Abstract
Resource exploitation and competition for food are important selective pressures in animal evolution. A number of recent investigations have focused on linkages between diversification, trophic morphology and diet in bats, partly because their roosting habits mean that for many bat species diet can be quantified relatively easily through faecal analysis. Dietary analysis in mammals is otherwise invasive, complicated, time consuming and expensive. Here we present evidence from insectivorous bats that analysis of three-dimensional (3-D) textures of tooth microwear using International Organization for Standardization (ISO) roughness parameters derived from sub-micron surface data provides an additional, powerful tool for investigation of trophic resource exploitation in mammals. Our approach, like scale-sensitive fractal analysis, offers considerable advantages over two-dimensional (2-D) methods of microwear analysis, including improvements in robustness, repeatability and comparability of studies. Our results constitute the first analysis of microwear textures in carnivorous mammals based on ISO roughness parameters. They demonstrate that the method is capable of dietary discrimination, even between cryptic species with subtly different diets within trophic guilds, and even when sample sizes are small. We find significant differences in microwear textures between insectivore species whose diet contains different proportions of ‘hard’ prey (such as beetles) and ‘soft’ prey (such as moths), and multivariate analyses are able to distinguish between species with different diets based solely on their tooth microwear textures. Our results show that, compared with previous 2-D analyses of microwear in bats, ISO roughness parameters provide a much more sophisticated characterization of the nature of microwear surfaces and can yield more robust and subtle dietary discrimination. ISO-based textural analysis of tooth microwear thus has a useful role to play, complementing existing approaches, in trophic analysis of mammals, both extant and extinct.
Keywords: insectivore microwear, carnivore, bats, dietary analysis, Rhinolophus, Pipistrellus, Plecotus, ISO roughness
Introduction
Dietary analysis of mammals is central to a wide range of evolutionary, ecological and conservation issues. Resource exploitation and competition for food are important selective pressures in animal evolution, and understanding the linkages between diversification, trophic morphology and diet are critical to testing hypotheses of adaptive radiation and the roles of dietary niche partitioning and competition in speciation (e.g. Darwin, 1859; Schluter, 2000; Dayan & Simberloff, 2005; Price et al., 2012). A number of such investigations in recent years have focused on bats (Freeman, 2000; Nogueira, Peracchi & Monteiro, 2009; Dumont et al., 2012; Santana, Grosse & Dumont, 2012). Among mammals, bats are ideally suited for such analyses because their roosting habits and the accessibility of roosts mean that for many species diet can be quantified relatively easily through faecal analysis (Kunz & Whitaker, 1983). Although such ‘scat analysis’ is becoming more widespread (Trites & Joy, 2005; Williams, Goodenough & Stafford, 2012), in some cases supplemented by DNA-based identification of prey species (Razgour et al., 2011), dietary analysis in mammals is otherwise invasive, complicated, and time consuming. It is therefore expensive, requiring detailed analysis of stomach or cheek-pouch contents, the contents of food stores or direct behavioural observation (e.g. Jordan, 2005). Analysis of stable isotopes of C and N can also be informative, especially in marine mammals (e.g. Kelly, 2000), but this usually provides only an indication of relative trophic levels.
Analysis of the patterns of wear on teeth that arise as a consequence of feeding provides an alternative route to dietary discrimination, with an established track record of application to mammals (e.g. Walker, Hoeck & Perez, 1978; Gordon, 1982; Teaford, 1988). In particular, analysis of microwear – the microscopic chipping and scratching within wear facets – can provide insights into the jaw kinematics and trophic ecology of species where other data are unavailable. It can be applied to historical museum specimens and extinct taxa for example. Furthermore, because the dietary signal of microwear accumulates over periods of days or weeks (Teaford & Oyen, 1989; Merceron et al., 2010) analysis of microwear avoids the problem of stomach contents recording only the ‘snapshot’ of what an animal ate in the few hours prior to capture (Merceron et al., 2010; Purnell, Seehausen & Galis, 2012). Microwear analysis has a long history of application to primates and ungulates in particular (e.g. Walker et al., 1978; Teaford, 1988; Scott et al., 2005), but new approaches to examination and quantification of wear patterns are allowing microwear analysis to be applied to new problems and to a broader range of taxa, including carnivorans, dinosaurs and fish (Scott et al., 2005, 2006; Purnell et al., 2006, 2007, 2012; Ungar, Merceron & Scott, 2007; Ungar, Grine & Teaford, 2008; Goillot, Blondel & Peigne, 2009; Williams, Barrett & Purnell, 2009; Merceron et al., 2010; Schubert, Ungar & DeSantis, 2010; Schulz, Calandra & Kaiser, 2013a 2013a; Schulz et al., 2013b 2013b).
Here we present evidence from insectivorous bats that analysis of three-dimensional (3-D) textures of tooth microwear using ISO roughness parameters derived from sub-micron surface elevation data (International Organization for Standardization, 2012) provides an additional, powerful tool for investigation of trophic resource exploitation in mammals. Our approach is based on the same type of high-resolution 3-D data and offers the same advantages as scale-sensitive fractal analysis (SSFA) of tooth microwear (Scott et al., 2005, 2006; Ungar et al., 2007, 2008; Merceron et al., 2010). These advantages include improvements in robustness, repeatability and comparability of studies, realized to a large extent because 3-D approaches are not dependent on operators to identify, measure and score scratches and pits on tooth surfaces, a problem that creates significant noise and error in two-dimensional (2-D) microwear analyses (Grine, Ungar & Teaford, 2002; Purnell et al., 2006; Mihlbachler et al., 2012).
