Abstract
Background
Maternal obesity has been linked to offspring asthma; however, other allergy-related diseases, as well as the association beyond early school age, are largely unstudied.
Objective
To examine the associations between maternal body mass index (BMI) in pregnancy and offspring asthma, rhinitis, eczema and sensitization up to 16 years of age.
Methods
A total of 3294 children from the Swedish birth cohort BAMSE were included in the analyses. Maternal BMI was assessed around week 10 in pregnancy. Information on asthma, rhinitis, eczema, lifestyle factors and environmental exposures was obtained by parental questionnaires at 1, 2, 4, 8, 12 and 16 years. Sensitization was defined from IgE levels of inhalant allergens at 4, 8 and 16 years in a subsample of 2850 children. Generalized estimated equation models were used to analyse the associations between maternal BMI and the outcomes at 1–16 years.
Results
Maternal BMI was positively associated with overall risk of asthma up to age of 16 years (adj OR per 5 kg/m2 increase: 1.23; 95% CI 1.07–1.40 for prevalent asthma) excluding underweight mothers. In contrast, no significant associations were found for rhinitis, eczema or sensitization. The association with asthma was restricted to obese, rather than overweight mothers, but was attenuated when adjusting for overweight in the offspring. A causal inference test at 16 years further indicated that the child’s own overweight is a mediator in the suggested association between maternal BMI and offspring asthma at 16 years.
Conclusions and Clinical Relevance
Maternal BMI is associated with an increased risk of asthma, but not rhinitis, eczema or sensitization; however, overweight in the offspring seems to have a mediating role. Prevention strategies of maternal pre-pregnancy and childhood obesity might be important to reduce the prevalence of childhood asthma.
Keywords: allergic disease, asthma, body mass index, children, maternal obesity, rhinitis, sensitization
Introduction
The prevalences of obesity and allergic disease (including asthma, rhinitis, eczema and sensitization) have increased in parallel during the last decades [1–3]. These conditions are influenced by genetic and environmental factors, and several studies have found obesity to be associated with asthma in both adult and paediatric populations [4,5].
In recent years, prenatal factors have been suggested to have an important role in the development of allergic disease, as the pre- and perinatal periods include critical developmental stages of the respiratory tract and the immune system [6,7]. A high maternal pre-pregnant body mass index (BMI) as well as estimated body fat mass has been linked to asthma, asthma symptoms, medication use and respiratory hospitalization in the offspring [8–16]. Several potential mechanisms for how maternal obesity may affect the development of asthma and allergic disease in the offspring have been proposed, including epigenetic programming and shared genetics [17,18].
Although a growing body of literature reports that high maternal BMI is associated with an increased risk of asthma in the offspring, the majority of these studies followed children up to preschool or early school age [8–10, 12, 14–16]. Less evidence of an association is present when children reach adolescence [11,13], and due to non-repeated or short follow-up, there is also little longitudinal information about disease phenotypes. Therefore, it remains unclear whether the risk of maternal overweight is attributed to early transient wheeze, which was indicated in one study [14], or to a more problematic persistent asthma. Previous studies have not always been able to adjust for overweight in the child, and no study has to our knowledge formally tested whether the child’s BMI could be a mediator in the association between maternal overweight and offspring asthma. Furthermore, few studies have investigated whether high maternal BMI influences the risk of rhinitis, eczema and sensitization, which could be plausible consequences if maternal overweight induces inflammation or modulates the fetal immune system.
The aim of this study was to explore the association between maternal BMI, objectively measured in early pregnancy, and offspring asthma, rhinitis, eczema and sensitization, as well as the role of the child’s overweight in this association, in a population-based birth cohort followed up to the age of 16 years.
Methods
Study design and study population
The study population consists of participants in the prospective birth cohort BAMSE. A detailed description of the study has been published elsewhere [19]. Briefly, the study consists of 4089 children born between 1994 and 1996 in pre-defined areas of Stockholm. Information on background exposures, socio-economic status, parental history of allergic disease and other factors was collected through a baseline questionnaire when the child was approximately 2 months old. Repeated follow-up questionnaires focusing on symptoms of allergic disease were sent to the parents when the children were approximately 1, 2, 4, 8, 12 and 16 years old. Response rates were 96%, 94%, 91%, 84%, 82% and 78%, respectively.
At 4, 8 and 16 years, all children were invited to a clinical examination including blood sampling and measurement of height and weight. Blood was obtained from 2605 children (64%) at the age of 4 years, 2470 (60%) at the age of 8 years and 2547 (62%) at the age of 16 years and was analysed for IgE antibodies to eight common inhalant allergens (Phadiatop®; Thermo Fischer Scientific, Uppsala, Sweden). The study was approved by the regional ethical review board in Stockholm (application number 2010/1474-31/3).
Anthropometric measures and co-variables
Maternal BMI was obtained through linkage to the Swedish Medical Birth Register containing information on weight and height recorded at the first visit to the antenatal-care clinic (usually around week 10 in pregnancy [20]). Information on weight and height was complete for 3533 mothers (86% of the total cohort). BMI was calculated as body weight in kilograms divided by height in metres squared (kg/m2).
