Short abstract
Heterogeneity of material properties is an important potential contributor to bone fracture resistance because of its putative contribution to toughness, but establishing the contribution of heterogeneity to fracture risk is still in an incipient stage. Experimental studies have demonstrated changes in distributions of compositional and nanomechanical properties with fragility fracture history, disease, and pharmacologic treatment. Computational studies have demonstrated that models with heterogeneous material properties predict apparent stiffness moderately better than homogeneous models and show greater energy dissipation. Collectively, these results suggest that microscale material heterogeneity affects not only microscale mechanics but also structural performance at larger length scales.
Introduction
Whole-bone fracture resistance depends on bone quantity (as assessed clinically by bone mineral density, BMD) and bone quality, which encompasses the additional geometric, microarchitectural, and material factors that contribute to skeletal integrity. Although BMD is currently used clinically to predict fracture risk, variation in BMD explains only 30–60% of the observed variation in bone strength [1,2,3,4,5,6,7]. As the limitations of BMD in predicting fracture have become apparent, clinical and scientific interest in bone quality factors have increased [8]. Recently, average tissue mineral and matrix properties assessed by Fourier transform infrared imaging (FTIRI), including the mineral to matrix ratio, the mineral crystallinity, and the collagen maturity, were identified as bone quality factors that contribute to fracture risk independently of changes in BMD [9]. This study demonstrated that average tissue compositional properties assessed at the microscale played a critical role in the macroscopic fracture behavior of whole bones. However, the material properties of bone are not homogeneous; rather, spatial variations in material properties within the tissue arise from the composite structure (Fig. 1) and continuous remodeling activity.
Fig. 1.
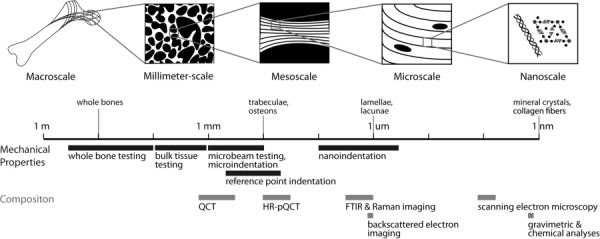
Diagram of the physiologically relevant length scales at which to measure heterogeneity in trabecular bone. This review focuses on heterogeneity at the millimeter-scale (bulk tissue), the mesoscale (single trabeculae), and the microscale. Techniques that can be used to assess both mechanical and compositional properties of bone are included, along with the length scale at which they are relevant. Adapted with permission from Ref. [8], License No. 3480580167028.
Nanoscale or microscale heterogeneity, i.e., spatial variation in material properties, is emerging as a potentially important contributor to bone quality because of its putative contribution to tissue-level toughening mechanisms [10]. The microstructure of bone encompasses numerous toughening mechanisms that act across multiple hierarchical levels. From a materials science perspective, heterogeneity of bone tissue material properties is expected to provide intrinsic toughening by promoting plasticity that resists crack initiation and propagation, in addition to “extrinsic” toughening provided by lamellar or osteonal interfaces that may deflect cracks once they have begun to propagate [11]. However, the current understanding of the contribution of heterogeneity of material properties to fracture risk is still in an incipient stage, in part due to the multiple levels of microstructural hierarchy involved.
Over the past decade, experimental evidence highlighting changes in microscale heterogeneity of tissue properties with patient fragility fracture history, disease, and pharmacologic treatment has suggested that these properties might be important sentinels of altered bone quality in pathologic tissue [8,12,13,14,15]. In parallel, analytical and computational studies incorporating heterogeneous material properties have begun to provide mechanistic insight into the effects of microscale material property variations on bone structural performance at the bulk tissue level [16,17,18,19,20].
Here, we review studies examining the contribution of heterogeneity of material properties to the structural behavior of cancellous bone. We focus primarily on experimental studies assessing the material properties of bone tissue in a spatially resolved fashion and secondarily on computational studies that integrate tissue-level material properties across multiple hierarchical levels. In this review, we do not address factors that contribute exclusively to cortical bone behavior, such as cortical microarchitecture, as reviewed elsewhere [21,22,23]. We do not address heterogeneity of trabecular architecture and density, although these properties clearly affect bone behavior at a structural level [24,25,26]. Here, we examine studies that probe bone compositional and mechanical properties across multiple hierarchical levels of the microstructure (Fig. 1). For consistency, we adopt the following terminology: (1) “microscale” refers to structures in bone approximately 1–10 μm, for instance, lamellae; (2) “mesoscale” refers to structures on the order of 100 μm, for instance, individual trabeculae; and (3) “millimeter scale” refers to structures approximately 1–10 mm or bulk cancellous tissue.
Experimental Assessment of Tissue Material Heterogeneity
To characterize the heterogeneity of bone tissue material properties at these length scales, a variety of materials characterization techniques can be used, as outlined in Fig. 1. At the microscale, mechanical properties can be directly assessed only by nanoindentation, which can measure both elastic and inelastic properties [27,28,29,30]. At the mesoscale, reference point indentation measures mechanical response to impact loading in vivo [31,32], and three-point bend tests on individual trabeculae or cortical microbeams assess stiffness, strength, and fracture properties [33,34,35]. Finally, at the millimeter scale, bulk cancellous tissue can be compressed to assess apparent stiffness, yield stress, and ultimate strength [36]. To measure composition of bone tissue at the microscale, techniques include scanning electron microscopy, quantitative backscattered electron imaging (qBEI), FTIRI, and Raman imaging. Of these techniques, the electron microscopy-based techniques assess only the mineralized components of the tissue; Raman imaging and FTIRI are the only techniques capable of assessing collagen matrix properties [37,38]. However, SEM and qBEI are higher throughput techniques that allow analysis of greater specimen numbers for large studies [39]. These techniques can be combined to generate complementary data on bone quality. The strengths and limitations of each of these methods have been reviewed previously [40].
Microscale.
Application of materials characterization techniques to bone tissue have allowed assessment of bone compositional and nanomechanical properties at spatial resolutions ∼1 μm. These characterization techniques have been used in a variety of studies evaluating the effect of several variables on tissue heterogeneity, including fragility fracture history, disease status, and pharmacologic treatment history (Table 1). Taken together the results of these studies suggest that microscale heterogeneity of bone tissue affects not only microscale mechanical properties but also structural performance at larger length scales.
Table 1.