Only a few studies have investigated microwear in carnivorous mammals (Taylor & Hannam, 1986; Van Valkenburgh, Teaford & Walker, 1990; Strait, 1993a 1993a; Anyonge, 1996; Goillot et al., 2009; Schubert et al., 2010; Bastl, Semprebon & Nagel, 2012; DeSantis et al., 2012). Of these, all except for Strait (1993a) are studies of large carnivores, and only two utilize the analytically more robust 3-D approaches (Schubert et al., 2010; DeSantis et al., 2012). These studies demonstrate the potential for analysis of microwear to discriminate between carnivores with different diets, but Strait’s (1993a) work remains the only previous analysis of microwear in bats. Using scanning electron microscopy (SEM) to quantify microwear in small-bodied bats and primates, Strait was unable to distinguish insectivores from flesh eaters, but found significant differences between species that consumed hard and soft prey. The hypothesis that microwear differs within a guild of small-bodied insectivores remains untested. This study, which explores this hypothesis, also provides the first application of 3-D textural analysis to small-bodied mammals.
Materials and methods
Insectivores with well-constrained differences in their diets were selected for this analysis. We analysed four species of bat: common pipistrelle Pipistrellus pipistrellus, soprano pipistrelle Pi. pygmaeus, brown long-eared bat Plecotus auritus and greater horseshoe bat Rhinolophus ferrumequinum. Specimens were all wild-found, and acquired from UK sources (see Supporting Information).
In considering the dietary differences between insectivorous animals, what matters is not the taxonomic identity of the prey, but the relative difficulty faced by the predator when attempting to pierce and chew the prey items. Terminology used to characterize the relevant properties of prey items is complicated (for discussion see Evans & Sanson, 2005; Freeman & Lemen, 2007). Evans & Sanson (2005) suggested the term ‘intractability’, but this is not widely used and can be confusing. Here we use ‘hard’ and ‘soft’ to mean prey that is more or less difficult to pierce and chew.
Information regarding diets of the bat species studied comes mainly from Barlow (1997) and Vaughan (1997) and references therein. Pipistrellus pipistrellus and Pi. pygmaeus were only recently recognized to be separate, cryptic species based on molecular, behavioural and echolocation differences (Jones & Van Parijs, 1993; Barratt et al., 1997); their diets are subtly different. Both are specialists on Diptera (flies) with a preference for Nematocera (mosquitoes, crane flies, gnats, and midges), but they consume different families in different proportions. Pipistrellus pipistrellus consumes more non-nematoceran dipterans and other insects with a wider range of cuticle ‘hardness’ in its diet [greater quantities of Trichoptera (caddisflies), Neuroptera (lacewings), Hymenoptera (sawflies, wasps, bees and ants), Lepidoptera (moths and butterflies) and Coleoptera (beetles)] (Swift, Racey & Avery, 1985; Barlow, 1997). This diet includes ‘harder’ prey than that of Pi. pygmaeus, the diet of which is made up mostly (c. 80%) of the ‘softer’ ‘biting’ and ‘non-biting’ midges (Table 1; Barlow, 1997). Also, Pi. pipistrellus is known to consume larger flies than Pi. pygmaeus, and the ‘hardness’ of insects is correlated with size (Aguirre et al., 2003; Freeman & Lemen, 2007). In summary, Pi. pipistrellus consumes prey that spans a broad range of ‘hardness’, whereas the prey of Pi. pygmaeus is narrower in range and generally ‘softer’.
Table 1.
Trophic categorization and diets of the British bat species analysed, modified from Vaughan (1997 and references therein) and Barlow (1997)
| Species | Trophic category | Diet |
|---|---|---|
| Common pipistrelle Pipistrellus pipistrellus | Prey of mixed ‘hardness’; Diptera specialist, but with some ‘harder’ species | Mostly suborder Nematocera: Psychodidae ‘moth flies’; Anisopodidae ‘wood gnats’; Muscidae ‘house flies’. |
| Soprano pipistrelle Pipistrellus pygmaeus | Diptera specialist, particularly midges; mainly ‘softer’ prey | Mostly suborder Nematocera: Chironomidae ‘non-biting midges’; Ceratopogonidae ‘biting midges’. |
| Greater horseshoe bat Rhinolophus ferrumequinum | Prey of mixed ‘hardness’; mixed feeder, including more ‘hard’ prey, especially Coleoptera | Mainly Lepidoptera & Coleoptera. Lepidopteran families: Noctuidae ‘owlet moths’; Nymphalidae ‘brush-footed butterflies’; Hepialidae ‘swift moths’; Sphingidae ‘hawk moths’; Geometridae ‘geometer moths’; Lasiocampidae ‘lappet moths’. Coleopteran families: Scarabaeidae ‘scarab beetles’; Geotrupidae ‘dor beetles’; Silphidae ‘carrion beetles’; Carabidae ‘ground beetles’. Diptera also consumed. |
| Brown long-eared bat Plecotus auritus | ‘Soft’ prey specialist; specializing on Lepidoptera | Almost entirely Lepidoptera: Noctuidae ‘owlet moths’; Hepialidae ‘swift moths’; Thyatiridae Nymphalidae ‘brush-footed butterflies’; Geometridae ‘geometer moths’; Sphingidae ‘hawk moths’; Notodontidae ‘prominents’; Arctiidae Pyralidae ‘snout moths’. |
Assessment of prey ‘hardness’ was based on published data (cited in text).
Rhinolophus ferrumequinum is a mixed forager, consuming Lepidoptera (butterflies and moths) and Coleoptera (beetles) in approximately equal amounts, together with dipterans (Jones, 1990). Like Pi. pipistrellus, this diet is a mixture of ‘soft’ prey, and prey that is among the ‘hardest’ of insects (i.e. coleopterans). Plecotus auritus specializes on Lepidoptera, with faecal studies indicating that Lepidoptera can constitute 99–100% of the diet: Lepidoptera are known from numerous studies to be among the ‘softest’ insects (Aguirre et al., 2003; Evans & Sanson, 2005; Freeman & Lemen, 2007).
