Abstract
The aim of this study was to investigate the impact of moderate aerobic training on functional, anthropometric, biochemical, and health-related quality of life (HRQOL) parameters on women with metabolic syndrome (MS). Fifteen untrained women with MS performed moderate aerobic training for 15 weeks, without modifications of dietary behaviours. Functional, anthropometric, biochemical, control diet record and HRQOL parameters were assessed before and after the training. Despite body weight maintenance, the patients presented decreases in waist circumference (P = 0.001), number of MS components (P = 0.014), total cholesterol (P = 0.049), HDL cholesterol (P = 0.004), LDL cholesterol (P = 0.027), myeloperoxidase activity (P = 0.002) and thiobarbituric acid-reactive substances levels (P = 0.006). There were no differences in total energy, carbohydrate, protein and lipid intake pre- and post-training. Furthermore, improvements in the HRQOL subscales of physical functioning (P = 0.03), role-physical (P = 0.039), bodily pain (P = 0.048), general health (P = 0.046) and social functioning scoring (P = 0.011) were reported. Despite the absence of weight loss, aerobic training induced beneficial effects on functional, anthropometric, biochemical and HRQOL parameters in women with MS.
Keywords: metabolic syndrome X, women, exercise, quality of life, weight loss
INTRODUCTION
Considered a cluster of several chronic diseases, the metabolic syndrome (MS) is defined as the combination of interrelated risk factors including abdominal obesity, insulin resistance, dyslipidaemia and high blood pressure [1]. This metabolic profile is highly related to a sedentary lifestyle, leading to an association between MS and cardiovascular diseases [1]. Evidence demonstrated a close link between MS, a state of chronic inflammation, and oxidative stress, which is also implicated in development of cardiovascular diseases [2]. In this line, recent evidence showed that women are more prone to present a low grade and subclinical internal inflammation compared to men [3].
Most of the studies concerning exercise training in obese populations have focused on the single analysis of anthropometric, physiological and/or biochemical parameters, without taking into consideration the possible health-related quality of life (HRQOL) afforded by exercise training. In this sense, a poor HRQOL is associated with a worse performance in the daily living activities [4]. Moreover, health-related increments are associated with increases in self-management, adherence to therapies, and reduced number of hospitalizations and use of medications in type 2 diabetes melli-tus (T2DM) patients [5].
It is known that moderate aerobic training improves the maximal oxygen uptake (VO2max) and lipid profile, reduces inflammation and oxidative stress markers, and ameliorates HRQOL parameters in overweight men and women [6, 7, 8]. These favourable changes are proved to reduce economic costs related to these health outcomes [5]. In spite of this, few studies have examined the impact of aerobic training as a single intervention on improvements in functional, biochemical, and HRQOL parameters in women with MS. Therefore, the aim of this study was to investigate the effects of moderate aerobic training on functional, anthropometric, biochemical, and HRQOL indices on women with MS.
MATERIALS AND METHODS
Participants
Fifteen untrained women aged 49.82 ± 8.03 years old were recruited to participate in the study. Inclusion criteria for participation were (1) the presence of MS [1]; 2) non-smokers; and (3) full medical screening with a sports science physician. The study was approved by the Ethics Committee of the Universidade Federal de Santa Maria (permit number 0032.0.243.000-07) and followed the statements of the Declaration of Helsinki and Ethical Standards in Sport and Exercise Science Research.
Study Design
Before and after 15 weeks of exercise training, participants underwent anthropometric measurements, a flexibility test, and aerobic power testing in order to assess functional capacity. Blood sampling for serum and plasma analysis was carried out and a self-perceived quality of life questionnaire was also applied. To minimize a possible nutritional bias, participants were encouraged to maintain the habitual dietary intake during the study and filled in a 3-day diet record..
Training Protocol
The training protocol was designed according to the exercise training recommendations for this specific population [9]. All training sessions were supervised and the heart rate (HR) was continuously monitored using heart rate monitors (Polar RS 400, Polar Electro Oy, Kempele, Finland). Exercise training consisted of brisk walking and/or slow jogging on a treadmill 3 days per week. Intensity was controlled across the target heart rate reserve (HRR). The training sessions started with a 5 min warm-up performed on the treadmill at approximately 40% HRR and ended with a 5 min recovery and standardized stretching routine. The exercise duration and intensity were in the range of 30-55 min and 50-65% of the HRR during the 15 weeks of the training protocol.
