository, routine quality assurance reports to each institution for rectification by the data managers, and on-site audits of a random sample of source documents against the submitted data, within the first few months of data collection (repeated annually). Each NCCN institution is audited for data completeness at routine intervals.
Statistical Analysis
The primary endpoint was MIS vs open surgery. The association between MIS and patient demographic and clinical features was characterized using descriptive statistics for two cohorts (colon cancer, rectal cancer). The median and range were reported for continuous variables, and the number and proportion of patients for categorical variables. A Chi-square test for association was used to compare proportions between MIS and open surgery groups. For median age and body mass index comparisons, a Wilcoxon rank-sum test was used.
To examine time trends of MIS and variation among institutions in adopting MIS, the number and proportion of patients undergoing MIS vs open surgery was stratified by diagnosis year and NCCN institution. A bar graph by diagnosis year was used to depict MIS time trends separately for colon and rectal cancer.
The association between MIS in colon cancer and each variable was assessed independently in a univariate logistic regression model. Parameters found to be potentially associated with MIS, based on a P value of less than or equal to .20, were included in the initial multivariable model.
The final multivariable model included predictors with two-sided P less than .05, and control variables defined a priori (NCCN institution, Charlson comorbidity score, sex). Point estimates of the multivariable model were reported as odds ratios (ORs) and 95% confidence intervals (CIs) with the two-sided P value for each odds ratio. Because the same data were used for model selection and evaluation, the P values may be smaller than actually the case. And because seven variables were included in the final model, to adjust for multiple comparisons, the Bonferroni cutpoint for statistical significance is 0.05/7 = 0.007. All statistical analyses were conducted using SAS software (SAS 9.3 Cary, NC, USA).
Results
Study Characteristics
Characteristics of the study cohort are shown in Table 1. A total of 4032 patients, diagnosed between September 1, 2005 and December 31, 2010, underwent elective colorectal resection at a CRC group NCCN center.
Table 1.
Baseline characteristics in patients undergoing definitive surgical resection for stage I-IV colon and rectal cancer at NCCN institutions, September 1, 2005 to December 31, 2010*
| Variable | Colon | Rectal | ||
|---|---|---|---|---|
| Overall No. (%) (n = 2493) | MIS No. (%) (n = 899) | Overall No. (%) (n= 1 539) | MIS No. (%) (n = 299) | |
| Median age at diagnosis (range), y | 62.6 (21–94) | 64.0 (27–94) | 55.9 (22–93) | 54.1 (29–88) |
| Sex | ||||
| Male | 1214 (49) | 461 (51) | 864 (56) | 158 (53) |
| Median household income |
||||
| <40K | 814 (33) | 266 (30) | 491 (32) | 85 (28) |
| 40-59K | 871 (35) | 337 (37) | 502 (33) | 103 (34) |
| 60-79K | 475 (19) | 170 (19) | 314 (20) | 66 (22) |
| ≥80K | 233 (9) | 102 (11) | 167 (11) | 31 (10) |
| Insurance | ||||
| Private | 1306 (52) | 477 (53) | 1028 (67) | 222 (74) |
| Medicare | 976 (39) | 356 (40) | 360 (23) | 46 (15) |
| Medicaid | 113 (5) | 44 (5) | 79 (5) | 18 (6) |
| Self-pay | 38 (2) | 7 (<1) | 24 (1) | 4 (1) |
| Median BMI Mean BMI ± SD | 27.3 | 27.2 | 27.1 | 26.7 |
| 28.3±6.5 | 28.1±6.2 | 28.0±6.0 | 27.4±5.5 | |
| ECOG performance Charlson comorbidity Score | ||||
| 0 | 1874 (75) | 699 (78) | 1237 (80) | 252 (84) |
| 1+ | 278 (11) | 67 (7) | 172 (11) | 25 (8) |
| 0 | 1637 (66) | 614 (68) | 1170 (76) | 247 (83) |
| 1 | 437 (17) | 159 (18) | 231 (15) | 37 (12) |
| 2 | 239 (10) | 69 (8) | 92 (6) | 8 (3) |
| 3+ | 180 (7) | 57 (6) | 46 (3) | 7 (2) |
| Pathologic TNM Stage | ||||
| 0/I | 338 (14) | 183 (20) | 550 (36) | 116 (39) |
| II | 769 (31) | 316 (35) | 228 (19) | 52 (17) |
| III | 771 (31) | 301 (33) | 428 (28) | 107 (36) |
| IV | 606 (24) | 95 (11) | 262 (17) | 21 (7) |
| Number of lymph nodes removed Neoadjuvant Procedure † | ||||
| <12 | 167 (7) | 53 (6) | 266 (17) | 47 (16) |
| 12+ | 2324 (93) | 846 (94) | 1273 (83) | 252 (84) |
| No | 2471 (99) | 895 (99) | 665 (43) | 174 (58) |
| Right hemicolectomy | 1297 (52) | 522 (58) | ||
| Left hemicolectomy | 252 (10) | 93 (11) | ||
| Transverse colectomy | 47 (2) | 12 (1) | ||
| Sigmoid colectomy | 479 (19) | 146 (16) | ||
| Subtotal colectomy | 97 (4) | 24 (3) | ||
| Low anterior resection | 275 (11) | 87 (10) | 1489 (97) | 285 (95) |
* Chi-square test for proportions comparing minimally invasive surgery with open colectomy. For median age, income, and body mass index, a Wilcoxon rank-sum test was used. All statistical tests were two-sided. BMI = body mass index; ECOG = Eastern Cooperative Oncology Group; MIS = minimally invasive surgery; NCCN = National Comprehensive Cancer Network.