The child’s BMI was calculated from height and weight measured at the clinical investigation (at 4, 8 and 16 years) or obtained from child health care/school records (at 1, 2 and 12 years). Overweight was defined using the age- and sex-specific cut-off values developed for children under 18 years of age (isoBMI ≥ 25 kg/m2) [21]. At 1 year, however, there is no reference value for overweight and the gender-specific 85th percentile, based on children in the BAMSE study, was therefore used. Number of children with available data on BMI status at any of the ages was 3545 (87% of the total cohort).
Information on other co-variables was obtained from the baseline questionnaire (gender, maternal age, parental history of allergic disease, socio-economic status and maternal smoking during pregnancy and/or in infancy), the 1 year questionnaire (duration of breastfeeding) and the Swedish Medical Birth Register (birth weight, older siblings, mode of delivery and newborn respiratory diagnosis).
Definition of health outcomes
All health outcomes were based on parental questionnaires, with the exception of sensitization. Complete age-specific definitions for all outcomes are found in Data S1. Briefly, asthma, rhinitis and eczema were defined as follows:
Asthma at 1 and 2 years: at least three episodes of wheeze in combination with inhaled steroids and/or hyper-reactivity without upper respiratory infection. At 4, 8, 12 and 16 years: at least four episodes of wheeze or at least one episode of wheeze in combination with inhaled steroids.
Early transient asthma: fulfilling the diagnosis of asthma at 1, 2 and/or 4 years but not at 8, 12 and 16 years.
Persistent asthma: fulfilling the diagnosis of asthma at 1, 2 and/or 4 years and at 8, 12 and/or 16 years.
School-age onset asthma: not fulfilling the diagnosis of asthma at 1, 2 and 4 years but at 8, 12 and/or 16 years.
Rhinitis: symptoms from eye/nose after exposure to allergens and/or a doctor’s diagnosis of allergic rhinitis.
Eczema: dry skin, itchy rashes at specific location and/or a doctor’s diagnosis of eczema.
Sensitization: at least one allergen-specific IgE result of ≥ 0.35 kU/L against inhalant allergens (Phadiatop®; cat, dog horse, birch, timothy, mugwort, Dermatophagoides pteronyssinus and Cladosporium herbarium).
Incident disease was defined as fulfilling the diagnosis of disease at the respective age without fulfilling it at any previous follow-up.
Statistical analyses
Differences in the distribution of potential confounders in relation to maternal BMI were analysed using the t-test (continuous variables) and the chi-square test (categorical variables). The associations between maternal BMI and allergic diseases were analysed longitudinally using generalized estimating equation (GEE) models with an unstructured correlation matrix. The GEE model calculates population average risks taking the correlation within individuals into account [22]. To analyse age-specific associations, an interaction between exposure and time was incorporated into the model.
Results are presented as odds ratios (OR) with 95% confidence intervals (95% CI). The associations between maternal BMI and phenotypes of asthma (early transient, persistent and school-age onset asthma) were analysed using multi-nomial logistic regression and the association between maternal BMI and asthma stratified for sensitization were analysed using ordinary logistic regression.
Maternal BMI was analysed as a continuous variable and classified according to the World Health Organization’s recommendations: underweight (< 18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2) and obesity (≥ 30 kg/m2). As there was indications of a U-shaped association between maternal BMI and asthma, underweight subjects (n = 117) were excluded in all analyses where maternal BMI was treated as a continuous variable. To fully explore the association between maternal BMI in early pregnancy and asthma in the offspring, we also flexibly modelled maternal BMI between 16 and 40 kg/m2 using restricted cubic splines with four knots (at BMI 19, 21, 23 and 29 kg/m2). Linearity was evaluated by testing the two curvilinear coefficients of the spline transformations equal to 0 [23].
Covariates were identified from previous literature and separated into two models. Model 1 includes gender and the following potential confounders: maternal age, older siblings, parental history of allergic disease, socio-economic status and maternal smoking during pregnancy and/or in infancy. Model 2 includes the following potential mediators: birth weight, mode of delivery, duration of breastfeeding and newborn respiratory diagnoses. To explore the role of the child’s BMI on the association between maternal BMI and offspring asthma, adjustments for overweight in the offspring at each age were additionally performed. Testing for possible interaction between maternal BMI and gender, as well as with parental history of allergic disease and sensitization, was performed using an interaction term between these variables and maternal BMI.
To investigate a potential mediating effect of the child’s BMI in the association between maternal BMI in early pregnancy and asthma in the offspring, we applied the causal inference test (CIT) [24]. The CIT requires four criteria to be fulfilled: (1) the causal factor is associated with the outcome, (2) the causal factor is associated with the mediator, (3) the mediator is associated with the outcome after adjusting for the causal factor and (4) the causal factor is independent of the outcome after adjusting for the mediator. Logistic regression analyses were used to perform these steps with overweight in the child at 16 years as a potential mediator in the association between maternal BMI in early pregnancy and asthma at 16 years (N = 2110).
Children were included in the analyses if maternal BMI in early pregnancy was available together with information from ≥ 3 follow-up questionnaires (N = 3294 for analyses on asthma, rhinitis and eczema. N = 2850 for analyses on sensitization to inhalant allergens). All analyses were performed using the statistical software Stata version 11 (StataCorp, College Station LP, TX, USA).
Results
Descriptive results of exposure and outcomes
The 3294 children included in the analyses were comparable with the children in the total cohort regarding distribution of background characteristics including gender, maternal age, birth weight, gestational age, mode of delivery, older siblings, breastfeeding, parental history of allergic disease, maternal smoking during pregnancy and/or in infancy and socio-economic status (Table S1 in Data S1).