Flexibility Test and Anthropometric Measurements
The lumbar and hamstring muscles’ flexibility was assessed by the sit-and-reach test before and after the training protocol. The participants sat with their feet approximately hip-wide against the testing box, kept their knees extended, placed the right hand over the left, and slowly reached forward as far as they could by sliding their hands along the measuring board [9]. The longest distance reached on three attempts was registered. After that, subjects were weighed with a scale (Plenna, Sao Paulo, Brazil) and their height was measured with a stadiometer (Cardiomed, Curitiba, Brazil). Waist circumference was measured at the midpoint between the lowest rib and iliac crest, and hip circumference at the level of the greater trochanters of the femur.
Blood Pressure, Resting Heart Rate and Cardiorespiratory Fitness
The resting systolic and diastolic blood pressures were measured using a standard aneroid sphygmomanometer (BIC, Itupeva, Brazil) before and after the training protocol. The participant resting HR was measured during 5 min in the seated position and the lower value was registered. The cardiovascular fitness of participants was assessed by the one-mile track walk test on a track with Olympic dimensions [10]. The measurement of perceived exertion was also monitored in each completed lap of the test with a modified Borg scale [11]. Additionally, the HR and the time were recorded for each completed lap.
Blood Collection and Sample Preparation
Blood samples were collected following 12 h of fasting and participants were asked to avoid intense exercise during the previous 48 h. Blood samples were collected in 4 ml EDTA anticoagulant or serum separator tubes. Serum and plasma were separated by centrifugation at 600 x g for 15 min, and stored at -20°C for further biochemical analysis.
Biochemical Assays
Serum triglycerides (TG), total cholesterol (TC), high-density lipoprotein (HDL), glucose, creatinine, uric acid, urea and albumin were determined using commercially available assay kits (Labtest, Lagoa Santa, Brazil). Low-density lipoprotein (LDL) was calculated using the Friedewald equation [12]. The serum activity of enzymes aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (AP) and lactate dehydrogenase (LDH) was also measured following manufacturer protocols (Labtest, Lagoa Santa, Brazil). Plasma non-protein sulfhydryl groups (NPSH) were assessed according to Ellman [13] and expressed in nmol • mg−1 of glutathione (GSH). The formation of thiobarbituric acid-reactive substances (TBARS) in plasma was assayed as previously described [14] and expressed in pg • dL−1 of malondialdehyde (MDA). The plasma activity of the pro-inflammatory enzyme myeloperoxidase (MPO) was measured spectrophotometrically by a modified peroxidase-coupledassay system involving phenol, 4-aminoantipyrine and hydrogen peroxide (H2O2) [15]. The results were expressed in micromolar of quinoneimine produced at 30 min.
Quality of Life
The HRQOL was assessed in a face-to-face interview. Participants answered the 36-Item Short-Form Health Survey (SF-36) which was previously validated in the Brazilian population [16]. Eight subscales (physical functioning, role-physical, bodily pain, vitality, general health, social functioning, role-emotional, and mental health) were calculated by a standard scoring protocol [17]. Scores for each dimension range from 0 (poor state of HRQOL) to 100 (good state of HRQOL).
Dietary Assessment
Participants completed a 3-day diet record, in which they were instructed to measure and record the exact amount of food and drink consumed over 2 week days and 1 weekend day in order to determine total daily caloric intake before and after the training protocol. Dietary intake was determined using dietary analysis software (Dietwin, Sao Paulo, Brazil).
Statistical Analysis
Data were expressed as mean ± standard deviation (SD). Student's t-test for parametric and Wilcoxon signed rank test for nonparamet-ric data were used to determine significant differences between the mean for the individual response to the training protocol. A two-way ANOVA (laps vs. before and after training) was used to compare changes in the cardiorespiratory test. SPSS 15.0 (Statistical Package for Social Sciences, Chicago, USA) was used, and statistical significance was set at P < 0.05.
RESULTS
Fifteen participants aged 49.46 ± 8.51 years and 164 ± 0.07 cm high completed the training protocol. Table 1 shows the functional and anthropometric data related to before and after training measurements. Among the 15 participants, the adherence to the training was 87% throughout the study.
TABLE 1.