Summary of the microscale heterogeneity data on changes in material parameter distribution widths associated with fragility fracture status, osteoporosis disease states, and antiresorptive treatment.
| Cause of change | Parameter (assessment method) | Distribution width change (+, −, or no change) | Reference |
|---|---|---|---|
| Fragility fracture status | Mineralization (qBEI) | − | Ciarelli et al. [41] |
| + | Tamminen et al. [44] | ||
| Mineralization (microradiography) | + | Bousson et al. [43] | |
| Mineral to matrix ratio (FTIRI) | − | Gourion-Arsiquaud et al. [12] | |
| Carbonate to phosphate ratio (FTIRI) | − | Gourion-Arsiquaud et al. [12] | |
| Crystallinity (FTIRI) | + | Gourion-Arsiquaud et al. [12] | |
| − | Wang et al. [42] | ||
| Collagen maturity (FTIRI) | − | Wang et al. [42] | |
| Osteoporosis (pooled) | Mineralization (qBEI) | − | Tjhia et al. [48] |
| + | Roschger et al. [15] | ||
| + | Busse et al. [49] | ||
| Mineral to matrix ratio (FTIRI) | − | Boskey et al. [47] | |
| Crystallinity (FTIRI) | − | Boskey et al. [47] | |
| Hardness (Nanoindentation) | − | Tjhia et al. [48] | |
| Osteoporosis (low-turnover) | Mineralization (qBEI) | + | Ciarelli et al. [41] |
| Mineral to matrix ratio (FTIRI) | No difference | Boskey et al. [13] | |
| Crystallinity (FTIRI) | + | Boskey et al. [13] | |
| Osteoporosis (high-turnover) | Mineralization (qBEI) | No difference | Ciarelli et al. [41] |
| Mineral to matrix ratio (FTIRI) | − | Boskey et al. [13] | |
| Crystallinity (FTIRI) | + | Boskey et al. [13] | |
| Antiresorptive treatment | Mineralization (qBEI) | − | Roschger et al. [14] |
| − | Roschger et al. [15] | ||
| Mineral to matrix ratio (FTIRI) | − | Gourion-Arsiquaud et al. [52] | |
| − | Donnelly et al. [53] | ||
| Crystallinity (FTIRI) | − | Gourion-Arsiquaud et al. [52] | |
| Collagen maturity (FTIRI) | − | Donnelly et al. [53] |
Association Between Fracture Status and Microscale Tissue Heterogeneity.
In human patients, comparison of bone tissue properties in cohorts of patients with and without fragility fractures is the only experimental approach that enables microscale tissue material properties to be related to whole-bone structural behavior in vivo. Assessments of heterogeneity in patient groups with and without fragility fractures using several analytical techniques have shown differences between groups in the widths of material property distributions. However, while most studies show significant differences in distribution widths between fracture and nonfracture groups, their results are in conflict regarding which group is characterized by wider distributions of material properties.
Several studies comparing properties of tissue from patients with and without histories of fragility fractures have observed narrower distributions of tissue material properties in the fracture group versus the nonfracture group (Fig. 2). When the mineral content of bone tissue from patients with and without fractures was assessed with qBEI, cancellous iliac crest tissue from patients with fractures had a 9% smaller coefficient of variation than patients without fractures. This result held for both superficial and deep remodeling packets of trabeculae from the iliac crest [41]. Similarly, cancellous and cortical bone from the femoral necks of patients with fragility fractures characterized with FTIRI had narrower distributions of mineral to matrix ratio and carbonate to phosphate ratio compared to fracture-free cadaveric controls [12]. However, cortical tissue from the fracture group had wider distributions of crystallinity values compared to those of the nonfracture group [12]. Finally, FTIRI studies of iliac crest biopsies from perimenopausal women with and without fragility fractures recently showed that the distributions of cortical crystallinity and trabecular collagen maturity were, respectively, 26% and 33% narrower in the fracture group relative to the nonfracture group [42]. These data sets point to an emerging trend toward reduced heterogeneity of material properties in patients with a history of fragility fracture.
Fig. 2.
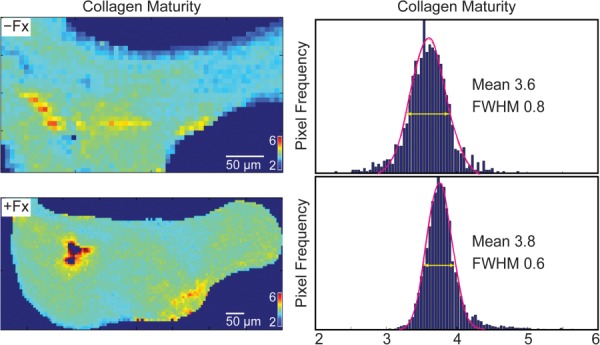
Representative FTIR images and pixel histograms of collagen maturity in trabeculae from perimenopausal women without history of fragility fracture (−Fx) and with history of fragility fracture (+Fx). The mean and the full width at half maximum values of the Gaussian fits to the distributions are indicated on each histogram.
In contrast, a substantial minority of studies comparing mineralization distributions in tissue from patients with and without fragility fractures have observed broader distributions of mineral properties in the fracture group versus the nonfracture group. A microradiograpic study of the femoral neck showed that the heterogeneity of mineralization was increased in patients with a history of fragility fracture versus nonfracture controls [43]. In a qBEI study of iliac crest biopsies from pediatric patients with idiopathic osteoporosis who had had at least one vertebral fragility fracture, the fracture group had a similar mean mineralization, but a significantly higher heterogeneity of mineralization, compared to a previously assessed normal BMD distribution (BMDD) for children and young adults [44,45]. Thus, consistent trends in bone tissue heterogeneity with fracture status have not yet coalesced, in part because variation in anatomic site, patient age, and experimental technique likely contributes to the observed variation in material property distributions.
Effect of Osteoporosis on Microscale Tissue Heterogeneity.
Osteoporosis alters the properties of bone tissue, and these changes in tissue properties contribute to fracture risk independently of changes in BMD [9]. Comparison of distributions of material properties in tissue from osteoporotic patients to those in healthy controls has also revealed altered microscale heterogeneity in osteoporotic tissue. However, just as in the studies comparing tissue from patients with fragility fractures to that of nonfracture controls, there is an apparent divergence in the results between those that find that tissue from patients with osteoporosis has narrower distributions of material properties compared to that of healthy patients, and those that find that tissue from osteoporotic patients has wider distributions of material properties compared to controls. This divergence in results could be due to the fact that bone turnover, expected to have a substantial effect on bone tissue heterogeneity, has not been assessed in the majority of studies that compare patients with and without osteoporosis.
FTIRI studies of iliac crest biopsies revealed narrower distributions of mineral crystallinity and mineral to matrix ratio in cortical bone tissue from untreated osteoporotic patients compared to healthy age-matched controls [46,47]. A comparison of nanomechanical and compositional properties of iliac crest biopsies from osteoporotic patients with age-matched healthy controls using qBEI and nanoindentation showed an 8% lower standard deviation in trabecular hardness and a corresponding trend toward a reduced standard deviation of mineralization that did not reach statistical significance in osteoporotic versus control bone. No significant differences in the mean mechanical properties or in tissue mineral content were observed [48].
On the other hand, qBEI analysis of iliac crest biopsies of osteoporotic patients and those of healthy controls revealed a wider BMDD in the osteoprotic tissue compared to that of the control bone [15]. Similarly, qBEI analysis of cadaveric bone from the iliac crest and vertebrae showed that individual trabecular rods from osteoporosis patients, while thinner, were more highly mineralized and had a wider distribution of mineralization than control trabeculae [49]. In addition, this study was unique in mechanically testing individual trabeculae along with assessing compositional properties. In three-point bending tests of individual dry trabeculae, even after normalizing for smaller size, osteoporotic trabeculae had significantly lower stiffness, strength, and work to fracture. While these results from dry trabeculae likely do not reflect absolute in vivo values, because drying increases stiffness and decreases work to fracture [50,51], the relative differences between osteoporotic and non-osteoporotic groups may be preserved [49]. These differences in material property distributions may reflect a reparative response. In osteoporotic bone, overloading may arise in the thinner and sparser trabecular architecture, which could cause microdamage in the bone and stimulate resorption and microfracture repair. The subsequent increase in new bone formation would be expected to increase the heterogeneity of mineralization by adding new low-mineral-content tissue to the mineralization distributions.