Maternal BMI in early pregnancy ranged from 14.7 to 44.4 kg/m2 with a median of 22.3 kg/m2 and a mean of 22.9 kg/m2 (SD 3.2). About 4% of the women were classified as underweight, 76% as normal weight, 16% as overweight and 4% as obese. An early pregnancy BMI of ≥ 25 kg/m2, compared with < 25 kg/m2, was associated with higher birth weight in the offspring, Caesarean section, smoking during pregnancy and/or in infancy, lower socio-economic status, shorter duration of breastfeeding and overweight in the offspring at 16 years (Table1). Compared with mothers who were normal weight in early pregnancy, underweight mothers were younger, gave birth to children with lower birth weight and were less likely to have any previous children (data not shown). The prevalence of allergic disease at 16 years was 6% for asthma, 26% for rhinitis, 9% for eczema and 43% for sensitization to inhalant allergens.
Table 1.
Distribution of potential confounding factors in relation to maternal body mass index (BMI) in early pregnancy for children included in the analyses (N = 3294)
| Maternal BMI | ||||||
|---|---|---|---|---|---|---|
| < 25 kg/m2 N = 2633 (79.9%) | ≥ 25 kg/m2 N = 661 (20.1%) | |||||
| Mean | SD | Mean | SD | P-value | ||
| Maternal age (years) | 30.7 | 4.4 | 30.8 | 4.7 | 0.54 | |
| Birth weight† (g) | 3514 | 540 | 3608 | 580 | < 0.001 | |
| n | % | n | % | P-value | |
|---|---|---|---|---|---|
| Gender | |||||
| Boy | 1316 | 50.0 | 347 | 52.5 | |
| Girl | 1317 | 50.0 | 314 | 47.5 | 0.25 |
| Caesarean section | |||||
| No | 2321 | 88.2 | 546 | 82.6 | |
| Yes | 312 | 11.9 | 115 | 17.4 | < 0.001 |
| Newborn respiratory diagnosis, | |||||
| No | 2491 | 94.6 | 625 | 94.6 | |
| Yes | 142 | 5.4 | 36 | 5.5 | 0.96 |
| Older siblings | |||||
| 0 | 1478 | 56.1 | 343 | 51.9 | |
| ≥ 1 | 1155 | 43.9 | 318 | 48.1 | 0.05 |
| Breastfeeding | |||||
| < 4 months | 486 | 18.7 | 175 | 27.1 | |
| ≥ 4 months | 2108 | 81.3 | 472 | 73.0 | < 0.001 |
| Parental history of allergic disease | |||||
| No | 1846 | 70.8 | 441 | 67.3 | |
| Yes | 762 | 29.2 | 214 | 32.7 | 0.08 |
| Maternal smoking during pregnancy and/or in infancy | |||||
| No | 2297 | 87.3 | 546 | 82.7 | |
| Yes | 335 | 12.7 | 114 | 17.3 | 0.002 |
| Socio-economic status | |||||
| Blue-collar worker | 413 | 15.9 | 138 | 21.2 | |
| White-collar worker | 2184 | 84.1 | 513 | 78.8 | 0.001 |
| Child’s overweight at 16 years | |||||
| No | 1572 | 87.5 | 296 | 67.6 | |
| Yes | 224 | 12.5 | 142 | 32.4 | < 0.001 |
P-value obtained from t-test.
Obtained from the medical birth registry.
P-value obtained from chi-square test.
Includes hypoxia, asphyxia, respiratory distress syndrome and other respiratory conditions of fetuses and newborns (ICD 9: 768–770).
Doctor diagnosed asthma and/or hayfever in combination with reported allergy to pollen or pets in one or both parents.
The mother smoked at least one cigarette per day at any point of time during pregnancy and/or in infancy.
Defined as isoBMI ≥ 25 kg/m2.
Association with allergic diseases
Figure1 shows the associations between maternal BMI in early pregnancy and prevalent and incident asthma, rhinitis, eczema and sensitization to inhalant allergens with maternal BMI treated as a continuous variable and underweight mothers excluded (n = 117, leaving 3177 children in the analyses). Maternal BMI was associated with an increased overall risk (using GEE) of asthma in the offspring (adj OR per 5 kg/m2 increase, e.g. BMI 25 kg/m2 compared with 20 kg/m2: 1.23; 95% CI 1.07–1.40 for prevalent asthma and 1.17; 95% CI 1.03–1.32 for incident asthma). No statistically significant associations were found for the association between maternal BMI and rhinitis, eczema or sensitization (adj OR: 1.07; 95% CI 0.96–1.19 for prevalent rhinitis, 1.03; 95% CI 0.93–1.14 for prevalent eczema and 1.05; 95% CI 0.93–1.19 for prevalent sensitization). No significant interactions were found between maternal BMI and gender or parental history of allergic disease. Additional adjustments for potential mediators (birth weight, mode of delivery, breastfeeding and newborn respiratory diagnosis) had no major influence on the observed ORs (data not shown) and were not included in the subsequent analyses.
Figure 1.