Functional, anthropometric and biochemical characterization of female MS patients before and after the training protocol.
| Parameter | Before | After |
|---|---|---|
| Body Weight (kg) | 86.20±16.22 | 86.27 ± 15.80 |
| Waist Circumference (cm) | 96.30 ± 12.51 | 93.24 ± 11.83 ** |
| Hip Circumference (cm) | 114.77 ± 9.40 | 112.62 ± 11.03* |
| Resting Heart Rate (bpm) | 75.06 ± 9.33 | 69.33 ± 9.90** |
| Systolic Blood Pressure (mmHg) | 135.33 ± 18.94 | 127 ± 10.65 |
| Diastolic Blood Pressure (mmHg) | 82 ± 9.41 | 77.33 ± 7.98 |
| VO2max (mL·kg−1·min−1) | 23.89 ± 6.42 | 26.76 ± 5.80** |
| Flexibility (cm) | 22.19 ± 9.45 | 25.85 ± 8.55* |
| Total Cholesterol (mg·dL−1) | 171.44 ± 27.23 | 158.44 ± 26.83** |
| Triglycerides (mg·dL−1) | 136.17 ± 79.87 | 114.20 ± 62.23 |
| HDL (mg·dL−1) | 43.30 ± 11.65 | 54.09 ± 12.76** |
| LDL (mg·dL−1) | 100.90 ± 23.35 | 81.50 ± 25.60* |
| Glucose (mg·dL−1) | 106.24 ± 72.21 | 102.31 ± 28.21 |
| Creatinine (mg·dL−1) | 1 ± 0.21 | 0.82 ± 0.18** |
| Uric Acid (mg·dL−1) | 4.68 ± 1.62 | 4.17 ± 1.44 |
| Urea (mg·dL−1) | 34.98 ± 12.46 | 25.34 ± 5.43* |
| Albumin (g·dL−1) | 3.81 ± 0.36 | 4.3 ± 0.78* |
| LDH (U·L−1) | 294.34 ± 187.83 | 140.84± 117.3** |
| AP (U·L−1) | 56.51 ± 21.28 | 48.85 ± 16.06 |
| AST (U·L−1) | 30.12 ± 21.81 | 29.53 ± 8.81 |
| ALT (U·L−1) | 45.41 ± 30.58 | 29.42 ± 17.34 |
Note: Values are expressed as mean ± SD. BMI = body mass index. VO2max = maximal oxygen uptake. HDL: high-density lipoprotein. LDL: low-density lipoprotein. LDH: lactate dehydrogenase. AP: alkaline phosphatase. AST: aspartate aminotransferase. ALT: alanine aminotransferase.
P< 0.05
P< 0.01 after vs. before training.