While there appears to be a contradiction in the data regarding both fracture and osteoporosis studies, this contradiction may be explained in part by controlling for turnover. In the absence of an imbalance of bone formation and resorption, increased turnover generates increased compositional heterogeneity in bone tissue. However, in osteoporotic patients, resorption exceeds formation, and differences in rates of turnover across patient groups affect not only the measured tissue heterogeneity across groups but can also shift the mean values of the distributions of material properties. For instance, when qBEI mineralization data from patients with and without fragility fractures was further subdivided into low-turnover and high-turnover groups, only the low-turnover subgroup showed a significantly wider distribution in the nonfracture group versus the fracture group [41]. Similarly, when iliac crest biopsies from patients with low-turnover and high-turnover osteoporosis were compared to healthy controls using FTIRI, the standard deviation of the mineral to matrix ratio was significantly lower in high-turnover biopsies compared to control biopsies. However, the standard deviation in low-turnover biopsies was lower but did not reach statistical significance [13]. In the three FTIRI parameters studied (mineral to matrix ratio, carbonate substitution, crystallinity, and acid phosphate content), coefficients of variation showed trends toward the narrowest distributions being high-turnover osteoporosis biopsies, and the widest distributions being from normal controls, with low-turnover osteoporosis biopsies having intermediate values. While these trends were not statistically significant, they were consistent across all parameters. The one exception to these trends was crystallinity, which was greater in both high- and low-turnover osteoporosis patients [13]. Generally, these studies point to trends toward wider distributions with lower turnover disease states and narrower distributions with high-turnover disease states. The trends toward narrower distributions in high-turnover osteoporosis suggest that in high-turnover disease states, resorption outpaces formation, leading to disproportionate loss of relatively recently formed tissue of low mineral content and collagen maturity on bone surfaces, resulting in narrowed distributions of tissue material properties.
In contrast, a study of juvenile patients with vertebral fractures due to idiopathic osteoporosis found that FTIRI parameter distribution widths increased with an increase in serum bone turnover markers [44]. However, interpretation of results of this study are complicated by the potential effects of fracture healing on turnover, the young patient population, and the putatively pathologic baseline turnover values in this patient population. In fact, the authors concluded that this particular group of patients likely had suppressed bone turnover prior to their vertebral fractures, because turnover rates immediately after fracture were not above average for a healthy control. In growing patients biopsied within 18 months of fragility fracture, bone tissue heterogeneity is expected to increase due to the increase in turnover associated in remodeling, particularly when the imbalance in resorption and formation associated with osteoporosis may be attenuated by skeletal growth.
Effect of Antiresorptive Treatment on Microscale Tissue Heterogeneity.
Antiresorptive agents used to treat osteoporosis are expected to alter tissue property distributions through their modulation of bone turnover. In particular, studies of postmenopausal osteoporosis patients treated with bisphosphonates using multiple assessment techniques have shown that bisphosphonate treatment narrows the distributions of tissue material properties. Iliac crest tissue of patients taking alendronate assessed with qBEI had mean mineralization higher than that of patients taking placebo and had significantly narrower mineralization distribution compared to placebo group [14,15]. A canine model assessed with FTIRI showed that several dose levels of alendronate and risedronate increased the mean values of the mineral to matrix ratio and the collagen maturity in both cortical and cancellous bone compared to the vehicle control group. The heterogeneity of the mineral to matrix ratio and crystallinity was also significantly lower in the alendronate- and risedronate-treated groups in both cortical and cancellous bone [52]. Similarly, in an FTIRI analysis of biopsies from patients taking alendronate, risedronate, and ibandronate, the distribution widths of cortical mineral crystallinity and collagen maturity were significantly narrower compared to bisphosphonate-naïve controls [53]. Thus, by reducing bone turnover, antiresorptive therapy decreases formation of new bone that would otherwise broaden the distribution of tissue material properties, and the distributions of bone tissue properties becomes narrower relative to those in untreated tissue.
Mesoscale.
Trabecular bone, while lacking some of the extrinsic toughening mechanisms associated with cortical bone (as reviewed previously in Refs. [11,54,55]), has heterogeneity at the length scale of hundreds of microns that affects the mechanical properties at larger length scales. The primary effect of compositional heterogeneity at the length scale of single trabeculae is the variation in tissue material properties arising from variation in tissue age. The variations in material properties from the outer, more recently formed trabecular surface, to the older inner core of the trabeculae creates a gradient similar to concentric composite beams, with an older, stiffer core and a newer, more compliant shell.
High-resolution (HR) spatially resolved qBEI and micro computed tomography (CT) measurements of mineral content in trabecular bone have revealed gradients of mineralization from the inside to the outside of trabeculae [16,56,57,58]. Specifically, trabecular remodeling packets that are in contact with the trabecular surface are less mineralized than packets that are in the interior [41,59]. A similar gradient has been shown to exist in collagen cross-linking through FTIRI studies, with greater collagen maturity on the interior of trabeculae than at the surface [47,60]. In parallel with the gradient of compositional properties with distance from the trabecular surface, there is a gradient of mechanical properties, with nanoindentation studies showing that the inner trabecular packets are stiffer than those at the surface [59]. This gradient of compositional and nanomechanical properties arises from differences in tissue age. Remodeling packets near the center of trabeculae are older than those near the surface, which have been recently remodeled.
This variation of material properties across the thickness of a single trabecula is critical to the mechanical properties of cancellous bone because it acts as an intrinsic toughening mechanism. In trabeculae that are damaged, microdamage accumulates in the highly mineralized, stiffer core, while the less mineralized and stiff surface layer hinders crack propagation and maintains the structural integrity of the trabecula [61,62].
The importance of the gradient in trabecular composition as an intrinsic toughening mechanism is exemplified in studies of iliac crest biopsies from patients with high- and low-turnover osteoporosis. In these cases, it was found that while normal bone showed a large amount of variation in compositional properties from the surface to the core of trabeculae, low-turnover osteoporotic patients showed significantly less variation in properties from the surface to the core, and high-turnover osteoporotic patients showed no detectable variation in properties [13]. The loss of this toughening mechanism of cancellous bone could therefore contribute to the increased fracture risk in osteoporotic patients. Conversely, restoration of normal turnover rates and balance between formation and resorption could reestablish a broad distribution of tissue properties and potentially restore this toughening mechanism to diseased tissue.
Millimeter-Scale.
On the scale of a whole-bone cross section, trabeculae are loaded variably across the cross section, particularly in long bones subjected to complex bending loads during physiologic loading. When whole bones are loaded in bending, certain regions of the cross section are subjected to compression, while others are subjected to tension. Additionally, variations in microdamage arising from the differential loading are expected to cause local variations in remodeling. This variation in both strain and remodeling within the bone is expected to give rise to complex spatial variation in material properties, but the material properties of trabeculae subjected to different loading modes have not been examined systematically until recently. In a study of deer calcanei, trabecular regions subjected to compression during physiologic loading had 3% greater mineral content than those in tension, suggesting an adaptive response in tissue material properties to the loading environment [63]. When the heterogeneity of the mineralization was examined in these areas, differences between compressive and tensile trabecular regions were observed only in juvenile growing bone—in adult bone, there were no significant differences in trabecular heterogeneity between tensile and compressive regions.