Rather than extract individual teeth, mandibles were removed from entire cadavers (see Supporting Information for preparation details). For all specimens, data were acquired from the distal wear facet of the M2 protoconid – as near to the cusp tip as possible without compromising surface flatness – because of the significant role it plays in food processing (Strait, 1993b).
Our methods for data acquisition and analysis are modified slightly from those of Purnell et al. (2012). Before sputter coating with gold (SC650, Bio-Rad, Hercules, CA, USA), specimens were mounted onto 12.7 mm SEM stubs [using carbon disks and Leit-C plastic carbon cement (Fluka, Buchs, Switzerland)], with the M2 facet of interest oriented horizontally in order to maximize the quality of data acquired.
High-resolution 3-D surface data were captured using an Alicona Infinite Focus microscope G4b (IFM; Alicona GmbH, Graz, Austria; software version 2.1.2), using ×100 objective to give a field of view of 145 × 110 μm. Recent work (Merceron et al., 2010; Purnell et al., 2012) has show that this is a large enough area to extract dietarily informative texture data; furthermore, many of the teeth analysed here are too small for a larger area to be sampled. The Alicona Infinite Focus microscope G4b has a CCD of 1624 × 1232 pixels. In theory, for a field of view of 145 μm, this equates to a lateral sampling distance of 0.09 μm, but the limits imposed by the wavelength of white light mean that lateral optical resolution is actually about 0.35–0.4 μm. For all samples, vertical resolution was set at 20 nm, and the lateral resolution factor for the IFM was set at 0.3. Exposure and contrast settings were adjusted to maximize data quality in terms of measurement repeatability (this is estimated automatically by the IFM software during data capture) for each sample. Adjusting exposure and contrast do not affect the values for 3-D measurements. Prior to generation of roughness surfaces, captured 3-D surface data for each specimen was examined visually to ensure that only those surfaces which preserved primary tooth microwear textures were subject to analysis. Data showing evidence of post-mortem artefacts or with extraneous material obscuring the surface were rejected.
All 3-D data were processed using the Alicona IFM software (version 2.1.2) to remove dirt and dust particles from the surface (by manual deletion), and were then exported as .sur files for processing using SurfStand software (version 5.0.0 Centre for Precision Technologies, University of Huddersfield, West Yorkshire, UK). Measurement errors (anomalous peaks and low points) were deleted, and data were levelled (subtraction of least squares plane) to remove variation caused by differences in orientation of tooth surfaces at the time of data capture. Scale-limited roughness surfaces were generated from the data through application of a fifth-order robust polynomial (which finds and removes the least squares fifth-order polynomial surface for the levelled data) and a robust Gaussian wavelength filter (λc = 0.025 mm; to remove long wavelength features of the tooth surface (gross tooth form; Fig. 1). ISO 25178-2 texture parameters (International Organization for Standardization, 2012) were then generated from the resulting roughness surface. These include: height parameters (quantifying the distribution of height values along the z-axis); spatial parameters (quantifying direction and spatial periodicity of the surface); hybrid parameters (combining the information present on the x-, y-and z-axes of the surface, quantifying aspects of the spatial shape of the data), and parameters related to measures of volumes, such as peak material, calculated from the areal material ratio curve (see Supporting Information). Sample sizes used in this study are relatively small (five individuals of each species), but as demonstrated by Purnell et al. (2012), this does not prevent detection of dietary signals through textural analysis of microwear.
Figure 1.
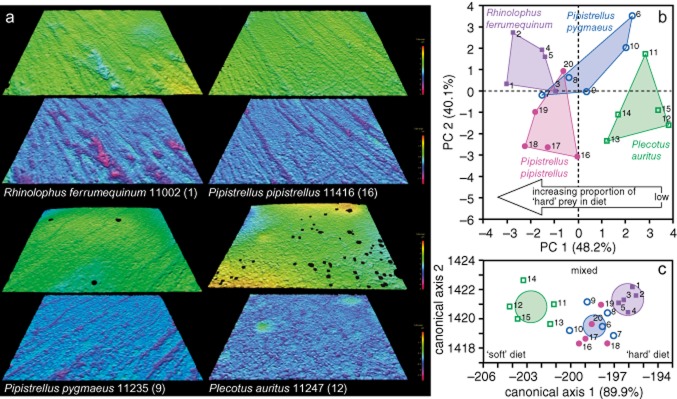
Tooth microwear textures of bats, and multivariate analysis of International Organization for Standardization (ISO) roughness parameters. (a) Digital elevation models showing levelled surface data (above) and scale-limited roughness surfaces (below) for the four species of bats. See text for details of data processing; numbers in brackets identify specimens in (b) and (c). Measured areas are 146-μm wide. (b) Principal components (PC) analysis of ISO roughness parameters that differ between species. Species form largely non-overlapping clusters; PC axis 1 correlates with dietary differences between species. For details of loadings (eigenvectors) of roughness parameters onto PC axes 1 and 2 see Supporting Information Table S3. (c) Linear discriminant analysis of ISO roughness parameters that differ between species. Analysis correctly assigns all specimens to one of three trophic groups (groups based on the amount of ‘hard’ prey consumed; probability of correct assignment >0.9 for all but one Pipistrellus pygmaeus (0.64) and one Pipistrellus pipistrellus (0.63); Wilks’ Lambda = 0.07; F = 2.72; P = 0.02). Canonical axis 1 correlates with dietary differences between species. Ellipses show 95% confidence limits for means.