† Abdominal perineal resections were not evaluated in this analysis.
A total of 2493 had colon surgery (median age, 62.6 years; 49% male [1214/2493]). Median household income was $47692 (range = $14642-$185466). Most had private insurance: 52% (1306/2493). Mean BMI was 28.3 (+/-6.5). The majority had an ECOG score of 0, 75% (1874/2493), and a Charlson score of 0, 66% (1637/2493). Most had right-sided resections: 52% (1297/2493).
A total of 1539 patients underwent rectal surgery during the same time period (median age 55.9 years [range = 22–93]; 56% male [864/1539]). Median household income was $49394 (range = $12708-$173368). Most had private insurance: 67% (1028/1539). Mean BMI was 28 (+/-6). The majority had an ECOG score of 0, 80% (1237/1539), and a Charlson score of 0, 76% (1170/1539). Nearly all had low anterior resections (97%, n = 1489). Final pathologic stage was most often 0/I (36%, n = 550).
Comparing MIS with open colectomy for colon cancer across all stages, 899 patients (36%) underwent MIS; 1594 (64%) had an open procedure. MIS patients were more likely to have a Charlson score of 0 (68% vs 64%, P = .04), household income greater than or equal to $80000 (11% vs 8%, P < .001); the majority were older (age 64.0 vs 61.5 years, P < .001), and male (51% vs 47%, P = .06). Colon cancer patients undergoing an open procedure had a higher percentage of pathologic stage IV disease (32% vs 11%, P < .001). There was no statistically significant difference in median BMI or insurance.
Comparing MIS with open colectomy for stage I-IV rectal cancer, 299 (19%) patients had MIS; 1240 (81%) had an open procedure. MIS patients were generally younger (age 54.1 vs 56.6 years, P = .02), with a Charlson score of 0 (83% vs 74%, P = .01), and more likely to have private insurance (74% vs 65%, P = .01) and a lower median BMI (26.7 vs 27.3, P = .04).