In human femoral neck samples analyzed with FTIRI as a function of anatomical quadrant, mean FTIRI parameters were similar across quadrants, but there were many differences among quadrants in compositional heterogeneity [12]. Specifically, the heterogeneity of the mineral to matrix ratio was 7–10% greater posteriorly than in the superior and anterior quadrants. The heterogeneity of the mineral crystallinity was also highest posteriorly, with a 12% increase over the superior quadrant. The heterogeneity of the crystallinity was lowest in the inferior quadrant, with a 12–19% reduction in heterogeneity from the superior quadrant. Although the variation in material property distributions across the femoral neck may reflect an adaptive response to the local loading environments, the variation in the heterogeneity is not straightforwardly correlated with loading during walking. The greatest variations in tissue material properties were observed in the posterior quadrant, which during walking lies near the neutral axis, where the bone is under neither tension nor compression [64]. In contrast, in a sideways fall, compression loading is maximized posterosuperiorly [64], where greater heterogeneity may contribute to crack arrest processes.
Computational Modeling Incorporating Experimentally Assessed Tissue Material Properties
While experimental studies are the sole method for direct assessment of bone material heterogeneity, computational modeling has important advantages. Experimentally derived parameters measured at the microscale can be incorporated into multiscale models to predict properties at larger length scales, and the effects of different material property distributions on bone structural performance can be explored while holding other variables constant, enabling isolation of the effects of property heterogeneity on bone structural properties.
Microscale.
Analytical and computational models of the effects of inelastic mechanical property heterogeneity on the properties of cortical bone tissue demonstrate that nano- to microscale heterogeneity in material properties increases toughness. Analytical models of nanoscale variation in mechanical properties suggest that for bone and other biological composites, there is a characteristic length scale of approximately 200 nm at which heterogeneity acts as a toughening mechanism rather than a stress concentrator [65,66]. At this length scale, mineral platelets are insensitive to cracklike flaws and behave similarly to perfect crystals, whereas above this length scale, cracklike flaws in mineralized tissue cause the platelet to fail by stress concentration at crack tips. This was confirmed through finite element models incorporating both arbitrarily heterogeneous nanoscale material properties [65], and experimentally determined heterogeneous material properties [10,19,67]. Finite element analyses (FEA) of cortical bone created from nanomechanical property maps measured by contact atomic force microscopy and nanoindentation demonstrated that a model incorporating heterogeneous elastic properties dissipated more energy than a model incorporating homogeneous elastic properties when inelastic deformation was applied [10]. In an extension of this work, a model incorporating heterogeneous inelastic mechanical properties dissipated more energy than a model incorporating heterogeneous elastic mechanical properties [67]. Thus, models incorporating heterogeneity of elastic and inelastic material properties at a length scale smaller than 200 nm have shown increased energy dissipation, leading to the hypothesis that this nanoscale heterogeneity could be an important intrinsic toughening mechanism in bone.
In addition to studies of nanoscale heterogeneity in bone, computational models of crack propagation in iliac crest biopsies suggest that trabecular bone lamellar-level compositional properties have an important effect on crack propagation [68]. Two-dimensional finite element models created from qBEI maps of transiliac biopsies from osteoporotic and nonosteoporotic patients demonstrated that even when mean mineralization was held constant, the osteoporotic fracture group had a wider mineralization distribution and greater lamellar mineral variation than the healthy control group, leading to increased crack number, but decreased crack length and crack area [68]. Thus, differences in compositional variation in lamellae at the microscale could lead to differences in microcrack propagation in trabecular bone.
Mesoscale.
At the scale of a single trabecula, computational studies have not yet addressed the effect of heterogeneity. Current computational studies at this length scale address the effect of osteoclast remodeling lacunae on trabecular strut mechanics [69,70,71,72] and the effects of loading orientation on trabecular mechanical behavior [73].
Millimeter-Scale.
At the millimeter scale, the trabecular architecture dominates structural properties in experimental studies. Thus, at this length scale, computational modeling is ideally suited to address the effect of microscale material property heterogeneity on larger-scale mechanics, as the material properties in finite element models created from patient data can be varied while keeping architecture constant. The studies presented here share the same general methodology. Micro-CT is used to generate a three-dimensional image of a sample of cancellous bone, with grayscale values representing the x-ray attenuation at each point in the sample. These gray-scale values are then thresholded and converted, through various algorithms, into modulus values for each voxel of the model. The finite element model is then compressed, and the resulting data are validated in some way against experimental results.
Computational studies incorporating experimentally obtained heterogeneous material property values are motivated by computational studies that arbitrarily increased specimen heterogeneity [16,17]. These studies used a voxel-based finite element model to compare cancellous bone with homogenous properties to models in which the coefficient of variation of the modulus had been arbitrarily increased. Homogenous models overpredicted apparent stiffness by up to 20% and underpredicted the percentage of bone that had been damaged, thus indicating the possibility that heterogeneous intraspecimen material properties have a meaningful effect on the mechanical properties of bulk cancellous tissue.
Thus, several groups used varying methods to incorporate heterogeneous tissue properties into finite element models to predict apparent stiffness. When specimen x-ray attenuation was used to scale the modulus values of the elements, a homogeneous model overpredicted experimental compression apparent modulus by up to 20%. A heterogeneous model had at least 13% greater explanatory power for the subset of specimens with the greatest variation in mineralization [18]. However, a specimen-specific homogenous model based on average attenuation was also a better predictor of apparent modulus than a homogeneous model with a reference modulus, with at least 8% more explanatory power [18]. A similar study using synchrotron micro-CT showed that a homogeneous model overpredicted apparent stiffness by 4% compared to a specimen-specific model with heterogeneous material properties [74]. Thus, incorporation of specimen-specific heterogeneous material properties improves prediction of apparent modulus and is most critical for specimens with large variability in tissue modulus.
Incorporating experimentally determined nanomechanical property data, Harrison and coworkers used nanoindentation measurements of specimen modulus to map mechanical properties to voxels, rather than using CT attenuation, and found that a scaled heterogeneous model was a very good predictor of tissue apparent modulus, with an average error of less than 10% compared to experimental results. They also found that the heterogeneous scaled model predicted highest tissue stresses in struts that were observed to fail first experimentally [75]. Furthermore, finite element studies have also demonstrated that models with heterogeneous material properties that scale tissue modulus from CT attenuation have altered distributions of tissue stress and strain as compared to homogenous models [75,76,77]. For example, an increase in the coefficient of variation in tissue modulus from 20% to 50% resulted in up to a 28-fold increase in the volume of failed tissue [67], suggesting that large variations in tissue modulus may substantially alter the failure behavior of cancellous bone.
Finite element models have also been used to examine the effects of larger-scale heterogeneity, by applying a gradient of properties from the inside to the outside of individual trabeculae. When modulus was scaled linearly from CT attenuation, heterogeneous models were 8% less stiff than homogeneous models; however, when a power law relation was used, heterogeneous models were stiffer than their homogeneous counterparts [16,78]. In FEA models incorporating heterogeneous trabecular surface and core properties, specimen-specific models performed better at predicting experimental moduli than a homogenous model, with an average of 9% greater explanatory power [18].