There were no differences in the body weight, body mass index (BMI), or systolic and diastolic blood pressures values after the intervention. However, the participants’ waist and hip circumference values were decreased after the training protocol (P = 0.001 andP = 0.039, respectively). The training protocol also induced decreases in the resting heart rate (P = 0.007). In addition, the training protocol resulted in higher levels of predicted VO2max (P = 0.001) and lumbar and hamstring muscle flexibility (P = 0.004) when compared with baseline values. The exercise training reduced the serum levels of TC (P = 0.049), creatinine (P = 0.001), and urea (P = 0.007), while increases in HDL (P = 0.004), and albumin (P = 0.043) were found in the participants. The LDH activity (P = 0.005) was also decreased after the exercise training.
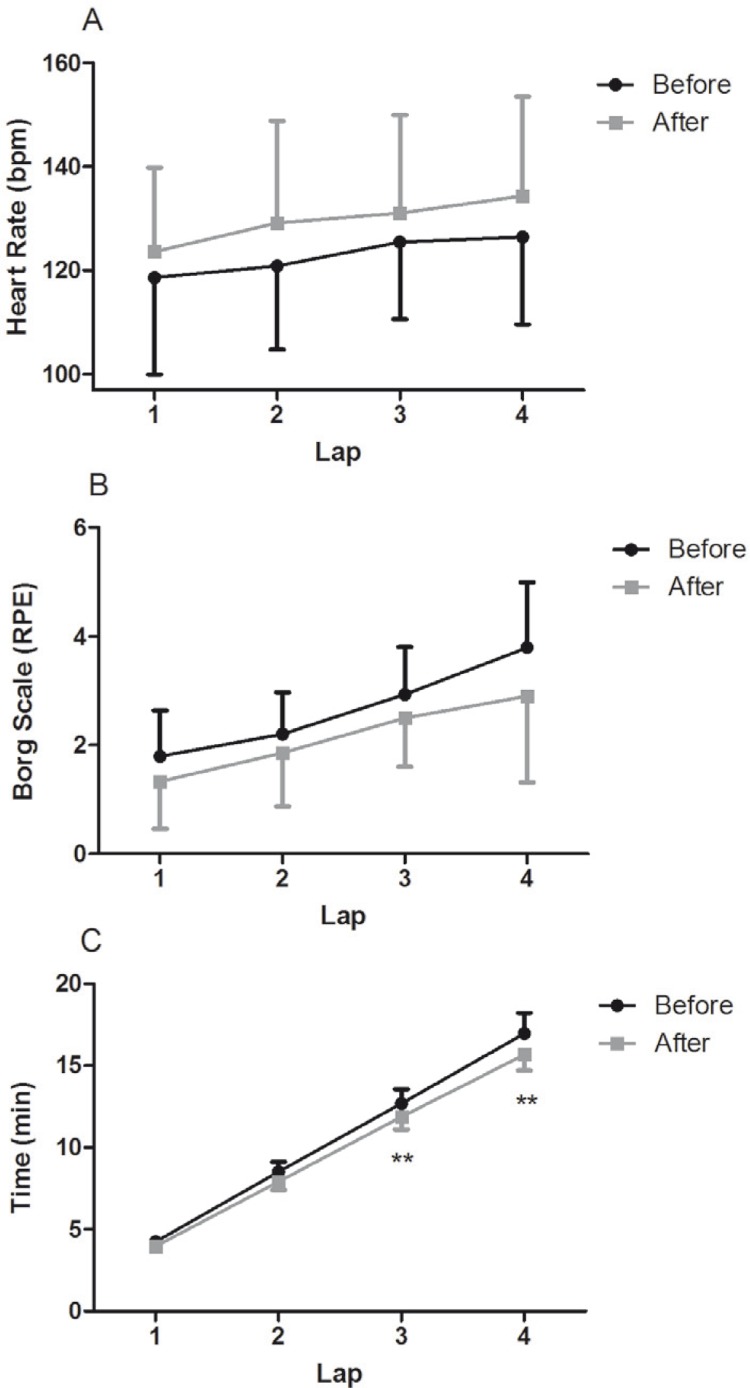
There were no significant changes in the HR and perceived effort (Figure 1A and B) along the 4 laps of the cardiorespiratory test when compared to baseline values. However, the time elapsed between pre- and post-exercise training in the third and fourth laps was decreased (P = 0.001 and P = 0.001, respectively) (Figure 1C).
FIG. 1.
Cardiorespiratory test characteristics before and after AT in women with MS.
Note: Heart rate (A), Borg scale (B) and time elapsed (C) along each lap of cardiorespiratory test.
* P < 0.05 and **P < 0.01 for difference between before and after AT. Bpm: heart beats per minute. Borg Scale RPE: Borg rating of perceived exertion.
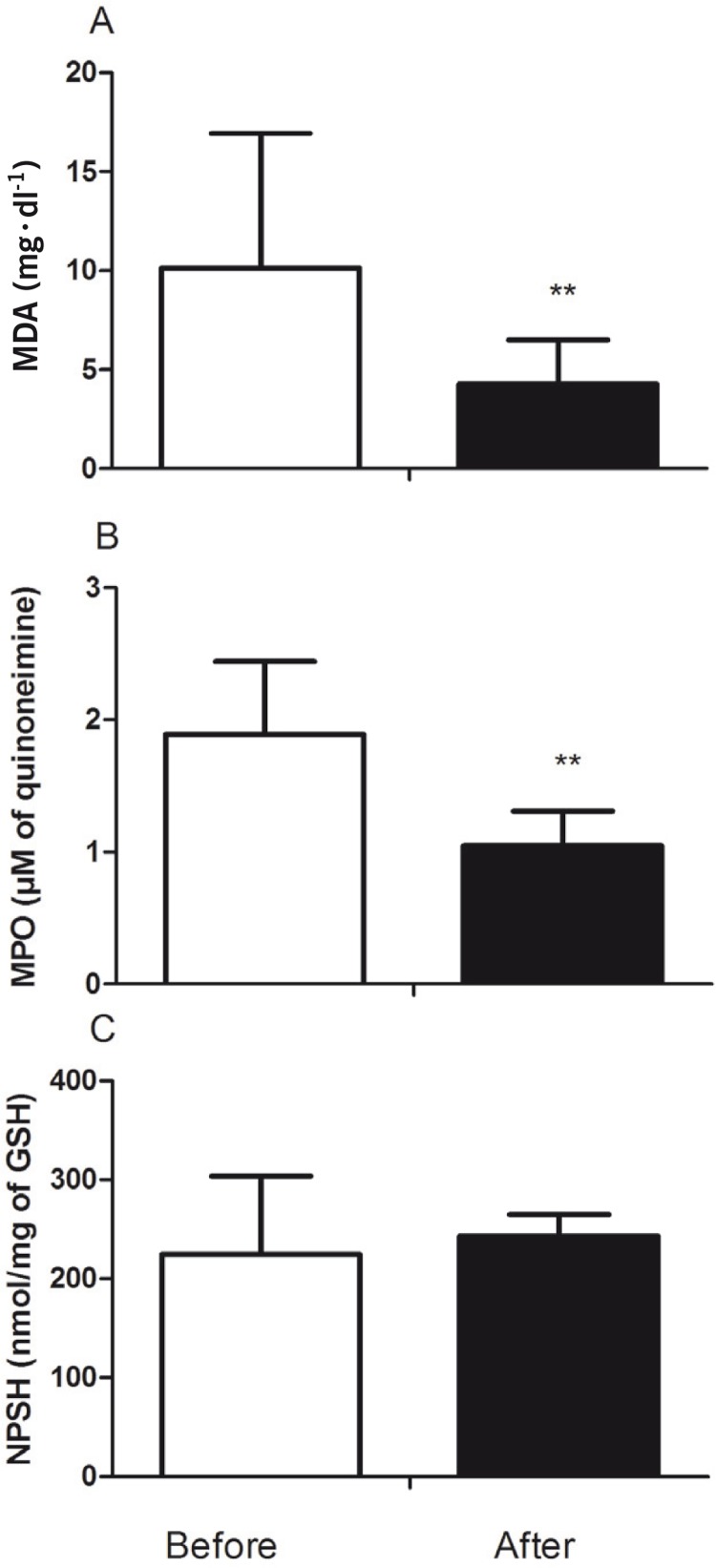
Analysis of oxidative stress and inflammation markers in plasma are shown in Figure 2. The TBARS levels (P = 0.006) and the MPO activity (P = 0.002) were decreased after the training protocol in female patients with MS (Figure 2A and B, respectively).
