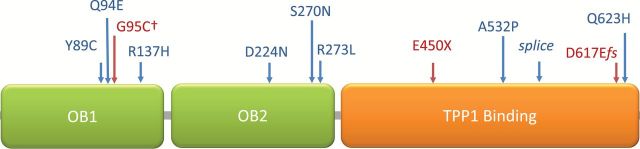
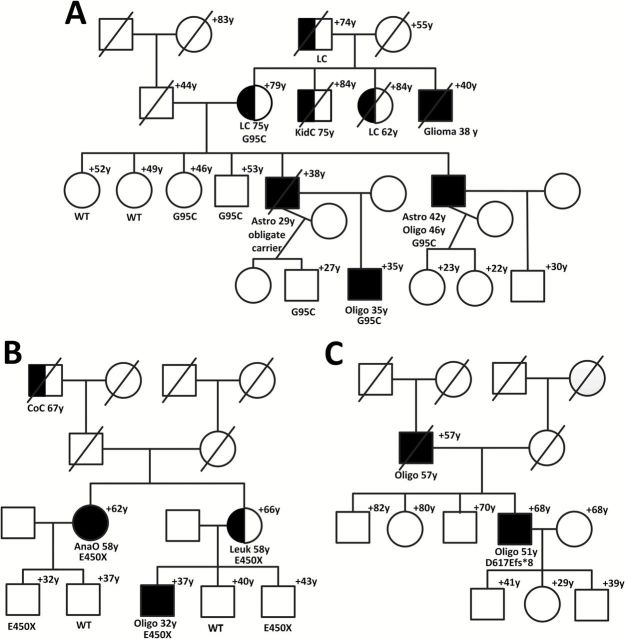
Matthew N Bainbridge
Matthew N Bainbridge
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Georgina N Armstrong
Georgina N Armstrong
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
M Monica Gramatges
M Monica Gramatges
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Alison A Bertuch
Alison A Bertuch
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Shalini N Jhangiani
Shalini N Jhangiani
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Harsha Doddapaneni
Harsha Doddapaneni
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Lora Lewis
Lora Lewis
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Joseph Tombrello
Joseph Tombrello
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Spyros Tsavachidis
Spyros Tsavachidis
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Yanhong Liu
Yanhong Liu
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Ali Jalali
Ali Jalali
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Sharon E Plon
Sharon E Plon
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Ching C Lau
Ching C Lau
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Donald W Parsons
Donald W Parsons
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Elizabeth B Claus
Elizabeth B Claus
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Jill Barnholtz-Sloan
Jill Barnholtz-Sloan
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Dora Il’yasova
Dora Il’yasova
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Joellen Schildkraut
Joellen Schildkraut
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Francis Ali-Osman
Francis Ali-Osman
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Siegal Sadetzki
Siegal Sadetzki
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Christoffer Johansen
Christoffer Johansen
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Richard S Houlston
Richard S Houlston
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Robert B Jenkins
Robert B Jenkins
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Daniel Lachance
Daniel Lachance
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Sara H Olson
Sara H Olson
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Jonine L Bernstein
Jonine L Bernstein
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Ryan T Merrell
Ryan T Merrell
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Margaret R Wrensch
Margaret R Wrensch
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Kyle M Walsh
Kyle M Walsh
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Faith G Davis
Faith G Davis
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Rose Lai
Rose Lai
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Sanjay Shete
Sanjay Shete
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Kenneth Aldape
Kenneth Aldape
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Christopher I Amos
Christopher I Amos
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Patricia A Thompson
Patricia A Thompson
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Donna M Muzny
Donna M Muzny
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Richard A Gibbs
Richard A Gibbs
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Beatrice S Melin
Beatrice S Melin
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,
Melissa L Bondy
Melissa L Bondy
1Affiliations of authors: Human Genome Sequencing Center, Baylor College of Medicine, Houston, TX (MNB, SNJ, HD, LL, JT, DMM, RAG); Codified Genomics, LLC, Houston, TX (MNB); Department of Pediatrics, Division of Hematology-Oncology, Dan L. Duncan Cancer Center (GNA, MMG, AAB, ST, YL, SEP, CCL, DWP, MLB) and Department of Neurosurgery (AJ), Baylor College of Medicine, Houston, TX; Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, CT (EBC); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (EBC); Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH (JBS); Department of Epidemiology and Biostatistics, Georgia State University School of Public Health, Atlanta, GA (DI); Cancer Control and Prevention Program, Department of Community and Family Medicine, Duke University Medical Center, Durham, NC (DI, JS); Department of Surgery, Duke University Medical Center, Durham, North Carolina (FAO); Cancer and Radiation Epidemiology Unit, Gertner Institute, Chaim Sheba Medical Center, Tel Hashomer (SS); Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel (SiS); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (CJ); Section of Cancer Genetics, Institute of Cancer Research, Sutton, Surrey, UK (RSH); Mayo Clinic Comprehensive Cancer Center, Mayo Clinic, Rochester, MN (RBJ, DL); Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, NY (SHO, JLB); Department of Neurology, NorthShore University HealthSystem, Evanston, IL (RTM); Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA (MRW, KMW); Department of Public Health Services, University of Alberta, Edmonton, Alberta, Canada (FGD); Departments of Neurology, Neurosurgery, and Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA (RL); Department of Biostatistics (SaS) and Department of Pathology (KA), University of Texas MD Anderson Cancer Center, Houston, TX; Department of Community and Family Medicine, Department of Genetics, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Hanover, NH (CIA); Department of Cellular and Molecular Medicine, University of Arizona Cancer Center, Tucson, AZ (PAT); Department of Radiation Sciences Oncology, Umeå University, Umeå, Sweden (BSM).
1,✉;
The Gliogene Consortium1