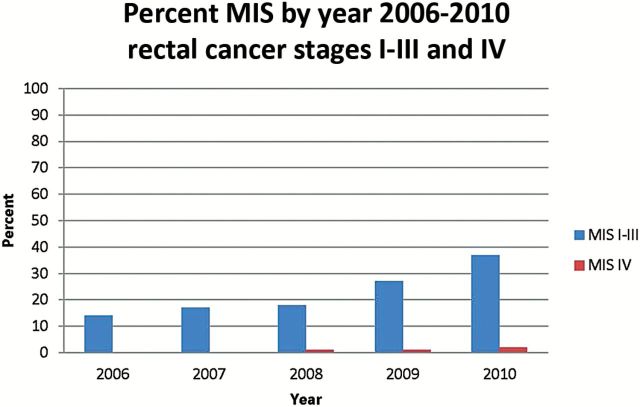
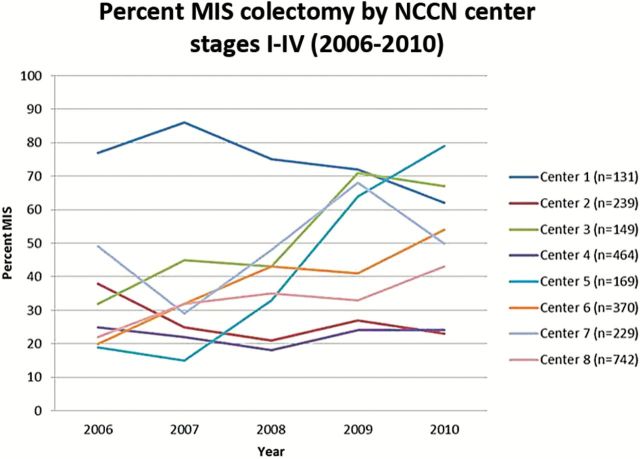
Figures 1 and 2 demonstrate the change in percent of patients undergoing MIS over time from 2006 to 2010 (the years when we have a complete year of data) looking at trends in combined stage I-III and then separately stage IV colon cancer. In 2006, 35% of patients with stage I-III colon cancer underwent MIS; by 2010, the rate was 51% (Figure 1). For patients with stage I-III rectal cancer, there was also increased use of MIS, from 14% in 2006 to 37% in 2010 (Figure 2). MIS for stage IV colon and rectal cancer has been relatively stable over time. Figure 3 shows variations in practice patterns at the eight different NCCN centers. Rates of MIS at individual centers ranged from 15% to 86% of patients across the time period; in the last two years, rates among institutions varied from 23% to 79%. While rates varied at individual institutions, there was an increase overall in percent MIS at NCCN centers.
Figure 1.
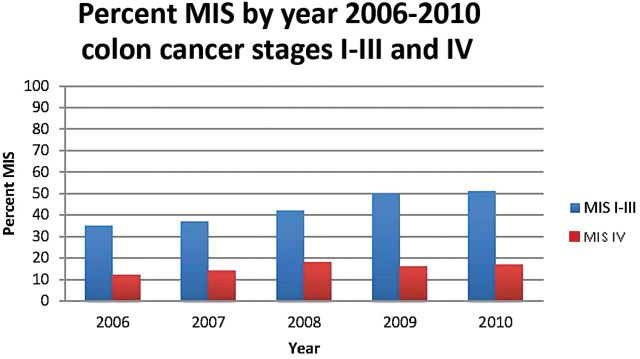
Percent minimally invasive surgery by Year: Colon Cancer Stage I-III and IV, 2006–2010. MIS = minimally invasive surgery.
Figure 2.
Percent minimally invasive surgery by Year: Rectal Cancer Stages I-III and IV, 2006–2010. MIS = minimally invasive surgery.
Figure 3.
Percent MIS Colectomy by National Comprehensive Cancer Network Center, 2006–2010. 2005 is not included, as data were not available for the complete year. MIS = minimally invasive surgery; NCCN = National Comprehensive Cancer Network.
Multivariable Analysis
On multivariable analysis (Table 2), factors associated with minimally invasive colectomy for stage I-IV colon cancer patients undergoing surgery at an NCCN institution were older age (P = .02), male sex (P = .006), fewer comorbidities (P ≤ .001), lower final T-stage (P < .001), median household income greater than $80000 (P < .001), ECOG performance status of 0 (P = .02), and NCCN institution (P < .001). The following factors were tested in the univariate logistic regression: age at diagnosis, sex, race/ethnicity, median household income, insurance, BMI (<25, 25–30, 30+), ECOG performance status, Charlson comorbidity score, pathologic TNM stage, number of lymph nodes positive, neoadjuvant therapy, and NCCN institution. Only race/ethnicity (P = .72 univariate), and BMI (P = .30 univariate) were not considered in the multivariable model. The final model has the following factors: age at diagnosis, sex, median household income, ECOG performance status, Charlson comorbidity score, pathologic TNM stage, neoadjuvant therapy, and center. Number of lymph nodes positive (P = .99 saturated multivariable model) and insurance (P = .09 saturated multivariable model) were excluded from the multivariable model, since the two-sided P value was not less than .05. Even after controlling for other parameters, NCCN institution was a statistically significant predictor of MIS. The institution-specific rates of MIS ranged from 23% to 75%. Patients undergoing surgery at institutions 1 (Adjusted OR = 6.02, 95% CI = 3.95 to 9.2), 3 (Adjusted OR = 1.95, 95% CI = 1.37 to 2.78), or 7 (Adjusted OR = 1.94, 95% CI = 1.44 to 2.62) were statistically significantly more likely to undergo minimally invasive CRC surgery than patients at institution 8 (referent group).