FIG. 2.
Effect of AT on oxidative and inflammatory plasma parameters. Thiobarbituric acid-reactive substances (TBARS) (A), myeloperoxidase (MPO) activity (B) and non-protein sulfhydryl groups (NPSH) (C) content.
**P < 0.01 before vs. after 15 weeks of AT.
The SF-36 showed increases in the physical functioning (P = 0.030), role-physical (P = 0.039), bodily pain (P = 0.048), general health (P = 0.046) and social functioning (P = 0.011) scoring scales after the training protocol in the female MS participants (Table 2].
TABLE 2.
SF-36 questionnaire subscales scoring of female MS patients before and after the training protocol.
| Scales | Before | After |
|---|---|---|
| Physical Functioning | 74.00 ± 23.84 | 85 ± 10.35* |
| Role-physical | 85.00 ± 26.39 | 98.33 ± 6.45* |
| Bodily Pain | 60.27 ± 28.34 | 75.67 ± 17.98* |
| Vitality | 59.33 ± 11.31 | 65.00 ± 8.66 |
| General Health | 69.00 ± 18.30 | 78.33 ± 11.87* |
| Social Functioning | 74.17 ± 20.30 | 90.83 ± 19.74* |
| Role-emotional | 88.88 ± 27.22 | 86.66 ± 30.34 |
| Mental Health | 66.67 ± 14 | 74.93 ± 19.09 |
Note: P< 0.05 after vs. before training.
P< 0.01 after vs. before training.
No differences were found in the total energy, carbohydrate, protein and lipid intake in the 3-day diet record of participants when comparing baseline and post-training values (Table 3)
TABLE 3.
Energy intake before and after the training protocol.
| Variables | Before | After |
|---|---|---|
| Energy intake (kcal) | 1313.30 ± 400.31 | 1313.87 ± 419.81 |
| Carbohydrate (kcal) | 767.33 ± 244.67 | 726.49 ± 277.17 |
| Protein (kcal) | 292.49 ± 273.26 | 276.12 ± 165.95 |
| Lipid (kcal) | 303.87 ± 156.46 | 301.67 ± 156.66 |
Note: Values are expressed as mean ± SD.