Data were explored using analysis of variance (ANOVA), correlations, principal components analysis (on correlations; PCA) and linear discriminant analyses (LDA). All statistical analysis of microtextural data was carried out using JMP 9 (SAS Institute, Cary, NC, USA). The results of Shapiro–Wilk tests indicated that some roughness parameters were non-normally distributed (P > 0.05), and log-transformed data were used for analysis (the only exception was Ssk, which included negative values and for which we could not reject the null hypothesis that data were drawn from a population with a normal distribution). Where homogeneity of variance tests (Bartlett and Levene tests) revealed evidence of unequal variances, Welch ANOVA was used. The significance of LDA was assessed using Wilks’ Lambda.
Results
ANOVA revealed that nine parameters differed significantly between bat species (Table 2 ). The nine parameters are: Ssk – skewness of the surface; Str – texture aspect ratio; Vmp – peak material volume; Vmc – core material volume; Vvc – core void volume; Vvv – dale void volume; Svk – reduced dale height; Smr1 and Smr2 – material ratio for peaks and dales, respectively. Core, peaks and dales are defined by the bearing area curve for the scale limited surface; for more detailed description and discussion of parameters see ISO 25178-2 (International Organization for Standardization, 2012) and Supporting Information. Tukey’s honestly significant difference (HSD) procedure indicates that R. ferrumequinum differs significantly from Pl. auritus for six of the nine parameters (lower Ssk, and Str, higher Vmc, Vvc, Vvv and Svk); it differs from Pi. pipistrellus for three (Vmc, Vvc, Vvv; all higher in R. ferrumequinum), but does not differ from Pi. pygmaeus. Plecotus auritus differs from Pi. pipistrellus, for four parameters (Ssk, Str, Smr1 and Smr2; all higher in Pl. auritus), and from Pi. pygmaeus for Vvc and Vvv (lower in Pl. auritus). The two Pipistrellus species differ only for Vmp and Vvc (higher in Pi. pygmaeus).
Table 2.
Results of analysis of ANOVA, bat roughness parameters (log transformed)
| d.f. | F | P | |
|---|---|---|---|
| Sq | 3, 16 | 1.715 | 0.204 |
| Ssk | 3, 16 | 6.733 | 0.004 |
| Suk | 3, 16 | 0.078 | 0.971 |
| Sp | 3, 16 | 0.457 | 0.716 |
| Sv | 3, 16 | 1.091 | 0.381 |
| Sz | 3, 16 | 0.358 | 0.784 |
| Sds | 3, 16 | 0.468 | 0.709 |
| Str | 3, 16 | 10.020 | 0.0006 |
| Sal | 3, 16 | 2.923 | 0.066 |
| Sdqa | 3, 8.24 | 2.231 | 0.160 |
| Ssca | 3, 7.54 | 0.833 | 0.514 |
| Sdra | 3, 8.23 | 1.688 | 0.244 |
| Vmp | 3, 16 | 3.364 | 0.045 |
| Vmc | 3, 16 | 10.413 | 0.0005 |
| Vvc | 3, 16 | 7.988 | 0.002 |
| Vvv | 3, 16 | 16.697 | <.0001 |
| Spka | 3, 8.47 | 2.221 | 0.159 |
| Sk | 3, 16 | 1.763 | 0.195 |
| Svk | 3, 16 | 5.508 | 0.009 |
| Smr1a | 3, 8.65 | 7.138 | 0.010 |
| Smr2 | 3, 16 | 5.704 | 0.007 |
| S5z | 3, 16 | 0.163 | 0.920 |
| Sa | 3, 16 | 2.140 | 0.135 |
Parameters in bold are those for which the null hypothesis of no difference between species can be rejected. aIndicates Welch test result (ANOVA, unequal variances, Bartlett and/or Levene test). ANOVA, analysis of variance; d.f., degrees of freedom.
PCA of these nine parameters (Fig. 1) reveals that bat species are separated according to dietary preferences in a space defined by PC axes 1 and 2. PC axis 1 (48.2% of variance) is strongly correlated with bats dietary preferences (rs = 0.81, P < 0.0001; bats ranked according to proportion of ‘hard’ prey in diet: R. ferrumequinum 1, Pi. pipistrellus 2, Pi. pygmaeus 3, Pl. auritus 4). The ‘soft’ diet specialist (Pl. auritus) has positive values, while R. ferrumequinum, which consumes the highest amounts of ‘hard’ prey, has negative values. The two Pipistrellus species span the gap between, with Pi. pipistrellus overlapping with R. ferrumequinum on PC axis 1, while the range of values for Pi. pygmaeus extends to include some that are similar to R. ferrumequinum and some that are similar to Pl. auritus.
Analysis of bat surface texture parameters thus defines a ‘dietary space’ in which increasingly negative values for PC axis 1 indicate higher proportions of ‘hard’ prey, while increasing positive values indicate decreasing proportions of ‘hard’ prey. ANOVA of the PCA results provides further support: PC axes 1 and 2 both differ between species [PC axis 1, F = 14.97; degrees of freedom (d.f.) = 3, 13; P < 0.0001; PC axis 2, F = 4.97; d.f. = 3, 13; P = 0.013]. Tukey’s HSD procedure reveals that for PC axis 1 R. ferrumequinum differs from Pl. auritus and Pi. pygmaeus, Pl. auritus differs from R. ferrumequinum and Pi. pipistrellus. The two Pipistrellus species do not differ from one another. For PC axis 2, Pi. pipistrellus differs from R. ferrumequinum and Pi. pygmaeus. That the bat species are separated into largely non-overlapping areas of space defined by the first two axes of a PCA based solely on ISO roughness parameters derived from worn tooth surfaces, and that there are significant differences between species, provides powerful evidence that microtextural analysis of tooth microwear can differentiate between species within trophic guilds, in this case between insectivores, for some of which dietary differences are quite subtle.