Table 2.
Patient and treatment factors associated with minimally invasive surgery for stage I-IV colon cancer, September 1, 2005 to December 31, 2010*
| Variable | Patients with MIS No. (%) | Unadjusted odds ratio (95% CI) | P | Adjusted odds ratio (95% CI) | P |
|---|---|---|---|---|---|
| Age at diagnosis, y |
|||||
| <50 | 134 (28) | referent | <.001 | Referent | .02 |
| 50–64 | 341 (37) | 1.50 (1.18 to 1.90) | 1.36 (1.05 to 1.77) | ||
| 65–74 | 209 (37) | 1.50 (1.16 to 1.95) | 1.39 (1.04 to 1.86) | ||
| 75+ | 215 (24) | 1.76 (1.35 to 2.30) | 1.61 (1.18 to 2.18) | ||
| Sex | |||||
| Male | 461 (38) | Referent | .05 | Referent | .006 |
| Female | 438 (34) | 0.85 (0.72 to 1.00) | 0.78 (0.65 to 0.93) | ||
| Median household income, USD |
|||||
| <40K | 266 (33) | Referent | <.001 | Referent | <.001 |
| 40-59K | 337 (39) | 1.30 (1.06 to 1.59) | 1.31 (1.05 to 1.64) | ||
| 60-79K | 170 (36) | 1.15 (0.91 to 1.46) | 1.19 (0.91 to 1.56) | ||
| ≥80K | 102 (44) | 1.60 (1.19 to 2.16) | 2.17 (1.55 to 3.03) | ||
| ECOG performance |
|||||
| 0 | 699 (37) | Referent | <.001 | Referent | .02 |
| 1+ | 67 (24) | 0.53 (0.40 to 0.71) | 0.64 (0.47 to 0.89) | ||
| Charlson comorbidity score |
|||||
| 0 | 614 (38) | Referent | .04 | Referent | <.001 |
| 1 | 159 (36) | 0.95 (0.77 to 1.19) | 0.81 (0.64 to 1.04) | ||
| 2 | 69 (29) | 0.68 (0.50 to 0.91) | 0.50 (0.36 to 0.70) | ||
| 3+ | 57 (32) | 0.77 (0.56 to1.07) | 0.50 (0.35 to 0.74) | ||
| Pathologic TNM Stage |
|||||
| 0/I | 183 (54) | 1.84 (1.42 to 2.39) | <.001 | 1.71 (1.29 to 2.28) | <.001 |
| II | 316 (41) | 1.09 (0.89 to 1.34) | 1.03 (0.83 to 1.27) | ||
| III | 301 (39) | Referent | Referent | ||
| IV | 95 (16) | 0.29 (0.22 to 0.38) | 0.26 (0.20 to 0.34) | ||
| Neoadjuvant therapy Center |
|||||
| No | 895 (36) | Referent | .09 | Referent | .05 |
| Yes | 4 (18) | 0.39 (0.13 to 1.16) | 0.32 (0.10 to 1.01) | ||
| 1 | 98 (75) | 6.02 (3.95 to 9.20) | <.001 | 6.14 (3.88 to 9.73) | <.001 |
| 2 | 63 (26) | 0.73 (0.52 to 1.01) | 0.66 (0.46 to 0.93) | ||
| 3 | 73 (50) | 1.95 (1.37 to 2.78) | 2.13 (1.44 to 3.16) | ||
| 4 | 105 (23) | 0.59 (0.46 to 0.77) | 0.54 (0.40 to 0.73) | ||
| 5 | 69 (41) | 1.39 (0.99 to 1.96) | 1.38 (0.94 to 2.01) | ||
| 6 | 134 (36) | 1.15 (0.89 to 1.50) | 1.28 (0.95 to 1.72) | ||
| 7 | 112 (49) | 1.94 (1.44 to 2.62) | 1.83 (1.31 to 2.55) | ||
| 8 | 245 (33) | Referent | Referent |
* Unadjusted odds ratio = univariate logistic regression. Adjusted odds ratio = multivariable logistic regression. The final model has the following factors: age at diagnosis, sex, median household income, Eastern Cooperative Oncology Group performance status, Charlson comorbidity, pathologic TNM stage, neoadjuvant therapy, and center. Sex, Charlson comorbidity, and center were defined as control variables a priori. All statistical tests were two-sided. CI = confidence interval; ECOG = Eastern Cooperative Oncology Group; MIS = minimally invasive surgery.