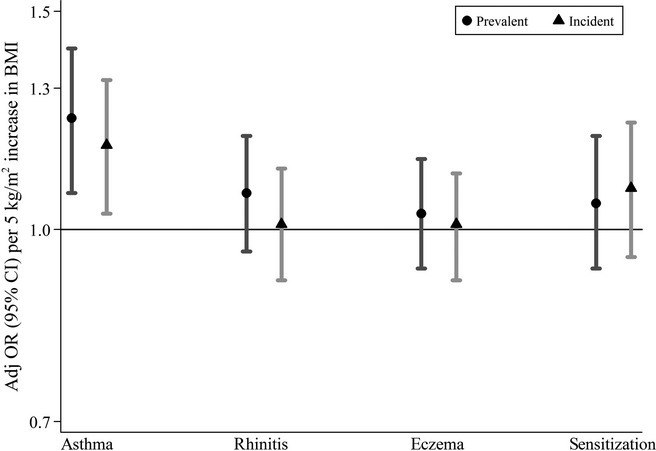
Associations between maternal body mass index (BMI) in early pregnancy (continuously measured) and prevalent (circles) and incident (triangles) asthma, rhinitis, eczema (n = 3177) and sensitization to inhalant allergens (n = 2748) in the offspring (overall effects 1–16 years) among mothers with BMI ≥ 18.5 kg/m2. Adjusted odds ratios (adj OR) and 95% confidence intervals (CI) represent 5 kg/m2 increase in BMI.
Age-specific associations and adjustments for overweight in the offspring
As the association between maternal BMI and allergic disease appeared to only be present for asthma, subsequent analyses were focused towards asthma only. The association between maternal BMI in early pregnancy and prevalent asthma was strongest in the first years of life, although non-significant, increased ORs were observed also at the ages of 12 and 16 years (Fig.2).
Figure 2.
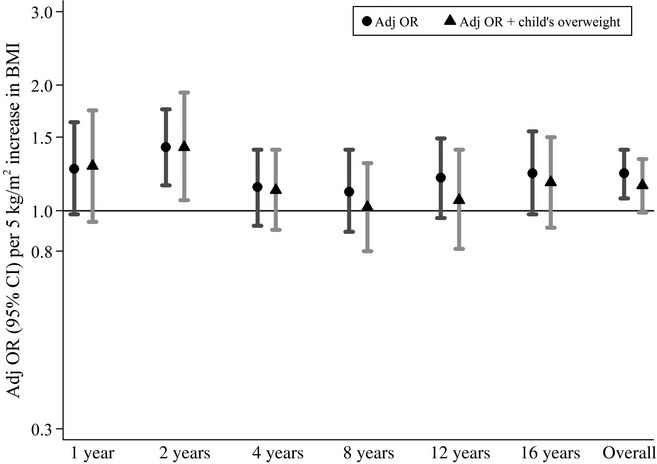
Association between maternal body mass index (BMI) in early pregnancy (continuously measured) and prevalent asthma in the offspring at 1–16 years among mothers with BMI ≥ 18.5 kg/m2 (n = 3177). Adjusted odds ratios (adj OR) and 95% confidence intervals (CI) represent 5 kg/m2 increase in BMI. Triangles are additionally adjusted for overweight in the offspring at each age.
Analyses between maternal BMI and asthma were further stratified for sensitization at 4, 8 and 16 years. Overall, there were no large differences in the association for asthma with and without sensitization (P-values for interaction between maternal BMI and sensitization were all non-significant).
As maternal BMI in early pregnancy was associated with overweight in the offspring at the age of 16 years (adj OR per 5 kg/m2 increase: 2.42; 95% CI 2.04–2.86), the analyses of maternal BMI in relation to asthma were additionally adjusted for overweight in the offspring at each age. In this model, the overall OR for prevalent asthma from age 1 to 16 years decreased and just failed to reach statistical significance (adj OR per 5 kg/m2 increase: 1.15; 95% CI 0.99–1.33). The associations were not attenuated at the early ages; however, from 8 years of age, the OR were somewhat decreased when adjusting for overweight in the child (Fig. 2).
Phenotypes of asthma
Maternal BMI in early pregnancy was associated with persistent asthma in the offspring (adj OR per 5 kg/m2 increase: 1.31; 95% CI 1.03–1.67). There was a tendency for an association also with school-age onset asthma (adj OR per 5 kg/m2 increase: 1.22; 95% CI 0.98–1.52) (Table2). No association was found between maternal BMI and early transient asthma. When adjusting for overweight in the offspring at age the of 16 years, the association for persistent asthma was somewhat attenuated and no longer statistically significant (Table2).
Table 2.
Association between maternal body mass index (BMI) in early pregnancy (continuously measured) and asthma phenotypes in the offspring among mothers with BMI ≥ 18.5 (N = 2343)
| Asthma phenotypes | n (%) | OR | 95% CI | OR | 95% CI |
|---|---|---|---|---|---|
| Early transient | 137 (5.9) | 1.02 | 0.77–1.36 | 0.92 | 0.66–1.29 |
| Persistent | 149 (6.4) | 1.31 | 1.03–1.67 | 1.29 | 0.98–1.70 |
| School-age onset | 213 (9.1) | 1.22 | 0.98–1.52 | 1.15 | 0.88–1.50 |
Information on outcome at all ages was required for the disease-free groups resulting in lower numbers of included children compared with the GEE analyses.
Odds ratio (OR) and 95% confidence intervals (CI) are obtained from logistic regression analyses and represent 5 kg/m2 increase in maternal BMI. Adjusted for gender, maternal age, parental history of allergic disease, older siblings, socio-economic status and maternal smoking in pregnancy and/or at baseline.