LDA produced a similar result to PCA analysis (Fig 1). Bat species were assigned to three dietary groups based on the amount of ‘hard’ prey consumed: higher (R. ferrumequinum) mixed (Pipistrellus species) and lower (Pl. auritus). LDA based on the nine roughness parameters assigned 100% of bat specimens to their correct dietary group. As with PCA, canonical axis 1 (89.9% variance) is strongly correlated with diet (species ranked 1–4; rs = −0.84, P < 0.0001).
Our results also allow us to explore the relationship between ISO roughness parameters and diet. Rank correlation of dietary preferences reveals that 10 parameters are correlated with diet (P < 0.05; Table 3). Ssk, Str, Smr1, Smr2 and Sa decrease as the proportion of ‘hard’ prey increases; these are parameters that capture aspects of the height (Ssk, Sa), spatial (Str), and areal material ratio attributes of the roughness surface (Smr1, Smr2). Sq, Vmc, Vvv, Sk and Svk increase with increasing proportion of ‘hard’ prey; these are parameters that capture aspects of the height (Sq) and areal material ratio attributes of the roughness surface (Sk, Svk, Vmc, Vvv; the latter two capturing core material and valley void volume). In simple terms, as the amount in ‘hard’ prey increases, tooth surfaces tend to have deeper valleys, the elevations of the surface and the core material (i.e. not peaks or valleys) are higher, there are fewer peaks, and there is more directionality to the surface texture. This is illustrated in Fig. 1a: R. ferrumequinum sample 11002 exhibits roughness values that, for seven of the 10 diet-correlated parameters, are towards the ‘hard’ end of the dietary scale; Pl. auritus sample 11247, on the other hand, exhibits values for seven of the parameters that are towards the ‘soft’ end.
Table 3.
Correlations between dietary rank and ISO roughness parameters (n = 20)
| Parameter | Spearman’s ρ | P |
|---|---|---|
| Sq | −0.465 | 0.039 |
| Ssk | 0.714 | 0.000 |
| Sku | 0.023 | 0.922 |
| Sp | 0.209 | 0.376 |
| Sv | −0.124 | 0.602 |
| Sz | 0.016 | 0.948 |
| Sds | 0.116 | 0.625 |
| Str | 0.683 | 0.001 |
| Sal | 0.241 | 0.306 |
| Sdq | −0.066 | 0.782 |
| Ssc | 0.023 | 0.922 |
| Sdr | 0.016 | 0.948 |
| Vmp | −0.147 | 0.535 |
| Vmc | −0.621 | 0.004 |
| Vvc | −0.372 | 0.106 |
| Vvv | −0.706 | 0.001 |
| Spk | 0.279 | 0.233 |
| Sk | −0.512 | 0.021 |
| Svk | −0.706 | 0.001 |
| Smr1 | 0.594 | 0.006 |
| Smr2 | 0.621 | 0.004 |
| S5z | −0.132 | 0.580 |
| Sa | −0.528 | 0.017 |
See Supporting Information for definitions of parameters. Significant correlations are shown in bold.
Discussion
Our method of data acquisition (focus variation microscopy) differs from previous applications of 3-D textural analysis of tooth microwear to dietary discrimination in mammals (which used confocal microscopy or interferometry). Most previous analyses employed SSFA, but our analysis is based on ISO parameters generated from scale-limited roughness surfaces (c.f. Purnell et al., 2012; Schulz et al., 2013a). Nevertheless, our results provide further confirmation of the power of 3-D textural analysis of tooth microwear as a tool for dietary discrimination. The results of PCA analysis, which requires no prior assumptions regarding dietary preferences or tooth wear in the species under investigation, are particularly compelling: the four bat species occupy largely non-overlapping areas in the space defined by PC axes 1 and 2, with clear separation between the species that eats the most ‘hard’ prey (R. ferrumequinum) and the ‘soft’ prey specialist (Pl. auritus). This result, coupled with statistical testing and linear discriminate analysis, demonstrates clearly that the 3-D texture of microwear as captured by ISO roughness parameters carries a strong dietary signal, and can detect subtle dietary differences between even cryptic species of insectivore.
Only a few previous analyses (Calandra et al., 2012; Purnell et al., 2012; Schulz et al., 2013a,b) have used ISO roughness parameters to investigate dietary differences between extant animals. The parameters found by Purnell et al. (2012) to differ significantly between cichlid fishes are not the same as for bats, but in both studies volume parameters (Vmp, Vmc, Vvc and Vvv for cichlid lower pharyngeal jaws; Vmp, Vmc, Vvc for cichlid oral teeth) differ between animals with different diets. In terms of diet, comparing results for cichlid lower pharyngeal jaws with the bats, it is the individuals that consume the most ‘hard’ food items (mollusc shells in the cichlids; beetle cuticle in bats) that have the higher values for volume parameters. Comparing insectivorous bats with cichlids that scrape-up algae (cichlid oral teeth analysis of Purnell et al., 2012) is a little more difficult, but for both groups, higher values for volume parameters occur in the animals in which teeth encounter more hard materials (rock scraping Neochromis gigas in the cichlids).
The texture parameters found by Schulz et al. (2013a) to differ between grazing and browsing ungulates also include three of the volume parameters that differ between bats. Vmc, Vvc, and Vvv are higher in grazers, interpreted by Schulz et al. (2013a) to reflect their more abrasive diet. Calandra et al. (2012) investigated six ISO roughness parameters in primates, only two of which (Sq and Vm) are directly comparable with parameters calculated here for bats. They found fairly weak statistical support for differences in parameter values between taxa, but observed that large hard particles produce tooth surfaces with more microscopic relief, with the highest Vm values found in species with a high proportion (>50%) of fruit (and therefore seeds) in their diet. This is consistent with our evidence of higher values for volume parameters in bats, which consume the highest amounts of ‘hard’ prey. Because they found few significant differences in ISO parameters between primates with different diets, Calandra et al. (2012) concluded that SSFA of microwear is a better tool for dietary discrimination. However, our results and those of Schultz et al. (2013a) suggest that analysis based on ISO parameters has comparable discriminatory power.