Given the much smaller proportion of rectal cancer patients undergoing MIS and the complex controversy in the use of MIS for rectal cancer, which is not yet recommended by NCCN guidelines, we did not do a multivariable analysis on this group.
Discussion
Over the last decade, numerous clinical trials and cohort studies have validated the use of MIS colectomy for patients with CRC. This study shows that the rates of laparoscopic colectomy in both colon and rectal cancer from September 1, 2005 to December 31, 2010 at the eight NCCN institutions participating in the Colorectal Outcomes Database Project has increased since the COST trial and is higher than rates reported from many US population-based series.
Over the past 15 years, randomized trials have demonstrated at least equivalent 30-day outcomes among patients undergoing laparoscopic colectomy for colon cancer (19). More recently, the COLOR, CLASSIC, and COST trials have confirmed the results of previous studies, showing at least equivalent long-term outcomes and multiple short-term benefits for laparoscopic colectomy (6,7,20).
While evidence supports MIS, rates on its use have varied. Rea et al. reviewed the National Inpatient Sample (NIS) data on 741817 colectomies performed for both benign and malignant disease from 2001 to 2003 and 2005 to 2007, demonstrating that the rate of MIS for colon cancer increased from 2.3% to 8.9% since publication of the COST trial—with 90% of CRC cases still being done via an open approach (15). Kemp et al. examined rates of laparoscopy using NIS data from 2000 to 2004, demonstrating increased rates of laparoscopy at academic vs nonacademic centers (21). Robinson et al. confirmed this with more recent NIS data, noting that changes have been slow, with academic centers adopting MIS technology more quickly (22). Fullum et al. report rates of MIS colectomy to be around 30%, without differentiating between colectomy for malignant vs nonmalignant disease (23). Most recently, Fox et al. and Kang et al. (23,24) published reviews of the NIS data, showing trends nearing 50% nationally (11). This is the first evidence that laparoscopic colectomy for cancer has become more mainstream (24,25). However, these studies, utilizing administrative databases, can only identify limited factors associated with MIS.
Interestingly, most groups noted substantial variation regionally and across center type (22,24,25). The fact that academic centers have led the way is not surprising, given that the majority of trials examining MIS have been performed at academic centers (9,26). More interesting is the regional variation, with higher rates reported in the US Northeast and South (24,25). We found statistically significant variability across institutions, even after accounting for stage and comorbidities. Patients are more likely to have MIS at certain centers than at others.
Variation in use of MIS among NCCN institutions is intriguing. All employ highly trained, specialized surgeons. The lag may be related, as many surgeons require retraining in laparoscopic techniques. Retraining of surgeons is costly and time consuming. The impetus for doing so includes clinicians’ desire to improve patient outcomes by way of the smaller incisions and quicker recovery associated with MIS. Patients undoubtedly play a role, and may demand MIS or seek out surgeons who offer it. Additionally, there may be a delay, as departments must invest in laparoscopic equipment in order to support an MIS program.
The lag in use of MIS in the community may also be because of the associated learning curve. The technical demands of laparoscopic colectomy are greater than in many more commonly performed laparoscopic procedures. Past literature suggests that the learning curve for laparoscopic colectomy ranges from 25 to 60 cases—more than most general surgeons perform in an entire year. At specialized centers, however, surgeons are trained to perform these procedures (27,28).
Our cohort, much like those in other nonrandomized studies, shows that MIS patients present with fewer comorbidities have higher incomes and have private medical insurance. This is likely related to the socioeconomic privilege associated with access to NCCN centers. Numerous studies have shown that patients with lower socioeconomic status are less likely to be treated at specialized centers (29,30). However, the differences demonstrated in our study are smaller than those in previous reports; in fact, the clinical differences are smaller than previously reported. This suggests that, as physicians become more comfortable with MIS, they may be expanding its use.
While we have do not have specific data looking at why older age would more likely be associated with MIS in our colectomy cohort, it is consistent with several published studies that show that the elderly may benefit the most from MIS (31,32). As MIS for colectomy has gained wider usage and more people are trained in MIS, physicians have noted the benefits of MIS, particularly for this older age group, and so it makes sense that its use is increasing. For rectal cancer, where the indications are not well defined, physicians are more likely to be cautious with their patient selection.