Additionally adjusted for overweight in the offspring at 16 years.
Maternal BMI categorized according to WHO’s definition
When categorizing maternal BMI in early pregnancy according to WHO’s definition of underweight, normal weight, overweight and obesity (n = 3294), there was a significant association between maternal obesity, compared with normal weight, and overall prevalent asthma in the offspring up to the age of 16 years (adj OR: 1.53; 95% CI 1.04–2.26) (Table3). No association was found for overweight mothers, although the trend for normal weight, overweight and obesity was significant (P = 0.03). When adjusting for overweight in the offspring, the association for maternal obesity and the trend across categories was no longer significant.
Table 3.
Association between categories of maternal body mass index (BMI) in early pregnancy and prevalent asthma in the offspring (overall effect 1–16 years*) (N = 3294)
| Maternal BMI (kg/m2) | OR | 95% CI | OR | 95% CI |
|---|---|---|---|---|
| < 18.5 | 1.29 | 0.83–2.01 | 1.20 | 0.73–1.97 |
| 18.5–24.9 | Referent | Referent | ||
| 25–29.9 | 1.14 | 0.90–1.45 | 1.12 | 0.87–1.45 |
| ≥ 30 | 1.53 | 1.04–2.26 | 1.24 | 0.80–1.94 |
| P for trend | 0.03 | 0.23 |
Odds ratio (OR) and 95% confidence intervals (CI) are obtained from Generalized estimating equation (GEE) analyses.
Adjusted for gender, maternal age, parental history of allergic disease, older siblings, socio-economic status and maternal smoking in pregnancy and/or at baseline.
Additionally adjusted for overweight in the offspring at each age.
Trend for normal weight, overweight and obese.
Maternal BMI flexibly modelled
The flexibly modelled dose–response association between maternal BMI in early pregnancy and asthma in the offspring up to 16 years (n = 3290) adjusted for model 1 covariates is illustrated in Fig. 3. Maternal BMI was rounded to whole numbers and BMI 20 kg/m2 was set as the reference. Increasing values was progressively associated with higher ORs of asthma in the offspring up to 16 years. Among mothers with a BMI below 20 kg/m2, there was an indication of an increased risk, although the association was uncertain with broad confidence intervals. Also, there was no significant evidence of departure from linearity (P = 0.18).
Figure 3.
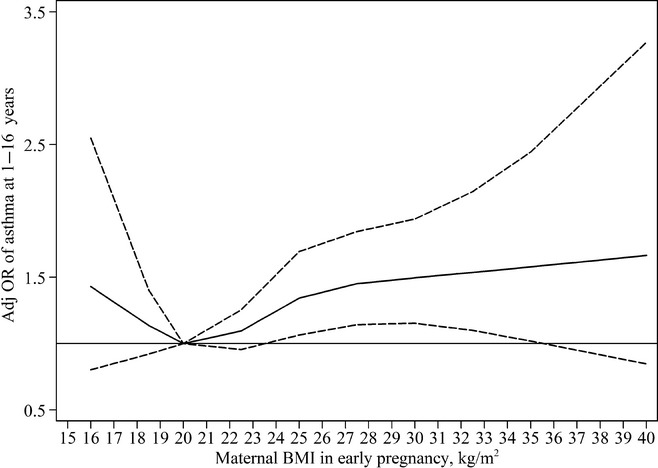
Adjusted odds ratios (adj OR) of prevalent asthma in the offspring at 1–16 years (solid line) by maternal body mass index (BMI), flexibly modelled using restricted cubic splines with four knots (at 19, 21, 23, 29 kg/m2). Data were fitted using generalized estimating equation models. Dashed lines represent 95% confidence interval. Horizontal line represents OR equal to one.
Causal inference test
The results of the CIT on overweight in the offspring at 16 years as a potential mediator in the association between maternal BMI in early pregnancy and asthma in the offspring are found in Table 4. Two of four criteria of the test were fulfilled and two criteria were borderline significant. Maternal BMI was close to significantly associated with asthma at 16 years (criterion 1), but not after adjusting for overweight in the child at 16 years (criterion 4). Maternal BMI was further associated with overweight in the child at 16 years after adjusting for asthma at 16 years (criterion 2). The association between overweight and asthma at 16 years including adjustment for maternal BMI (criterion 3), however, fell out just below the significance level. Taken together, this indicates that the child’s overweight, at least partly, might be a mediator in the association between maternal BMI in early pregnancy and asthma at 16 years.
Table 4.
Causal inference test (CIT) of overweight in the offspring at 16 years as a potential mediator in the association between maternal body mass index (BMI) in early pregnancy and asthma in the offspring at 16 years among mothers with BMI ≥ 18.5 (N = 2110)
| Test | OR | 95% CI |
|---|---|---|
| Maternal BMI† is associated with asthma | 1.27 | 0.99–1.63 |
| Maternal BMI† is associated with overweight in the child adjusted for asthma | 2.50 | 2.09–2.98 |
| Overweight in the child is associated with asthma adjusted for maternal BMI | 1.52 | 0.99–2.34 |
| Maternal BMI† is not associated with asthma adjusted for overweight in the child. | 1.19 | 0.91–1.54 |
Odds ratio (OR) and 95% confidence interval (CI) are obtained from logistic regression analyses and are additionally adjusted for gender, maternal age, parental history of allergic disease, older siblings, socio-economic status and maternal smoking in pregnancy and/or at baseline.