Comparing our results to the early work by Strait (1993a), her 2-D SEM-based approach went some way to demonstrating the potential of microwear analysis for dietary discrimination in bats: she was able to detect differences between hard object and soft object feeders, but was unable to discriminate between insectivores and flesh eaters. ISO roughness parameters provide a much more sophisticated characterization of the nature of microwear surfaces than is possible with 2-D analysis. Our results demonstrate that ISO-based analysis, in addition to avoiding the problems inherent in microwear analysis based on operator scoring, is capable of more robust and more subtle dietary discrimination.
Further work is required, including more detailed comparisons of different approaches to data acquisition, processing and analysis, with more comparative evaluations of ISO and SSFA approaches, but our results demonstrate the potential of ISO-based textural analysis for dietary discrimination, and establish the first set of ISO roughness data from extant small-bodied insectivorous mammals with known diets. These data provide textural reference points that will allow future studies to use ISO roughness characterization of microwear to test hypotheses of dietary specialization, niche partitioning and validation of functional models for taxa where dietary data are otherwise difficult to obtain, including extinct early mammals, many of which are assumed to have been insectivores.
Acknowledgments
We thank Matt Zeale (Bristol) for advice and access to bat specimens; Trudy Goddard (Veterinary Laboratories Agency) and Gail Armstrong (North Lancashire bat group) for providing specimens; Remmert Schouten and Martin Rücklin (Bristol), Laurent Darras and Rowan Dejardin (Leicester) for assistance with material preparation and methods; Trish Freeman (Nebraska-Lincoln), Scott Pederson and Gary Kwiecinski (South Dakota State), Peter Ungar (Arkansas), Jerry Hooker (NHM) and Peter Shellis (Bristol) for advice. NC thanks Izzy Way for her hospitality during visits to Leicester. Two anonymous referees and Ellen Schulz provided helpful reviews. M.A.P, E.J.R. and P.G.G. supported by NERC grants NE/G018189/1 and NE/E010431/1. M.A.P, E.J.R. and P.G.G. conceived and designed research programme; M.A.P and N.C. generated, analysed and interpreted data. All authors contributed to the manuscript.
Supporting Information
Figure S1 The areal material ratio curve (also referred to as the bearing area curve, or Abbot–Firestone curve) from which a number of height, volume, and material ratio parameters are derived. For definitions of parameters, see Supporting Information Table S1. The curve is a cumulative probability density function, derived from the scale-limited surface by plotting the cumulative percentage of the surface against height. Core, peaks and valleys within a surface are defined on the basis of this curve, with the core equivalent to the volume that lies between the heights of the surface delimited by the extrapolated intercept of the minimum slope of the curve as shown in the figure. Modified with permission from Alicona Infinite Focus Manual.
Figure S2 Cross-section through a surface showing how volume parameters relate to a surface. Note that this is a two-dimensional profile, but the parameters are volumes calculated for the whole surface. Modified with permission from Alicona Infinite Focus Manual.
Table S1 Specimens from which microtextural data were acquired. During preparation, the M3, coronoid crest and all other morphology posterior to the M2 was removed on both sides of the mandible to allow unobstructed examination of the M2. Soft tissues were removed from the teeth and jaws, carefully avoiding any contact between instruments and the M2 crowns, and specimens were further cleaned by boiling in individual beakers of water for 2 min. Prior to analysis, tooth surfaces were carefully cleaned with acetone applied with a soft synthetic brush. In a few cases the first attempts at data acquisition from the M2 facets revealed the presence of small crystals precipitated over the functional surface. These specimens were returned for a short period to the solution in which they had been stored, were gently brushed with de-ionized water on removal, and air-dried before examination.
Table S2 Short definitions and categorization of three-dimensional areal surface texture parameters. For further explanation see Supporting Information Figs S1 and S2.
Table S3 Loadings (eigenvectors) for roughness parameters onto PC axes 1 and 2 for the principal components (PC) analysis (nine parameters that differ significantly between bat species).