While the benefits of MIS are less clear in rectal cancer, recent retrospective studies have shown that total mesenteric excision can be safely performed using MIS, with no statistically significant differences in survival compared with an open approach (12,13). Data from the COLOR II trial demonstrate that carefully selected patients treated by skilled surgeons have similar short-term safety and oncologic outcomes, with shorter recovery. Long-term outcomes have not been established (14). Intraoperative conversion from laparoscopy to open surgery is markedly higher in rectal cancer, reflecting a greater level of procedural complexity (33–35). Studies show a higher learning curve for laparoscopic rectal resections, which likely explains why their use is greater at specialized high-volume centers (36). Although robotic techniques appear to facilitate rectal resection, optimal MIS for rectal cancer has yet to be determined (37,38).
Although several studies compare MIS with open colectomy—and there is a sense that its use is increasing—no study has specifically examined utilization of laparoscopy for CRC at specialized cancer centers. Furthermore, no study accounts for changes that have occurred since publication of the COST trial and other trials supporting MIS for CRC. Our study shows that these centers have increased use of MIS for CRC to over 50%.
We found that stage IV CRC patients were less likely to undergo MIS. There are several reasons for this. First, a comparatively larger proportion of NCCN patients have pathologic stage IV disease (1,39). Second, our analysis was based on final pathologic stage; perhaps these patients had more locally advanced tumors and, as a result, were deemed technically more challenging for laparscopic surgery. Such patients are more likely to have additional disease and may require combined resections. Because of their greater complexity, combined resections are less likely to be done using minimally invasive techniques. We observed that the number of MIS cases is higher in stage I-III patients alone than it is when stage IV patients are factored in. Stage IV CRC is really a different disease process, and other factors must be taken into consideration when treating these patients. Nevertheless, some stage IV cases can be managed using MIS, and these patients may benefit from it most.
The results of this study must be analyzed within the context of the limitations associated with any retrospective study using a large database. Surgeon choice regarding patient management shows a selection bias. To account for this, we made multivariable adjustments for comorbidities, age, and patient factors. But other factors not available in the database are clearly unaccounted for. Additionally, there are limitations in the measures of disease severity. Our multivariable analysis accounts for patient comorbidities using the Charlson Comorbidity Index and ECOG status, but these measures are only surrogates. Finally, as these data come from the nation’s leading cancer centers—which have highly specialized surgeons and disease-specific teams—the outcomes may not be comparable with those found in the general population. Physicians at cancer specialty centers may care for patients who present with more complex and reoperative cases; thus, the number of cases amenable to MIS may be lower than in the general population. Our goal was to examine trends at these specialized institutions, to ascertain whether they are on par with or greater than those found at the national level.
Use of MIS has increased nationally, and this trend is being seen at NCCN centers. Since publication of the COST trial results, nearly one half of all cases are now done laparoscopically. The rate of increase may have lagged because of regional variability, surgeon experience, a higher learning curve, or the fact that many colectomies continue to be done by general surgeons rather than specialists who are fellowship-trained in colon and rectal cancer. We expect that rates will continue to rise as MIS training disseminates and the technology advances.
As more centers offer MIS, its use in the general population will increase. While specialized centers increasingly utilize MIS, there remains a large variability between different centers. This is likely attributable to complexity and case mix, as well as surgeon skill and experience. Because most surgical training programs offer laparoscopy training and there are MIS requirements in surgical training (eg, Fundamentals of Laparoscopic Surgery), younger surgeons completing this training will more likely incorporate MIS into their practice.
Stage IV patients are less likely to be managed with MIS; however, these are the patients who may benefit from it most. MIS is strongly associated with less short-term morbidity and the potential to begin adjuvant therapy sooner. As techniques improve, combined procedures may become more feasible, increasing the number of stage IV patients who are MIS candidates. As surgeons gain technical proficiency, use of MIS is likely to increase—even in this higher-risk population.
Funding
This study was funded in part by the cancer center core grant P30 CA008748. The core grant provides funding to institutional cores, such as Biostatistics and Pathology, which were used in this study.
The authors have no conflicts of interest to declare.