While the FEA studies demonstrate the effect of heterogeneous elastic mechanical properties on the apparent stiffness of cancellous bone, they are limited in only looking at one facet of cancellous bone’s mechanical properties and capture only its elastic response. In predicting the fracture response of bone, toughness and crack growth are extremely important [79]. Analytical models of layered composite structures have demonstrated that in materials with periodically varying moduli, if the variation in modulus is great enough, the soft layers will act as crack arrest mechanisms [20]. Thus, in a layered composite material like lamellar bone, models that incorporate crack growth are important for understanding the role of heterogeneity of material properties in whole-bone fracture behavior. Recently, fracture mechanics criteria have begun to be incorporated in finite element models of bone, both in idealized models [80,81,82], and in HR peripheral quantitative CT (HR pQCT)-based models of cancellous bone [83]. An HR pQCT-based FEA model of distal radii in patients with and without fragility fractures demonstrated that although cortical thickness was the best single predictor of whole-bone fracture load in the nonfracture group (R 2 = 0.52, p = 0.019), trabecular thickness was the best single predictor of whole-bone fracture load in the fracture group (R 2 = 0.40, p < 0.05) [83], indicating that trabeculae contribute critically to fracture resistance in whole bones.
Although heterogeneous material properties have not yet been incorporated into HR pQCT-based FEA models of cancellous bone, arbitrarily heterogeneous material properties have recently been incorporated into a fracture-mechanics-based FE model of cortical bone [84]. To assess the influence of arbitrarily increased compositional heterogeneity in cortical bone, two simulations were performed by assigning homogeneous and heterogeneous material properties to the cortical microstructure based on values reported in the literature. When homogeneous and random heterogeneous models were compared, total crack growth in the interstitial bone and osteons was nearly double in the homogeneous model compared to the heterogeneous models, with extensive uncracked ligaments in the heterogeneous models [84]. This study demonstrated that heterogeneous microscale material properties increase fracture resistance at larger length scales. Incorporation of heterogeneous material properties into fracture-mechanics-based FE models of cancellous bone provide a promising avenue for further investigation of the role of heterogeneous material properties in the fracture behavior of cancellous tissue.
Conclusion
Experimental studies allow direct assessment of bone material properties, but the majority to date has measured only tissue composition. Few have related composition to material properties. At the microscale, analytical and computational studies suggest that material property heterogeneity at a length scale ∼200 nm intrinsically enhances bone toughness. Experimental results, however, diverge into those that show an increase in heterogeneity in patient cohorts known to be susceptible to fracture, and those that show a decrease in heterogeneity. Variation in turnover, anatomic site, and loading orientation further contribute to the compositional heterogeneity in these patient cohorts.
At the mesoscale, variation in properties in single trabeculae due to variation in tissue age act as an intrinsic toughening mechanism. The combination of a stiffer, more mineralized core and a more compliant, less mineralized trabecular surface increases toughness across computational and experimental studies.
At the macroscale, experimental studies have shown that the relationship between anatomic location and heterogeneity of material properties is complicated, but influenced by the loading mode that different bone areas experience during physiologic loading. Computational studies have shown that integrating microscale heterogeneity at the bulk tissue level in models leads to modestly better predictions of apparent stiffness. Incorporating fracture mechanics parameters into finite element models is expected to improve our understanding of the influence of microscale heterogeneity on the fracture toughness of cancellous bone at the macroscale.
Acknowledgment
The authors acknowledge Christopher Hernandez for his valuable comments on the manuscript.
E.D. received research support from an American Society for Bone and Mineral Research Junior Faculty Osteoporosis Research Award and NIH/NIAMS K01AR064314. This material is based upon the work supported by the National Science Foundation Graduate Research Fellowship under Grant No. NSF DGE-1144153.
Contributor Information
Ashley A. Lloyd, Department of Materials Science, and Engineering, Cornell University, B60 Bard Hall, Ithaca, NY 14853, e-mail: aal99@cornell.edu
Zhen Xiang Wang, Department of Materials Science, and Engineering, Cornell University, B60 Bard Hall, Ithaca, NY 14853, e-mail: zw55@cornell.edu.
Eve Donnelly, Assistant Professor, Department of Materials Science, and Engineering, Cornell University, 227 Bard Hall, Ithaca, NY 14853; Hospital for Special Surgery, 535 E. 70th Street, New York, NY 10021, e-mail: eve.donnelly@cornell.edu.
References
- [1]. Cefalu, C. A. , 2004, “Is Bone Mineral Density Predictive of Fracture Risk Reduction?,” Curr. Med. Res. Opin., 20(3), pp. 341–349. 10.1185/030079903125003062 [DOI] [PubMed] [Google Scholar]
- [2]. Moro, M. , Hecker, A. T. , Bouxsein, M. L. , and Myers, E. R. , 1995, “Failure Load of Thoracic Vertebrae Correlates With Lumbar Bone Mineral Density Measured by DXA,” Calcif. Tissue Int., 56(3), pp. 206–209. 10.1007/BF00298611 [DOI] [PubMed] [Google Scholar]
- [3]. Oden, Z. M. , Selvitelli, D. M. , Hayes, W. C. , and Myers, E. R. , 1998, “The Effect of Trabecular Structure on DXA-Based Predictions of Bovine Bone Failure,” Calcif. Tissue Int., 63(1), pp. 67–73. 10.1007/s002239900491 [DOI] [PubMed] [Google Scholar]