References
- Aguirre LF, Herrel A, Van Damme R. Matthysen E. The implications of food hardness for diet in bats. Funct. Ecol. 2003;17:201–212. &. [Google Scholar]
- Anyonge W. Microwear on canines and killing behavior in large carnivores: saber function in Smilodon fatalis. J. Mammal. 1996;77:1059–1067. [Google Scholar]
- Barlow K. The diets of two phonic types of the bat Pipistrellus pipistrellus in Britain. J. Zool. (Lond.) 1997;243:597–609. [Google Scholar]
- Barratt EM, Deaville R, Burland TM, Bruford MW, Jones G, Racey PA. Wayne RK. DNA answers the call of pipistrelle bat species. Nature. 1997;387:138–139. doi: 10.1038/387138b0. &. [DOI] [PubMed] [Google Scholar]
- Bastl K, Semprebon G. Nagel D. Low-magnification microwear in Carnivora and dietary diversity in Hyaenodon (Mammalia: Hyaenodontidae) with additional information on its enamel microstructure. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012;348:13–20. &. [Google Scholar]
- Calandra I, Schulz E, Pinnow M, Krohn S. Kaiser TM. Teasing apart the contributions of hard dietary items on 3D dental microtextures in primates. J. Hum. Evol. 2012;63:85–98. doi: 10.1016/j.jhevol.2012.05.001. &. [DOI] [PubMed] [Google Scholar]
- Darwin CR. On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. 1st edn. London: John Murray; 1859. [PMC free article] [PubMed] [Google Scholar]
- Dayan T. Simberloff D. Ecological and community-wide character displacement: the next generation. Ecol. Lett. 2005;8:875–894. &. [Google Scholar]
- DeSantis LRG, Schubert BW, Scott JR. Ungar PS. Implications of diet for the extinction of saber-toothed cats and American lions. PLoS ONE. 2012;7:e52453. doi: 10.1371/journal.pone.0052453. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont ER, Davalos LM, Goldberg A, Santana SE, Rex K. Voigt CC. Morphological innovation, diversification and invasion of a new adaptive zone. Proc. Biol. Sci. 2012;279:1797–1805. doi: 10.1098/rspb.2011.2005. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AR. Sanson GD. Biomechanical properties of insects in relation to insectivory: cuticle thickness as an indicator of insect ‘hardness’ and ‘intractability’. Aust. J. Zool. 2005;53:9–19. &. [Google Scholar]
- Freeman PW. Macroevolution in microchiroptera: recoupling morphology and ecology with phylogeny. Evol. Ecol. Res. 2000;2:317–335. [Google Scholar]
- Freeman PW. Lemen CA. Using scissors to quantify hardness of insects: do bats select for size or hardness? J. Zool. 2007;271:469–476. &. [Google Scholar]
- Goillot C, Blondel C. Peigne S. Relationships between dental microwear and diet in Carnivora (Mammalia) – implications for the reconstruction of the diet of extinct taxa. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2009;271:13–23. &. [Google Scholar]
- Gordon KD. A study of microwear on chimpanzee molars: implications for dental microwear analysis. Am. J. Phys. Anthropol. 1982;59:195–215. doi: 10.1002/ajpa.1330590208. [DOI] [PubMed] [Google Scholar]
- Grine FE, Ungar PS. Teaford MF. Error rates in dental microwear quantification using scanning electron microscopy. Scanning. 2002;24:144–153. doi: 10.1002/sca.4950240307. &. [DOI] [PubMed] [Google Scholar]
- International Organization for Standardization. 2012. pp. 1–42. Geometrical product specifications (GPS) – Surface texture: Areal – Part 2: Terms, definitions and surface texture parameters (ISO 25178-2)
- Jones G. Prey selection by the Greater horseshoe bat (Rhinolophus ferrumequinum) – optimal foraging by echolocation. J. Anim. Ecol. 1990;59:587–602. [Google Scholar]
- Jones G. Van Parijs SM. Bimodal echolocation in Pipistrelle bats – are cryptic species present. Proc. Biol. Sci. 1993;251:119–125. doi: 10.1098/rspb.1993.0017. &. [DOI] [PubMed] [Google Scholar]
- Jordan MJR. Dietary analysis for mammals and birds: a review of field techniques and animal-management applications. Int. Zoo Yearb. 2005;39:108–116. [Google Scholar]
- Kelly JF. Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can. J. Zool. 2000;78:1–27. [Google Scholar]
- Kunz TH. Whitaker JO. An evaluation of fecal analysis for determining food-habits of insectivorous bats. Can. J. Zool. 1983;61:1317–1321. &. [Google Scholar]
- Merceron G, Escarguel G, Angibault JM. Verheyden-Tixier H. Can dental microwear textures record inter-individual dietary variations? PLoS ONE. 2010;5:9. doi: 10.1371/journal.pone.0009542. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihlbachler MC, Beatty BL, Caldera-Siu A, Chan D. Lee R. Error rates and observer bias in dental microwear analysis using light microscopy. Palaeontol. Electron. 2012;15.1.12A &. [Google Scholar]
- Nogueira MR, Peracchi AL. Monteiro LR. Morphological correlates of bite force and diet in the skull and mandible of phyllostomid bats. Funct. Ecol. 2009;23:715–723. &. [Google Scholar]
- Price SA, Hopkins SSB, Smith KK. Roth VL. Tempo of trophic evolution and its impact on mammalian diversification. Proc. Natl. Acad. Sci. U.S.A. 2012;109:7008–7012. doi: 10.1073/pnas.1117133109. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnell MA, Hart PJB, Baines DC. Bell MA. Quantitative analysis of dental microwear in threespine stickleback: a new approach to analysis of trophic ecology in aquatic vertebrates. J. Anim. Ecol. 2006;75:967–977. doi: 10.1111/j.1365-2656.2006.01116.x. &. [DOI] [PubMed] [Google Scholar]
- Purnell MA, Bell MA, Baines DC, Hart PJB. Travis M. Correlated evolution and dietary change in fossil stickleback. Science. 2007;317:1887. doi: 10.1126/science.1147337. &. [DOI] [PubMed] [Google Scholar]
- Purnell MA, Seehausen O. Galis F. Quantitative three-dimensional microtextural analyses of tooth wear as a tool for dietary discrimination in fishes. J. R. Soc. Interface. 2012;9:2225–2233. doi: 10.1098/rsif.2012.0140. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razgour O, Clare EL, Zeale MRK, Hanmer J, Schnell IB, Rasmussen M, Gilbert T. Jones G. High-throughput sequencing offers insight into mechanisms of resource partitioning in cryptic bat species. Ecol. Evol. 2011;1:556–570. doi: 10.1002/ece3.49. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana SE, Grosse IR. Dumont ER. Dietary hardness, loading behavior, and the evolution of skull form in bats. Evolution. 2012;66:2587–2598. doi: 10.1111/j.1558-5646.2012.01615.x. &. [DOI] [PubMed] [Google Scholar]