Outcome represents 5 kg/m2 increase in maternal BMI.
Discussion
In the present population-based birth cohort of 3294 children, we examined whether maternal BMI in early pregnancy is associated with the development of allergic disease throughout childhood up until 16 years of age. Maternal BMI was associated with an increased risk of asthma, while no association was observed for rhinitis, eczema or sensitization. The risk was attributed to persistent asthma and not early transient asthma. Categorizing of maternal BMI revealed a significant association for obese, but not overweight mothers. The flexibly modelled dose–response association between maternal BMI and asthma confirmed the results observed in the categorical and the linear analyses, with a rather consistent continuous association starting from the lower range of normal weight. Although there was no evidence for nonlinearity, the association might mainly apply to women of normal weight and above, as the categorical and the spline model suggested an increased risk among underweight mothers. When adjusting for overweight in the offspring, the estimated ORs were reduced, suggesting that part of the association is mediated through the child’s own overweight. To further explore a potential mediating role of the child’s weight status in the association between maternal BMI and asthma in the offspring, we applied a CIT that confirmed this suggestion. Birth weight, mode of delivery, breastfeeding and newborn respiratory diagnoses did not explain the association between maternal BMI and asthma.
Our findings confirm the results of other studies that have linked maternal BMI to an increased risk of asthma or wheeze in the offspring [9–11,13,15]. We had the ability to investigate the association with asthma phenotypes and found that the association only was present for persistent asthma, which suggests that maternal BMI is a risk factor for a more problematic and clinically relevant asthma. This finding contradicts the result of a previous study that found a significant association for transient, but not persistent wheeze, although this study only based their definition on two time points up to 6 years of age [14].
When adjusting for overweight in the child, we observed reduced associations between maternal BMI and asthma, suggesting that the risk – at least partly – is mediated through the child’s own overweight. The attenuation appeared to be more pronounced in the older ages, indicating that the child’s BMI might be a more important factor in the association for school-age children, compared with younger children. This was further supported by the CIT suggesting that overweight in the child might be a mediator in the association between maternal BMI in early pregnancy and asthma in the offspring at 16 years. However, as all parts of the CIT were not definitely fulfilled (e.g. overweight was not significantly associated with asthma), the interpretation of the test is not straightforward. Also, as the estimate was not fully attenuated towards null when adjusting for the child’s overweight in the fourth step, we cannot rule out independent effects off maternal BMI also at 16 years. In previous studies that have adjusted for the child’s BMI, the association was not changed or only slightly attenuated [10,13–15]. This might be due to that most of these studies included younger children, or that self-reported BMI commonly was used.
Our results for rhinitis, eczema and sensitization are in line with the results from two previous cohorts showing no association between maternal BMI and childhood eczema, hayfever [9] or sensitization [15]. Together with the present study, these results indicate that maternal BMI is associated with pathways related to the respiratory system in the offspring. Obesity during pregnancy has also been associated with metabolic and birth complications (e.g. gestational diabetes, preeclampsia and preterm labour), which can cause health consequences, including overweight, in the offspring 25. We showed that the association might be explained by overweight passing over from the mother to the child. However, adjusting for birth-related factors such as birth weight, Caesarean section and newborn respiratory diagnosis did not affect the association between maternal BMI and asthma.
The association between maternal BMI and asthma in the offspring may be mediated through hormones or inflammatory factors such as leptin, TNF-α and cortisol [26–28], although the link between these markers and asthma in the offspring requires further investigation [4]. It is also possible that in utero factors associated with obesity influence fetal programming through epigenetic modifications predisposing the offspring to an increased risk of asthma. In mice models, maternal diet has been shown to affect methylation of genes that are associated with airway inflammation [29]. More studies are, however, needed to determine the mechanism behind the association between maternal BMI and offspring asthma.
The strengths of the present study include the population-based prospective birth-cohort design with repeated assessments of outcome up to 16 years of age, the large study sample size and the high response rate.
Some potential limitations require attention. Although we include 81% of the original cohort in our analyses, the possibility of selection bias cannot be ruled out completely. However, the children included in our analyses were comparable with the children in the original cohort regarding distribution of background characteristics such as parental history of allergic disease, socio-economic status and maternal smoking. Moreover, maternal BMI in early pregnancy was comparable to that in the general population, with a mean BMI of 22.9 kg/m2 in our study compared with 23.3 kg/m2 among all women in Stockholm in 1995 [30].
Maternal weight was measured or ascertained by trained nurses, which is a strength compared with many previous studies as weight usually is completely self-reported [9,13,15]. Although BMI was assessed during and not before pregnancy, it is likely to be very similar to pre-pregnant BMI. A study of 638 US women, showed a median weight gain of 1.6 kg at week 10 in pregnancy, which would only affect BMI marginally [31]. Also, as the exposure was assessed before onset of allergic symptoms, any misclassification is likely to be non-differential in relation to the outcomes.
The information on asthma, rhinitis and eczema is based on parental reports collected repeatedly throughout follow-up. Parental questionnaires are widely used and have shown to be consistent with everyday symptoms recorded in diaries [32], as well as with a physician diagnosis of asthma [33]. In addition to parental-reported symptoms, we also used repeated measures of sensitization to common allergens to assess allergic disease.