References
- 1. Weeks JC, Nelson H, Gelber S, Sargent D, Schroeder G. Short-term quality-of-life outcomes following laparoscopic-assisted colectomy vs open colectomy for colon cancer: a randomized trial. JAMA. 2002;287(3):321–328. [DOI] [PubMed] [Google Scholar]
- 2. Bilimoria KY, Bentrem DJ, Nelson H, et al. Use and outcomes of laparoscopic-assisted colectomy for cancer in the United States. Arch Surg. 2008;143(9):832–839; discussion 9–40. [DOI] [PubMed] [Google Scholar]
- 3. Jayne DG, Guillou PJ, Thorpe H, et al. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007;25(21):3061–3068. [DOI] [PubMed] [Google Scholar]
- 4. Tjandra JJ, Chan MK. Systematic review on the short-term outcome of laparoscopic resection for colon and rectosigmoid cancer. Colorectal Dis. 2006;8(5):375–388. [DOI] [PubMed] [Google Scholar]
- 5. Fleshman J, Sargent DJ, Green E, et al. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg. 2007;246(4):655–662; discussion 62–64. [DOI] [PubMed] [Google Scholar]
- 6. Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional vs laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365(9472):1718–1726. [DOI] [PubMed] [Google Scholar]
- 7. Veldkamp R, Kuhry E, Hop WC, et al. Laparoscopic surgery vs open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6(7):477–484. [DOI] [PubMed] [Google Scholar]
- 8. Jackson TD, Kaplan GG, Arena G, Page JH, Rogers SO., Jr Laparoscopic vs open resection for colorectal cancer: a metaanalysis of oncologic outcomes. J Am Coll Surg. 2007;204(3):439–446. [DOI] [PubMed] [Google Scholar]
- 9. Lacy AM, Delgado S, Castells A, et al. The long-term results of a randomized clinical trial of laparoscopy-assisted vs open surgery for colon cancer. Ann Surg. 2008;248(1):1–7. [DOI] [PubMed] [Google Scholar]
- 10. Braga M, Vignali A, Gianotti L, et al. Laparoscopic vs open colorectal surgery: a randomized trial on short-term outcome. Ann Surg. 2002;236(6):759–766; disscussion 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kang CY, Halabi WJ, Luo R, Pigazzi A, Nguyen NT, Stamos MJ. Laparoscopic colorectal surgery: a better look into the latest trends. Arch Surg. 2012;147(8):724–731. [DOI] [PubMed] [Google Scholar]
- 12. Glancy DG, Chaudhray BN, Greenslade GL, Dixon AR. Laparoscopic total mesorectal excision can be performed on a nonselective basis in patients with rectal cancer with excellent medium-term results. Colorectal Dis. 2012;14(4):453–457. [DOI] [PubMed] [Google Scholar]
- 13. Laurent C, Leblanc F, Wutrich P, Scheffler M, Rullier E. Laparoscopic vs open surgery for rectal cancer: long-term oncologic results. Ann Surg. 2009;250(1):54–61. [DOI] [PubMed] [Google Scholar]
- 14. van der Pas MH, Haglind E, Cuesta MA, et al. Laparoscopic vs open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14(3):210–218. [DOI] [PubMed] [Google Scholar]
- 15. Rea JD, Cone MM, Diggs BS, Deveney KE, Lu KC, Herzig DO. Utilization of laparoscopic colectomy in the United States before and after the clinical outcomes of surgical therapy study group trial. Ann Surg. 2011;254(2):281–288. [DOI] [PubMed] [Google Scholar]
- 16. Niland JC. NCCN outcomes research database: data collection via the Internet. Oncology (Williston Park). 2000;14(11A):100–103. [PubMed] [Google Scholar]
- 17. Niland JC. NCCN Internet-based data system for the conduct of outcomes research. Oncology (Williston Park). 1998;12(11A):142–146. [PubMed] [Google Scholar]
- 18. Temple LK, Romanus D, Niland J, et al. Factors associated with sphincter-preserving surgery for rectal cancer at national comprehensive cancer network centers. Ann Surg. 2009;250(2):260–267. [DOI] [PubMed] [Google Scholar]
- 19. Milsom JW, Bohm B, Hammerhofer KA, Fazio V, Steiger E, Elson P. A prospective, randomized trial comparing laparoscopic vs conventional techniques in colorectal cancer surgery: a preliminary report. J Am Coll Surg. 1998;187(1):46–54; discussion 54–55. [DOI] [PubMed] [Google Scholar]
- 20. Nelson H, Sargent DJ. Laparoscopically assisted vs open colectomy for colon cancer - Reply. New Engl J Med. 2004;351(9):933–934. [DOI] [PubMed] [Google Scholar]