- Schluter D. The ecology of adaptive radiations. Oxford: Oxford University Press; 2000. [Google Scholar]
- Schubert BW, Ungar PS. DeSantis LRG. Carnassial microwear and dietary behaviour in large carnivorans. J. Zool. 2010;280:257–263. &. [Google Scholar]
- Schulz E, Calandra I. Kaiser TM. Feeding ecology and chewing mechanics in hoofed mammals: 3D tribology of enamel wear. Wear. 2013a;300:169–179. &. [Google Scholar]
- Schulz E, Piotrowski V, Clauss M, Mau M, Merceron G. Kaiser TM. Dietary abrasiveness is associated with variability of microwear and dental surface texture in rabbits. PLoS ONE. 2013b;8:e56167. doi: 10.1371/journal.pone.0056167. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RS, Ungar PS, Bergstrom TS, Brown CA, Grine FE, Teaford MF. Walker A. Dental microwear texture analysis shows within-species diet variability in fossil hominins. Nature. 2005;436:693–695. doi: 10.1038/nature03822. &. [DOI] [PubMed] [Google Scholar]
- Scott RS, Ungar PS, Bergstrom TS, Brown CA, Childs BE, Teaford MF. Walker A. Dental microwear texture analysis: technical considerations. J. Hum. Evol. 2006;51:339–349. doi: 10.1016/j.jhevol.2006.04.006. &. [DOI] [PubMed] [Google Scholar]
- Strait SG. Molar microwear in extant small-bodied faunivorous mammals – an analysis of feature density and pit frequency. Am. J. Phys. Anthropol. 1993a;92:63–79. doi: 10.1002/ajpa.1330920106. [DOI] [PubMed] [Google Scholar]
- Strait S. Molar morphology and food texture among small-bodied insectivorous mammals. J. Mammal. 1993b;74:391–402. [Google Scholar]
- Swift SM, Racey PA. Avery MI. Feeding ecology of Pipistrellus pipistrellus (Chiroptera, Vespertilionidae) during pregnancy and lactation II. Diet. J. Anim. Ecol. 1985;54:217–225. &. [Google Scholar]
- Taylor ME. Hannam AG. Tooth microwear and diet in the African Viverridae. Can. J. Zool. 1986;65:1696–1702. &. [Google Scholar]
- Teaford MF. A review of dental microwear and diet in modern mammals. Scanning Microsc. 1988;2:1149–1166. [PubMed] [Google Scholar]
- Teaford MF. Oyen OJ. In vivo and in vitro turnover in dental microwear. Am. J. Phys. Anthropol. 1989;80:447–460. doi: 10.1002/ajpa.1330800405. &. [DOI] [PubMed] [Google Scholar]
- Trites AW. Joy R. Dietary analysis from fecal samples: how many scats are enough? J. Mammal. 2005;86:704–712. &. [Google Scholar]
- Ungar PS, Merceron G. Scott RS. Dental microwear texture analysis of varswater bovids and early pliocene paleoenvironments of Langebaanweg, Western Cape Province, South Africa. J. Mammal. Evol. 2007;14:163–181. &. [Google Scholar]
- Ungar PS, Grine FE. Teaford MF. Dental microwear and diet of the Plio-Pleistocene hominin Paranthropus boisei. PLoS ONE. 2008;3:e2044. doi: 10.1371/journal.pone.0002044. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Valkenburgh B, Teaford MF. Walker A. Molar microwear and diet in large carnivores: inferences concerning diet in the sabretooth cat, Smilodon fatalis. J. Zool. 1990;222:319–340. &. [Google Scholar]
- Vaughan N. The diets of British bats (Chiroptera) Mamm. Rev. 1997;27:77–94. [Google Scholar]
- Walker AC, Hoeck HN. Perez L. Microwear of mammal teeth as an indicator of diet. Science. 1978;201:808–810. doi: 10.1126/science.684415. &. [DOI] [PubMed] [Google Scholar]
- Williams RL, Goodenough AE. Stafford R. Statistical precision of diet diversity from scat and pellet analysis. Ecol. Inform. 2012;7:30–34. &. [Google Scholar]
- Williams VS, Barrett PM. Purnell MA. Quantitative analysis of dental microwear in hadrosaurid dinosaurs, and the implications for hypotheses of jaw mechanics and feeding. Proc. Natl. Acad. Sci. U.S.A. 2009;106:11194–11199. doi: 10.1073/pnas.0812631106. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 The areal material ratio curve (also referred to as the bearing area curve, or Abbot–Firestone curve) from which a number of height, volume, and material ratio parameters are derived. For definitions of parameters, see Supporting Information Table S1. The curve is a cumulative probability density function, derived from the scale-limited surface by plotting the cumulative percentage of the surface against height. Core, peaks and valleys within a surface are defined on the basis of this curve, with the core equivalent to the volume that lies between the heights of the surface delimited by the extrapolated intercept of the minimum slope of the curve as shown in the figure. Modified with permission from Alicona Infinite Focus Manual.
Figure S2 Cross-section through a surface showing how volume parameters relate to a surface. Note that this is a two-dimensional profile, but the parameters are volumes calculated for the whole surface. Modified with permission from Alicona Infinite Focus Manual.
Table S1 Specimens from which microtextural data were acquired. During preparation, the M3, coronoid crest and all other morphology posterior to the M2 was removed on both sides of the mandible to allow unobstructed examination of the M2. Soft tissues were removed from the teeth and jaws, carefully avoiding any contact between instruments and the M2 crowns, and specimens were further cleaned by boiling in individual beakers of water for 2 min. Prior to analysis, tooth surfaces were carefully cleaned with acetone applied with a soft synthetic brush. In a few cases the first attempts at data acquisition from the M2 facets revealed the presence of small crystals precipitated over the functional surface. These specimens were returned for a short period to the solution in which they had been stored, were gently brushed with de-ionized water on removal, and air-dried before examination.
Table S2 Short definitions and categorization of three-dimensional areal surface texture parameters. For further explanation see Supporting Information Figs S1 and S2.
Table S3 Loadings (eigenvectors) for roughness parameters onto PC axes 1 and 2 for the principal components (PC) analysis (nine parameters that differ significantly between bat species).