- [4]. Bjarnason, K. , Hassager, C. , Svendsen, O. L. , Stang, H. , and Christiansen, C. , 1996, “Anteroposterior and Lateral Spinal Dxa for the Assessment of Vertebral Body Strength: Comparison With Hip and Forearm Measurement,” Osteoporos. Int., 6(1), pp. 37–42. 10.1007/BF01626536 [DOI] [PubMed] [Google Scholar]
- [5]. Cheng, X. G. , Nicholson, P. H. , Boonen, S. , Lowet, G. , Brys, P. , Aerssens, J. , Van Der Perre, G. , and Dequeker, J. , 1997, “Prediction of Vertebral Strength In Vitro by Spinal Bone Densitometry and Calcaneal Ultrasound,” J. Bone Miner. Res., 12(10), pp. 1721–1728. 10.1359/jbmr.1997.12.10.1721 [DOI] [PubMed] [Google Scholar]
- [6]. Linde, F. , Gothgen, C. B. , Hvid, I. , and Pongsoipetch, B. , 1988, “Mechanical Properties of Trabecular Bone by a Non-Destructive Compression Testing Approach,” Eng. Med., 17(1), pp. 23–29. 10.1243/EMED_JOUR_1988_017_008_02 [DOI] [PubMed] [Google Scholar]
- [7]. Lochmuller, E. M. , Eckstein, F. , Kaiser, D. , Zeller, J. B. , Landgraf, J. , Putz, R. , and Steldinger, R. , 1998, “Prediction of Vertebral Failure Loads From Spinal and Femoral Dual-Energy X-Ray Absorptiometry, and Calcaneal Ultrasound: An In Situ Analysis With Intact Soft Tissues,” Bone, 23(5), pp. 417–424. 10.1016/S8756-3282(98)00127-6 [DOI] [PubMed] [Google Scholar]
- [8]. Donnelly, E. , Lane, J. M. , and Boskey, A. L. , 2014, “Research Perspectives: The 2013 AAOS/ORS Research Symposium on Bone Quality and Fracture Prevention,” J. Orthop. Res., 32(7), pp. 855–864. 10.1002/jor.22626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Gourion-Arsiquaud, S. , Faibish, D. , Myers, E. , Spevak, L. , Compston, J. , Hodsman, A. , Shane, E. , Recker, R. R. , Boskey, E. R. , and Boskey, A. L. , 2009, “Use of FTIR Spectroscopic Imaging to Identify Parameters Associated With Fragility Fracture,” J. Bone Miner. Res., 24(9), pp. 1565–1571. 10.1359/jbmr.090414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Tai, K. , Dao, M. , Suresh, S. , Palazoglu, A. , and Ortiz, C. , 2007, “Nanoscale Heterogeneity Promotes Energy Dissipation in Bone,” Nat. Mater., 6(6), pp. 454–462. 10.1038/nmat1911 [DOI] [PubMed] [Google Scholar]
- [11]. Ritchie, R. O. , 2011, “The Conflicts Between Strength and Toughness,” Nat. Mater., 10(11), pp. 817–822. 10.1038/nmat3115 [DOI] [PubMed] [Google Scholar]
- [12]. Gourion-Arsiquaud, S. , Lukashova, L. , Power, J. , Loveridge, N. , Reeve, J. , and Boskey, A. L. , 2013, “Fourier Transform Infrared Imaging of Femoral Neck Bone: Reduced Heterogeneity of Mineral-to-Matrix and Carbonate-to-Phosphate and More Variable Crystallinity in Treatment-Naive Fracture Cases Compared With Fracture-Free Controls,” J. Bone Miner. Res., 28(1), pp. 150–161. 10.1002/jbmr.1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Boskey, A. L. , Dicarlo, E. , Paschalis, E. , West, P. , and Mendelsohn, R. , 2005, “Comparison of Mineral Quality and Quantity in Iliac Crest Biopsies From High- and Low-Turnover Osteoporosis: An FT-IR Microspectroscopic Investigation,” Osteoporos. Int., 16(12), pp. 2031–2038. 10.1007/s00198-005-1992-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Roschger, P. , Rinnerthaler, S. , Yates, J. , Rodan, G. A. , Fratzl, P. , and Klaushofer, K. , 2001, “Alendronate Increases Degree and Uniformity of Mineralization in Cancellous Bone and Decreases the Porosity in Cortical Bone of Osteoporotic Women,” Bone, 29(2), pp. 185–191. 10.1016/S8756-3282(01)00485-9 [DOI] [PubMed] [Google Scholar]
- [15]. Roschger, P. , Lombardi, A. , Misof, B. M. , Maier, G. , Fratzl-Zelman, N. , Fratzl, P. , and Klaushofer, K. , 2009, “Mineralization Density Distribution of Postmenopausal Osteoporotic Bone is Restored to Normal After Long-Term Alendronate Treatment: QBEI and SSAXS Data From the Fracture Intervention Trial Long-Term Extension (Flex),” J. Bone Miner. Res., 25(1), pp. 48–55. 10.1359/jbmr.090702 [DOI] [PubMed] [Google Scholar]
- [16]. Van Der Linden, J. C. , Birkenhager-Frenkel, D. H. , Verhaar, J. A. , and Weinans, H. , 2001, “Trabecular Bone's Mechanical Properties Are Affected by Its Non-Uniform Mineral Distribution,” J. Biomech., 34(12), pp. 1573–1580. 10.1016/S0021-9290(01)00146-4 [DOI] [PubMed] [Google Scholar]
- [17]. Jaasma, M. J. , Bayraktar, H. H. , Niebur, G. L. , and Keaveny, T. M. , 2002, “Biomechanical Effects of Intraspecimen Variations in Tissue Modulus for Trabecular Bone,” J. Biomech., 35(2), pp. 237–246. 10.1016/S0021-9290(01)00193-2 [DOI] [PubMed] [Google Scholar]
- [18]. Bourne, B. C. , and Van Der Meulen, M. C. , 2004, “Finite Element Models Predict Cancellous Apparent Modulus When Tissue Modulus Is Scaled From Specimen CT-Attenuation,” J. Biomech., 37(5), pp. 613–621. 10.1016/j.jbiomech.2003.10.002 [DOI] [PubMed] [Google Scholar]
- [19]. Tai, K. , Qi, H. J. , and Ortiz, C. , 2005, “Effect of Mineral Content on the Nanoindentation Properties and Nanoscale Deformation Mechanisms of Bovine Tibial Cortical Bone,” J. Mater. Sci. Mater. Med., 16(10), pp. 947–959. 10.1007/s10856-005-4429-9 [DOI] [PubMed] [Google Scholar]
- [20]. Fratzl, P. , Gupta, H. S. , Fischer, F. D. , and Kolednik, O. , 2007, “Hindered Crack Propagation in Materials With Periodically Varying Young's Modulus–Lessons From Biological Materials,” Adv. Mater., 19(18), pp. 2657–2661. 10.1002/adma.200602394 [DOI] [Google Scholar]
- [21]. Nalla, R. K. , Stolken, J. S. , Kinney, J. H. , and Ritchie, R. O. , 2005, “Fracture in Human Cortical Bone: Local Fracture Criteria and Toughening Mechanisms,” J. Biomech., 38(7), pp. 1517–1525. 10.1016/j.jbiomech.2004.07.010 [DOI] [PubMed] [Google Scholar]
- [22]. Augat, P. , and Schorlemmer, S. , 2006, “The Role of Cortical Bone and Its Microstructure in Bone Strength,” Age Ageing, 35(Suppl 2), pp. ii27–ii31. 10.1093/ageing/afl081 [DOI] [PubMed] [Google Scholar]
- [23]. Rho, J. Y. , Kuhn-Spearing, L. , and Zioupos, P. , 1998, “Mechanical Properties and the Hierarchical Structure of Bone,” Med. Eng. Phys., 20(2), pp. 92–102. 10.1016/S1350-4533(98)00007-1 [DOI] [PubMed] [Google Scholar]