- 21. Kemp JA, Finlayson SRG. Nationwide trends in laparoscopic colectomy from 2000 to 2004. Surg Endosc. 2008;2(5):1181–1187. [DOI] [PubMed] [Google Scholar]
- 22. Robinson CN, Chen GJ, Balentine CJ, et al. Minimally invasive surgery is underutilized for colon cancer. Ann Surg Oncol. 2011;18(5):1412–1418. [DOI] [PubMed] [Google Scholar]
- 23. Fullum TM, Ladapo JA, Borah BJ, Gunnarsson CL. Comparison of the clinical and economic outcomes between open and minimally invasive appendectomy and colectomy: evidence from a large commercial payer database. Surg Endosc. 2010;24(4):845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fox JP, Desai MM, Krumholz HM, Gross CP. Hospital-Level Outcomes Associated with Laparoscopic Colectomy for Cancer in the Minimally Invasive Era. J Gastrointest Surg. 2012;16(11):2112–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fox J, Gross CP, Longo W, Reddy V. Laparoscopic colectomy for the treatment of cancer has been widely adopted in the United States. Diseases of the colon and rectum 2012;55(5):501–8. [DOI] [PubMed] [Google Scholar]
- 26. Braga M, Vignali A, Zuliani W, Frasson M, Di Serio C, Di Carlo V. Laparoscopic vs open colorectal surgery: cost-benefit analysis in a single-center randomized trial. Ann Surg. 2005;242(6):890–5, discussion 5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schlachta CM, Mamazza J, Seshadri PA, Cadeddu M, Gregoire R, Poulin EC. Defining a learning curve for laparoscopic colorectal resections. Dis Colon Rectum. 2001;44(2):217–222. [DOI] [PubMed] [Google Scholar]
- 28. Tekkis PP, Senagore AJ, Delaney CP, Fazio VW. Evaluation of the learning curve in laparoscopic colorectal surgery: comparison of right-sided and left-sided resections. Ann Surg. 2005;242(1):83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ward E, Halpern M, Schrag N, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58(1):9–31. [DOI] [PubMed] [Google Scholar]
- 30. Schrag D, Cramer LD, Bach PB, Cohen AM, Warren JL, Begg CB. Influence of hospital procedure volume on outcomes following surgery for colon cancer. JAMA. 2000;284(23): 3028–3035. [DOI] [PubMed] [Google Scholar]
- 31. Senagore AJ, Madbouly KM, Fazio VW, Duepree HJ, Brady KM, Delaney CP. Advantages of laparoscopic colectomy in older patients. Arch Surg. 2003;138(3):252–256. [DOI] [PubMed] [Google Scholar]
- 32. Mutch MG. Laparoscopic colectomy in the elderly: when is too old? Clin Colon Rectal Surg. 2006;19(1):33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kang J, Bae BN, Gwak G, et al. Comparative study of a single-incision laparoscopic and a conventional laparoscopic appendectomy for the treatment of acute appendicitis. J Korean Soc Coloproctology. 2012;28(6):304–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kang CY, Chaudhry OO, Halabi WJ, et al. Outcomes of laparoscopic colorectal surgery: data from the Nationwide Inpatient Sample 2009. Am J Surg. 2012;204(6):952–957. [DOI] [PubMed] [Google Scholar]
- 35. Buunen M, Bonjer HJ, Hop WC, et al. COLOR II. A randomized clinical trial comparing laparoscopic and open surgery for rectal cancer. Dan Med Bull. 2009;56(2):89–91. [PubMed] [Google Scholar]
- 36. Park IJ, Choi GS, Lim KH, Kang BM, Jun SH. Multidimensional analysis of the learning curve for laparoscopic resection in rectal cancer. J Gastrointest Surg. 2009;13(2):275–281. [DOI] [PubMed] [Google Scholar]
- 37. Park JS, Choi GS, Lim KH, Jang YS, Jun SH. Robotic-assisted vs laparoscopic surgery for low rectal cancer: case-matched analysis of short-term outcomes. Ann Surg Oncol. 2010;17(12):3195–202. [DOI] [PubMed] [Google Scholar]
- 38. deSouza AL, Prasad LM, Marecik SJ, et al. Total mesorectal excision for rectal cancer: the potential advantage of robotic assistance. Dis Colon Rectum. 2010;53(12):1611–1617. [DOI] [PubMed] [Google Scholar]
- 39. Tinmouth J, Tomlinson G. Laparoscopically assisted vs open colectomy for colon cancer. N Engl J Med. 2004;351(9):933–934. [DOI] [PubMed] [Google Scholar]