Although taking several variables previously shown to be important confounders such as maternal smoking and socio-economic status into account, we cannot rule out the role of residual or unmeasured confounding. Studying the association between prenatal characteristics and allergic disease in the offspring is challenging as several factors might be involved in disease development. As in many previous studies, we lack information on maternal diet and physical activity, factors that may both serve as markers for obesity and have a role in allergic predisposition. In addition, we were not able to examine the influence of gestational weight gain, which has been associated with asthma symptoms in the offspring [9,10]. However, gestational weight gain is usually lower among obese compared with normal weight subjects [34]. If a high gestational weight gain is associated with asthma in the offspring, taking this variable into account would thus possibly strengthen rather than attenuating the association between maternal BMI and asthma.
In summary, in this population-based birth cohort, we observed an association between maternal BMI in early pregnancy and asthma, but not rhinitis, eczema and sensitization in the offspring up to the age 16 years. The association seems to be partly, but not fully, mediated through overweight in the offspring. These findings have significant public health importance as asthma is the most common chronic disease among children and contributes to a large burden for both the individual and the society [35]. Obesity, both during pregnancy and childhood, is a modifiable risk factor, and prevention strategies might be important to be able to reduce the prevalence of asthma.
Acknowledgments
We would like to thank all participants and staff in the BAMSE study. The authors are also indebted to Niklas Andersson for statistical consultation.
Sources of funding: The study was funded by the Swedish Research Council, the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet, the Swedish Heart and Lung Foundation, the Swedish Research Council for Health, Working Life and Welfare, the Swedish Research Council Formas, the Swedish Asthma and Allergy Foundation, the European Commission’s Seventh Framework 29 Program MeDALL under grant agreement No. 261357 and the Strategic Research Program (SFO) in Epidemiology at Karolinska Institutet.
Conflicts of interest: The authors declare no conflict of interest.
Supporting Information
Table S1. Distribution of selected exposure characteristics among children in the total BAMSE cohort and among children included in the analyses.
Data S1. Definition of health outcomes.
References
- Asher MI, Montefort S, Bjorksten B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–43. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- Hansen TE, Evjenth B, Holt J. Increasing prevalence of asthma, allergic rhinoconjunctivitis and eczema among schoolchildren: three surveys during the period 1985–2008. Acta Paediatr. 2013;102:47–52. doi: 10.1111/apa.12030. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:1–253. [PubMed] [Google Scholar]
- Bergstrom A, Melen E. On childhood asthma, obesity and inflammation. Clin Exp Allergy. 2012;42:5–7. doi: 10.1111/j.1365-2222.2011.03902.x. [DOI] [PubMed] [Google Scholar]
- Ford ES. The epidemiology of obesity and asthma. J Allergy Clin Immunol. 2005;115:897–909. doi: 10.1016/j.jaci.2004.11.050. ; quiz 10. [DOI] [PubMed] [Google Scholar]
- Peters JL, Boynton-Jarrett R, Sandel M. Prenatal environmental factors influencing IgE levels, atopy and early asthma. Curr Opin Allergy Clin Immunol. 2013;13:187–92. doi: 10.1097/ACI.0b013e32835e82d3. [DOI] [PubMed] [Google Scholar]
- Rusconi F, Galassi C, Forastiere F, et al. Maternal complications and procedures in pregnancy and at birth and wheezing phenotypes in children. Am J Respir Crit Care Med. 2007;175:16–21. doi: 10.1164/rccm.200512-1978OC. [DOI] [PubMed] [Google Scholar]
- Guerra S, Sartini C, Mendez M, et al. Maternal prepregnancy obesity is an independent risk factor for frequent wheezing in infants by age 14 months. Paediatr Perinat Epidemiol. 2013;27:100–8. doi: 10.1111/ppe.12013. [DOI] [PubMed] [Google Scholar]
- Harpsoe MC, Basit S, Bager P, et al. Maternal obesity, gestational weight gain, and risk of asthma and atopic disease in offspring: a study within the Danish National Birth Cohort. J Allergy Clin Immunol. 2013;131:1033–40. doi: 10.1016/j.jaci.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Leermakers ET, Sonnenschein-van der Voort AM, Gaillard R, et al. Maternal weight, gestational weight gain and preschool wheezing: the Generation R Study. Eur Respir J. 2013;42:1234–43. doi: 10.1183/09031936.00148212. [DOI] [PubMed] [Google Scholar]
- Lowe AJ, Ekeus C, Braback L, Rajaleid K, Forsberg B, Hjern A. Impact of maternal obesity on inhaled corticosteroid use in childhood: a registry based analysis of first born children and a sibling pair analysis. PLoS One. 2013;8:e67368. doi: 10.1371/journal.pone.0067368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons EC, Patel K, Tran BT, Littman AJ. Maternal pre-gravid obesity and early childhood respiratory hospitalization: a population-based case-control study. Matern Child Health J. 2013;17:1095–102. doi: 10.1007/s10995-012-1092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SP, Rodriguez A, Little MP, et al. Associations between pre-pregnancy obesity and asthma symptoms in adolescents. J Epidemiol Community Health. 2012;66:809–14. doi: 10.1136/jech.2011.133777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike KC, Inskip HM, Robinson SM, et al. The relationship between maternal adiposity and infant weight gain, and childhood wheeze and atopy. Thorax. 2013;68:372–9. doi: 10.1136/thoraxjnl-2012-202556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtens S, Wijga AH, Brunekreef B, et al. Maternal overweight before pregnancy and asthma in offspring followed for 8 years. Int J Obes (Lond) 2010;34:606–13. doi: 10.1038/ijo.2009.194. [DOI] [PubMed] [Google Scholar]