DISCUSSION
In this study we found that moderate exercise training positively alters most of the functional and biochemical parameters together with reported HRQOL in female MS participants. The results revealed that 15 weeks of exercise training without diet prescription reduced cardiovascular risk factors, such as central obesity, lipid and lipoprotein profile. It is noteworthy that these positive changes were found despite body weight reduction in this group, indicating possible body adaptations that may precede weight loss. Moreover, exercise training intensity and frequency were sufficient to induce functional and metabolic favourable changes, such as increases in VO2max and flexibility, and decreases in LDH and MPO activities and TBARS levels.
The present results corroborate previous studies which have demonstrated that weight loss is not mandatory to obtain exercise training beneficial effects and cardiovascular fitness regarding total and abdominal fat obesity [18], lipoprotein profile [8], and HRQOL [7] in overweight and obese participants. In this sense, it was found that 13 weeks of exercise training reduced waist circumference without a corresponding decrease in body weight in obese men [18], suggesting that this variable may be assumed as the main precursor to favourable lifestyle changes [18]. In the present study, most lipid and lipoprotein serum parameters demonstrated positive alterations after the training protocol. In this line, the effects of exercise training on LDL levels are controversial: while studies did not find a difference in longer interventions [19], shorter training duration also resulted in lower levels of LDL in postmenopausal women without weight loss detection [20]. Furthermore, an important finding of our study was that 15 weeks of exercise training at 50-65% of HRR may increase the serum HDL levels. Higher levels of HDL are of clinical relevance, considering that HDL is thought to have anti-inflammatory and antioxidant activities, among others [21]. On the other hand, in accordance with other studies, changes in fasting glucose levels were not found after the exercise training [19].
In this study, serum creatinine and urea levels were decreased after training, while albumin levels increased. It was recently hypothesized that exercise training enhances the renal profile, contributing to increased plasma renin activity, estimated glomerular filtration rate, and decreased albuminuria in MS subjects without chronic kidney disease [22]. Additionally, a recent study has pointed out that serum albumin acts as a ligand binding and free radical-trapping molecule, presenting important circulating antioxidant activities [23]. On the other hand, no changes in serum ALT activity were found in our study; it seems that this regular exercise effect may strongly relate to changes in body weight [6]. Similarly, recent evidence highlighted that obesity reflects a chronic state of oxidative stress and persistent low systemic inflammation, in association with insulin resistance and cardiovascular diseases (CVD) [2, 20]. Decreased TBARS levels without weight loss have been previously reported for a similar population [20], pointing to lower oxidative stress. Regarding inflammation, the decreased MPO activity induced by exercise training in our study is a remarkable finding, considering that HDL is known to be oxidized by the leukocyte and phagocyte-derived enzyme MPO [24].
The above discussed biochemical effects induced by exercise training are well described in the literature. However, this study showed that 15 weeks of exercise training improved the physical functioning, role-physical, bodily pain, general health, and social functioning subscales of HRQOL in female MS participants without weight loss detection. In this context, a recent study with young adults described an association of higher physical fitness level with increased scores in physical functioning, general health, mental health, and vitality dimensions of HRQOL [25]. This finding is clinically relevant considering that MS patients usually report physical appearance disappointment and daily physical functioning impairments as a result of breath shortness, pain on the weight-bearing joints, and/or reduced mobility [26]. Furthermore, a decreased quality of life has been recognized as a predictor of higher DM and CVD risk [27]. On the other hand, exercise training may improve quality of life, thus reducing higher health public costs [5].
The results presented here are in accordance with previous studies that found exercise training and/or changes in lifestyle to ameliorate physical and mental health components through SF-36 application in patients with MS and T2DM, independently of changes in body fat, weight loss or aerobic power [4, 7]. This is a relevant finding, considering that most studies have focused on functional and biochemical profiles, leaving aside the HRQOL and its positive impact on free living. In fact, a better quality of life is associated with daily living activities such as rising, climbing stairs, walking several blocks and dressing [4]. The effects of exercise training on physical functioning and general health are important, as the worsening in these domains is associated with higher insulin resistance, waist-to-hip ratio, TG, and reduced HDL levels [28]. In this sense, we have found improvements in exercise capacity together with favourable biochemical adaptations and increased self-reported quality of life. Moreover, studies indicate that some of the exercise-related improvements in health, well-being and the psychological profile result from the interpersonal and emotional connections developed during the exercise training sessions in the group [29]. Thereby, reduced quality of life may also have an impact on family and professional relationships, apart from individual happiness, considering that obesity is associated with a decreased self-concept and self-image, and increased anxiety and depression [30]. The study must include as limitations the lack of parameters such as body composition assessment and the lack of a control group. Also, seasonal factors may have influenced blood pressure levels along the intervention.