- [24]. Dempster, D. W. , 2000, “The Contribution of Trabecular Architecture to Cancellous Bone Quality,” J. Bone Miner. Res., 15(1), pp. 20–23. 10.1359/jbmr.2000.15.1.20 [DOI] [PubMed] [Google Scholar]
- [25]. Keaveny, T. M. , Morgan, E. F. , Niebur, G. L. , and Yeh, O. C. , 2001, “Biomechanics of Trabecular Bone,” Annu. Rev. Biomed. Eng., 3(1), pp. 307–333. 10.1146/annurev.bioeng.3.1.307 [DOI] [PubMed] [Google Scholar]
- [26]. Zysset, P. K. , 2003, “A Review of Morphology-Elasticity Relationships in Human Trabecular Bone: Theories and Experiments,” J. Biomech., 36(10), pp. 1469–1485. 10.1016/S0021-9290(03)00128-3 [DOI] [PubMed] [Google Scholar]
- [27]. Rho, J. Y. , Tsui, T. Y. , and Pharr, G. M. , 1997, “Elastic Properties of Human Cortical and Trabecular Lamellar Bone Measured by Nanoindentation,” Biomaterials, 18(20), pp. 1325–1330. 10.1016/S0142-9612(97)00073-2 [DOI] [PubMed] [Google Scholar]
- [28]. Rho, J. Y. , Zioupos, P. , Currey, J. D. , and Pharr, G. M. , 1999, “Variations in the Individual Thick Lamellar Properties Within Osteons by Nanoindentation,” Bone, 25(3), pp. 295–300. 10.1016/S8756-3282(99)00163-5 [DOI] [PubMed] [Google Scholar]
- [29]. Donnelly, E. , Williams, R. W. , Downs, S. A. , Dickinson, M. E. , Baker, S. P. , and van der Meulen, M. C. H. , 2006, “Quasistatic and Dynamic Nanomechanical Properties of Cancellous Bone Tissue Relate to Collagen Content and Organization,” J. Mater. Res., 21(8), pp. 2106–2117. 10.1557/jmr.2006.0259 [DOI] [Google Scholar]
- [30]. Zysset, P. K. , Guo, X. E. , Hoffler, C. E. , Moore, K. E. , and Goldstein, S. A. , 1999, “Elastic Modulus and Hardness of Cortical and Trabecular Bone Lamellae Measured by Nanoindentation of the Human Femur,” J. Biomech., 32, pp. 1005–1012. 10.1016/S0021-9290(99)00111-6 [DOI] [PubMed] [Google Scholar]
- [31]. Jepsen, K. J. , and Schlecht, S. H. , 2014, “Biomechanical Mechanisms: Resolving the Apparent Conundrum of Why Individuals With Type II Diabetes Show Increased Fracture Incidence Despite Having Normal Bmd,” J. Bone Miner. Res., 29(4), pp. 784–786. 10.1002/jbmr.2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Hansma, P. , Turner, P. , Drake, B. , Yurtsev, E. , Proctor, A. , Mathews, P. , Lulejian, J. , Randall, C. , Adams, J. , Jungmann, R. , Garza-De-Leon, F. , Fantner, G. , Mkrtchyan, H. , Pontin, M. , Weaver, A. , Brown, M. B. , Sahar, N. , Rossello, R. , and Kohn, D. , 2008, “The Bone Diagnostic Instrument II: Indentation Distance Increase,” Rev. Sci. Instrum., 79(6), p. 064303. 10.1063/1.2937199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Choi, K. , Kuhn, J. L. , Ciarelli, M. J. , and Goldstein, S. A. , 1990, “The Elastic Moduli of Human Subchondral, Trabecular, and Cortical Bone Tissue and the Size-Dependency of Cortical Bone Modulus,” J. Biomech., 23(11), pp. 1103–1113. 10.1016/0021-9290(90)90003-L [DOI] [PubMed] [Google Scholar]
- [34]. Kuhn, L. T. , Grynpas, M. D. , Rey, C. C. , Wu, Y. , Ackerman, J. L. , and Glimcher, M. J. , 2008, “A Comparison of the Physical and Chemical Differences Between Cancellous and Cortical Bovine Bone Mineral at Two Ages,” Calcif. Tissue Int., 83(2), pp. 146–154. 10.1007/s00223-008-9164-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Tseng, K. F. , Bonadio, J. F. , Stewart, T. A. , Baker, A. R. , and Goldstein, S. A. , 1996, “Local Expression of Human Growth Hormone in Bone Results in Impaired Mechanical Integrity in the Skeletal Tissue of Transgenic Mice,” J. Orthop. Res., 14(4), pp. 598–604. 10.1002/jor.1100140414 [DOI] [PubMed] [Google Scholar]
- [36]. Morgan, E. F. , and Keaveny, T. M. , 2001, “Dependence of Yield Strain of Human Trabecular Bone on Anatomic Site,” J. Biomech., 34(5), pp. 569–577. 10.1016/S0021-9290(01)00011-2 [DOI] [PubMed] [Google Scholar]
- [37]. Boskey, A. , and Mendelsohn, R. , 2005, “Infrared Analysis of Bone in Health and Disease,” J. Biomed. Opt., 10(3), p. 031102. 10.1117/1.1922927 [DOI] [PubMed] [Google Scholar]
- [38]. Carden, A. , and Morris, M. D. , 2000, “Application of Vibrational Spectroscopy to the Study of Mineralized Tissues (Review),” J. Biomed. Opt., 5(3), pp. 259–268. 10.1117/1.429994 [DOI] [PubMed] [Google Scholar]
- [39]. Judex, S. , Boyd, S. , Qin, Y. X. , Miller, L. , Muller, R. , and Rubin, C. , 2003, “Combining High-Resolution Micro-Computed Tomography With Material Composition to Define the Quality of Bone Tissue,” Curr. Osteoporos Rep., 1(1), pp. 11–19. 10.1007/s11914-003-0003-x [DOI] [PubMed] [Google Scholar]
- [40]. Donnelly, E. , 2011, “Methods for Assessing Bone Quality: A Review,” Clin. Orthop. Relat. Res., 469(8), pp. 2128–2138. 10.1007/s11999-010-1702-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Ciarelli, T. E. , Tjhia, C. , Rao, D. S. , Qiu, S. , Parfitt, A. M. , and Fyhrie, D. P. , 2009, “Trabecular Packet-Level Lamellar Density Patterns Differ by Fracture Status and Bone Formation Rate in White Females,” Bone, 45(5), pp. 903–908. 10.1016/j.bone.2009.07.002 [DOI] [PubMed] [Google Scholar]
- [42]. Wang, Z. X. , and Donnelly, E. , “Altered Heterogeneity of Tissue Mineral and Collagen Properties in Perimenopausal Women With Fragility Fractures,” (in review). [DOI] [PubMed] [Google Scholar]
- [43]. Bousson, V. , Bergot, C. , Wu, Y. , Jolivet, E. , Zhou, L. Q. , and Laredo, J. D. , 2011, “Greater Tissue Mineralization Heterogeneity in Femoral Neck Cortex From Hip-Fractured Females Than Controls. A Microradiographic Study,” Bone, 48(6), pp. 1252–1259. 10.1016/j.bone.2011.03.673 [DOI] [PubMed] [Google Scholar]
- [44]. Tamminen, I. S. , Misof, B. M. , Roschger, P. , Mayranpaa, M. K. , Turunen, M. J. , Isaksson, H. , Kroger, H. , Makitie, O. , and Klaushofer, K. , 2014, “Increased Heterogeneity of Bone Matrix Mineralization in Pediatric Patients Prone to Fractures: A Biopsy Study,” J. Bone Miner. Res., 29(5), pp. 1110–1117. 10.1002/jbmr.2124 [DOI] [PubMed] [Google Scholar]