- Watson PE, McDonald BW. Subcutaneous body fat in pregnant New Zealand women: association with wheeze in their infants at 18 months. Matern Child Health J. 2013;17:959–67. doi: 10.1007/s10995-012-1124-6. [DOI] [PubMed] [Google Scholar]
- Melen E, Granell R, Kogevinas M, et al. Genome-wide association study of body mass index in 23 000 individuals with and without asthma. Clin Exp Allergy. 2013;43:463–74. doi: 10.1111/cea.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmatz M, Madan J, Marino T, Davis J. Maternal obesity: the interplay between inflammation, mother and fetus. J Perinatol. 2010;30:441–6. doi: 10.1038/jp.2009.182. [DOI] [PubMed] [Google Scholar]
- Wickman M, Kull I, Pershagen G, Nordvall SL. The BAMSE project: presentation of a prospective longitudinal birth cohort study. Pediatr Allergy Immunol. 2002;13(Suppl 15):11–3. doi: 10.1034/j.1399-3038.13.s.15.10.x. [DOI] [PubMed] [Google Scholar]
- Center for Epidemiology, Swedish National Board of Health and Welfare. The Swedish medical birth registry: a summary of content and quality. Stockholm: Swedish National Board of Health and Welfare; 2003. . Availiable at http://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/10655/2003-112-3_20031123.pdf (Last accessed 19 December, 2013) [Google Scholar]
- Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–3. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmaurice GMLN, Ware JH. Applied longitudinal analysis. 2nd edn. Hoboken: Wiley; 2011. [Google Scholar]
- Orsini N, Greenland S. A procedure to tabulate and plot results after flexible modeling of a quantitative covariate. Stata J. 2011;11:1–29. [Google Scholar]
- Millstein J, Zhang B, Zhu J, Schadt EE. Disentangling molecular relationships with a causal inference test. BMC Genet. 2009;10:23. doi: 10.1186/1471-2156-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogev Y, Visser GH. Obesity, gestational diabetes and pregnancy outcome. Semin Fetal Neonatal Med. 2009;14:77–84. doi: 10.1016/j.siny.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Halonen M, Lohman IC, Stern DA, Ellis WL, Rothers J, Wright AL. Perinatal tumor necrosis factor-alpha production, influenced by maternal pregnancy weight gain, predicts childhood asthma. Am J Respir Crit Care Med. 2013;188:35–41. doi: 10.1164/rccm.201207-1265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malli F, Papaioannou AI, Gourgoulianis KI, Daniil Z. The role of leptin in the respiratory system: an overview. Respir Res. 2010;11:152. doi: 10.1186/1465-9921-11-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RJ, Fisher K, Chiu YH, et al. Disrupted prenatal maternal cortisol, maternal obesity, and childhood wheeze. Insights into prenatal programming. Am J Respir Crit Care Med. 2013;187:1186–93. doi: 10.1164/rccm.201208-1530OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth JW, Maruoka S, Boon K, et al. In utero supplementation with methyl donors enhances allergic airway disease in mice. J Clin Invest. 2008;118:3462–9. doi: 10.1172/JCI34378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Swedish National Board of Health and Welfare. Pregnancies, deliveries and newborn infants – the Swedish medical birth register 1973–2010. Stockholm: Swedish National Board of Health and Welfare; 2009. , Appendix 1. Availiable at http://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/17862/2009-12-11_Bilaga1.pdf (Last accessed 19 December, 2013) [Google Scholar]
- Hutcheon JA, Platt RW, Abrams B, Himes KP, Simhan HN, Bodnar LM. A weight-gain-for-gestational-age z score chart for the assessment of maternal weight gain in pregnancy. Am J Clin Nutr. 2013;97:1062–7. doi: 10.3945/ajcn.112.051706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley MJ, Chalmers A, Clover K, Gibson PG, Toneguzzi R, Lewis PR. Symptoms of asthma: comparison of a parent-completed retrospective questionnaire with a prospective daily symptom diary. Pediatr Pulmonol. 2003;36:509–13. doi: 10.1002/ppul.10360. [DOI] [PubMed] [Google Scholar]
- Jenkins MA, Clarke JR, Carlin JB, et al. Validation of questionnaire and bronchial hyperresponsiveness against respiratory physician assessment in the diagnosis of asthma. Int J Epidemiol. 1996;25:609–16. doi: 10.1093/ije/25.3.609. [DOI] [PubMed] [Google Scholar]
- The Swedish National Food Agency. Energi och vikt vid graviditet och amning. Vetenskapligt underlag inför revideringen av Livsmedelsverkets kostråd för gravida och ammande. 2008 Report 25-2008. Availiable at http://www.slv.se/upload/dokument/rapporter/mat_naring/Energi_vikt_graviditet_amning_rapp25.pdf (Last accessed 13 March, 2014) (in Swedish)
- van Schayck OC. Global strategies for reducing the burden from asthma. Prim Care Respir J. 2013;22:239–43. doi: 10.4104/pcrj.2013.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Distribution of selected exposure characteristics among children in the total BAMSE cohort and among children included in the analyses.
Data S1. Definition of health outcomes.