CONCLUSIONS
In conclusion, in this study we found that 15-week moderate aerobic training induces positive effects on functional and biochemical parameters, together with favourable quality of life improvements in women with MS despite weight loss detection. Further research concerning the mechanisms related to this enhanced quality of life despite weight loss should be carried out in order to increase the feedback to the physical trainees.
Acknowledgements
We greatly thank Dr. Carlos Bolli Mota and Proximus Tecnologia for the technical support and research incentive. J.B.F, G.B. and S.TS. received fellowship from CAPES (Coordenagao de Aperfeicpamento de Pessoal de Nivel Superior). F.D.M. received a fellowship from UFSM/FIPE (Fundo de Incentivo a Pesquisa). F.A.A.S. received a fellowship from CNPq (Conselho Nacional de Desenvolvimento Cientifico e Tecnologico). Additional financial support was given by FAPERGS/CNPq - PRONEM #11/2029-1 (Programa de Apoio a Nucleos Emergentes).
Conflict of interests
The authors declared no conflict of interests regarding the publication of this manuscript.
REFERENCES
- 1.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23(5):469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 2.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115(5):1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogowski O, Shapira I, Berliner S. Exploring the usefulness of inflammation-sensitive biomarkers to reveal potential sex differences in relation to low-grade inflammation in individuals with the metabolic syndrome. Metabolism. 2008;57(9):1221–1226. doi: 10.1016/j.metabol.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 4.Levinger I, Goodman C, Hare DL, Jerums G, Selig S. The effect of resistance training on functional capacity and quality of life in individuals with high and low numbers of metabolic risk factors. Diabetes Care. 2007;30(9):2205–2210. doi: 10.2337/dc07-0841. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Norris SL, Chowdhury FM, Gregg EW, Zhang P. The effects of interventions on health-related quality of life among persons with diabetes: a systematic review. Med Care. 2007;45(9):820–834. doi: 10.1097/MLR.0b013e3180618b55. [DOI] [PubMed] [Google Scholar]
- 6.Devries MC, Samjoo IA, Hamadeh MJ, Tarnopolsky MA. Effect of endurance exercise on hepatic lipid content, enzymes, and adiposity in men and women. Obesity (Silver Spring) 2008;16(10):2281–2288. doi: 10.1038/oby.2008.358. [DOI] [PubMed] [Google Scholar]
- 7.Rejeski WJ, Lang W, Neiberg RH, Van Dorsten B, Foster GD, Maciejewski ML, Rubin R, Williamson DF. Correlates of health-related quality of life in overweight and obese adults with type 2 diabetes. Obesity (Silver Spring) 2006;14(5):870–883. doi: 10.1038/oby.2006.101. [DOI] [PubMed] [Google Scholar]
- 8.Slentz CA, Houmard JA, Johnson JL, Bateman LA, Tanner CJ, McCartney JS, Duscha BD, Kraus WE. Inactivity, exercise training and detraining, and plasma lipoproteins. STRRIDE: a randomized, controlled study of exercise intensity and amount. J Appl Physiol. 2007;103(2):432–442. doi: 10.1152/japplphysiol.01314.2006. [DOI] [PubMed] [Google Scholar]
- 9.American College of Sports Medicine. ACSM's guidelines for Exercise testing and prescription; Philadelphia: Lippincott Williams & Wilkins; 2006. [DOI] [PubMed] [Google Scholar]
- 10.Kline GM, Porcari JP, Hintermeister R, Freedson PS, Ward A, McCarron RF, Ross J, Rippe JM. Estimation of VO2max from a one-mile track walk, gender, age, and body weight. Med Sci Sports Exerc. 1987;19(3):253–259. [PubMed] [Google Scholar]
- 11.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 12.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 13.Ellman GL. Tissue sulfhydryl groups. Arch. Biochem Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 14.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 15.Pimentel VC, Pinheiro FV, Kaefer M, Moresco RN, Moretto MB. Assessment of uric acid and lipid peroxidation in serum and urine after hypoxia-ischemia neonatal in rats. Neurol Sci. 2011;32(1):59–65. doi: 10.1007/s10072-010-0393-3. [DOI] [PubMed] [Google Scholar]
- 16.Ciconelli RM, Ferraz MB, Santos W, Meinao I, Quaresma MR. Brazilian-portuguese version of SF-36: a reliable and valid quality of life outcome measure. Rev Bras Reumatol. 1999;39(3):143–150. [Google Scholar]
- 17.Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Qual Life Res. 2001;10(5):405–413. doi: 10.1023/a:1012588218728. [DOI] [PubMed] [Google Scholar]
- 18.Lee S, Kuk JL, Davidson LE, Hudson R, Kilpatrick K, Graham TE, Ross R. Exercise without weight loss is an effective strategy for obesity reduction in obese individuals with and without Type 2 diabetes. J Appl Physiol. 2005;99(3):1220–1225. doi: 10.1152/japplphysiol.00053.2005. [DOI] [PubMed] [Google Scholar]
- 19.Thomas EL, Brynes AE, McCarthy J, Goldstone AP, Hajnal JV, Saeed N, Frost G, Bell JD. Preferential loss of visceral fat following aerobic exercise, measured by magnetic resonance imaging. Lipids. 2000;35(7):769–776. doi: 10.1007/s11745-000-0584-0. [DOI] [PubMed] [Google Scholar]
- 20.Karolkiewicz J, Michalak E, Pospieszna B, Deskur-Smielecka E, Nowak A, Pilaczynska-Szczesniak L. Response of oxidative stress markers and antioxidant parameters to an 8-week aerobic physical activity program in healthy, postmenopausal women. Arch Gerontol Geriatr. 2009;49(1):67–71. doi: 10.1016/j.archger.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Nofer JR, Kehrel B, Fobker M, Levkau B, Assmann G, von Eckardstein A. HDL and arteriosclerosis: beyond reverse cholesterol transport. Atherosclerosis. 2002;161(1):1–16. doi: 10.1016/s0021-9150(01)00651-7. [DOI] [PubMed] [Google Scholar]
- 22.Straznicky NE, Grima MT, Lambert EA, Eikelis N, Dawood T, Lambert GW, Nestel PJ, Masuo K, Sari CI, Chopra R, Mariani JA, Schlaich MP. Exercise augments weight loss induced improvement in renal function in obese metabolic syndrome individuals. J Hypertens. 2011;29(3):553–564. doi: 10.1097/HJH.0b013e3283418875. [DOI] [PubMed] [Google Scholar]
- 23.Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E. The antioxidant properties of serum albumin. FEBS Lett. 2008;582(13):1783–1787. doi: 10.1016/j.febslet.2008.04.057. [DOI] [PubMed] [Google Scholar]
- 24.Podrez EA, Febbraio M, Sheibani N, Schmitt D, Silverstein RL, Hajjar DP, Cohen PA, Frazier WA, Hoff HF, Hazen SL. Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J Clin Invest. 2000;105(8):1095–1108. doi: 10.1172/JCI8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hakkinen A, Rinne M, Vasankari T, Santtila M, Hakkinen K, Kyrolainen H. Association of physical fitness with health-related quality of life in Finnish young men. Health Qual Life Outcomes. 2010;8:15. doi: 10.1186/1477-7525-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kushner RF, Foster GD. Obesity and quality of life. Nutrition. 2000;16(10):947–952. doi: 10.1016/s0899-9007(00)00404-4. [DOI] [PubMed] [Google Scholar]
- 27.Lidfeldt J, Nyberg P, Nerbrand C, Samsioe G, Schersten B, Agardh CD. Socio-demographic and psychosocial factors are associated with features of the metabolic syndrome. The Women's Health in the Lund Area (WHILA) study. Diabetes Obes Metab. 2003;5(2):106–112. doi: 10.1046/j.1463-1326.2003.00250.x. [DOI] [PubMed] [Google Scholar]
- 28.Kumari M, Seeman T, Marmot M. Biological predictors of change in functioning in the Whitehall II study. Ann Epidemiol. 2004;14(4):250–257. doi: 10.1016/j.annepidem.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Lavie CJ, Thomas RJ, Squires RW, Allison TG, Milani RV. Exercise training and cardiac rehabilitation in primary and secondary prevention of coronary heart disease. Mayo Clin Proc. 2009;84(4):373–383. doi: 10.1016/S0025-6196(11)60548-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blissmer B, Riebe D, Dye G, Ruggiero L, Greene G, Caldwell M. Health-related quality of life following a clinical weight loss intervention among overweight and obese adults: intervention and 24 month follow-up effects. Health Qual Life Outcomes. 2006;4:43. doi: 10.1186/1477-7525-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]