- [45]. Fratzl-Zelman, N. , Roschger, P. , Misof, B. M. , Pfeffer, S. , Glorieux, F. H. , Klaushofer, K. , and Rauch, F. , 2009, “Normative Data on Mineralization Density Distribution in Iliac Bone Biopsies of Children, Adolescents and Young Adults,” Bone, 44(6), pp. 1043–1048. 10.1016/j.bone.2009.02.021 [DOI] [PubMed] [Google Scholar]
- [46]. Paschalis, E. P. , Betts, F. , Dicarlo, E. , Mendelsohn, R. , and Boskey, A. L. , 1997, “FTIR Microspectroscopic Analysis of Human Iliac Crest Biopsies From Untreated Osteoporotic Bone,” Calcif. Tissue Int., 61(6), pp. 487–492. 10.1007/s002239900372 [DOI] [PubMed] [Google Scholar]
- [47]. Boskey, A. L. , and Mendelsohn, R. , 2005, “Infrared Spectroscopic Characterization of Mineralized Tissues,” Vib. Spectrosc., 38(1–2), pp. 107–114. 10.1016/j.vibspec.2005.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Tjhia, C. K. , Odvina, C. V. , Rao, D. S. , Stover, S. M. , Wang, X. , and Fyhrie, D. P. , 2011, “Mechanical Property and Tissue Mineral Density Differences Among Severely Suppressed Bone Turnover (Ssbt) Patients, Osteoporotic Patients, and Normal Subjects,” Bone, 49(6), pp. 1279–1289. 10.1016/j.bone.2011.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Busse, B. , Hahn, M. , Soltau, M. , Zustin, J. , Puschel, K. , Duda, G. N. , and Amling, M. , 2009, “Increased Calcium Content and Inhomogeneity of Mineralization Render Bone Toughness in Osteoporosis: Mineralization, Morphology and Biomechanics of Human Single Trabeculae,” Bone, 45(6), pp. 1034–1043. 10.1016/j.bone.2009.08.002 [DOI] [PubMed] [Google Scholar]
- [50]. Nyman, J. S. , Roy, A. , Shen, X. , Acuna, R. L. , Tyler, J. H. , and Wang, X. , 2006, “The Influence of Water Removal on the Strength and Toughness of Cortical Bone,” J. Biomech., 39(5), pp. 931–938. 10.1016/j.jbiomech.2005.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Sedlin, E. D. , and Hirsch, C. , 1966, “Factors Affecting the Determination of the Physical Properties of Femoral Cortical Bone,” Acta. Orthop. Scand., 37(1), pp. 29–48. 10.3109/17453676608989401 [DOI] [PubMed] [Google Scholar]
- [52]. Gourion-Arsiquaud, S. , Allen, M. R. , Burr, D. B. , Vashishth, D. , Tang, S. Y. , and Boskey, A. L. , 2010, “Bisphosphonate Treatment Modifies Canine Bone Mineral and Matrix Properties and Their Heterogeneity,” Bone, 46(3), pp. 666–672. 10.1016/j.bone.2009.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53]. Donnelly, E. , Meredith, D. S. , Nguyen, J. T. , Gladnick, B. P. , Rebolledo, B. J. , Shaffer, A. D. , Lorich, D. G. , Lane, J. M. , and Boskey, A. L. , 2012, “Reduced Cortical Bone Compositional Heterogeneity With Bisphosphonate Treatment in Postmenopausal Women With Intertrochanteric and Subtrochanteric Fractures,” J. Bone Miner. Res., 27(3), pp. 672–678. 10.1002/jbmr.560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54]. Launey, M. E. , Buehler, M. J. , and Ritchie, R. O. , 2010, “On the Mechanistic Origins of Toughness in Bone,” Annu. Rev. Mater. Res., 40(1), pp. 25–53. 10.1146/annurev-matsci-070909-104427 [DOI] [Google Scholar]
- [55]. Wang, X. , and Puram, S. , 2004, “The Toughness of Cortical Bone and Its Relationship With Age,” Ann. Biomed. Eng., 32(1), pp. 123–135. 10.1023/B:ABME.0000007797.92559.5e [DOI] [PubMed] [Google Scholar]
- [56]. Ciarelli, T. E. , Fyhrie, D. P. , and Parfitt, A. M. , 2003, “Effects of Vertebral Bone Fragility and Bone Formation Rate on the Mineralization Levels of Cancellous Bone From White Females,” Bone, 32(3), pp. 311–315. 10.1016/S8756-3282(02)00975-4 [DOI] [PubMed] [Google Scholar]
- [57]. Renders, G. A. , Mulder, L. , Van Ruijven, L. J. , and Van Eijden, T. M. , 2006, “Degree and Distribution of Mineralization in the Human Mandibular Condyle,” Calcif. Tissue Int., 79(3), pp. 190–196. 10.1007/s00223-006-0015-5 [DOI] [PubMed] [Google Scholar]
- [58]. Mulder, L. , Koolstra, J. H. , De Jonge, H. W. , and Van Eijden, T. M. , 2006, “Architecture and Mineralization of Developing Cortical and Trabecular Bone of the Mandible,” Anat. Embryol. (Berl), 211(1), pp. 71–78. 10.1007/s00429-005-0054-0 [DOI] [PubMed] [Google Scholar]
- [59]. Smith, L. J. , Schirer, J. P. , and Fazzalari, N. L. , 2010, “The Role of Mineral Content in Determining the Micromechanical Properties of Discrete Trabecular Bone Remodeling Packets,” J. Biomech., 43(16), pp. 3144–3149. 10.1016/j.jbiomech.2010.07.038 [DOI] [PubMed] [Google Scholar]
- [60]. Paschalis, E. P. , Betts, F. , Dicarlo, E. , Mendelsohn, R. , and Boskey, A. L. , 1997, “FTIR Microspectroscopic Analysis of Normal Human Cortical and Trabecular Bone,” Calcif. Tissue Int., 61(6), pp. 480–486. 10.1007/s002239900371 [DOI] [PubMed] [Google Scholar]
- [61]. Fyhrie, D. P. , and Schaffler, M. B. , 1994, “Failure Mechanisms in Human Vertebral Cancellous Bone,” Bone, 15(1), pp. 105–109. 10.1016/8756-3282(94)90900-8 [DOI] [PubMed] [Google Scholar]
- [62]. Mulder, L. , Koolstra, J. H. , Den Toonder, J. M. , and Van Eijden, T. M. , 2007, “Intratrabecular Distribution of Tissue Stiffness and Mineralization in Developing Trabecular Bone,” Bone, 41(2), pp. 256–265. 10.1016/j.bone.2007.04.188 [DOI] [PubMed] [Google Scholar]
- [63]. Skedros, J. G. , Knight, A. N. , Farnsworth, R. W. , and Bloebaum, R. D. , 2012, “Do Regional Modifications in Tissue Mineral Content and Microscopic Mineralization Heterogeneity Adapt Trabecular Bone Tracts for Habitual Bending? Analysis in the Context of Trabecular Architecture of Deer Calcanei,” J. Anat., 220(3), pp. 242–255. 10.1111/j.1469-7580.2011.01470.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64]. Lotz, J. C. , Cheal, E. J. , and Hayes, W. C. , 1995, “Stress Distributions Within the Proximal Femur During Gait and Falls: Implications for Osteoporotic Fracture,” Osteoporos. Int., 5(4), pp. 252–261. 10.1007/BF01774015 [DOI] [PubMed] [Google Scholar]
- [65]. Gao, H. , Ji, B. , Jager, I. L. , Arzt, E. , and Fratzl, P. , 2003, “Materials Become Insensitive to Flaws at Nanoscale: Lessons From Nature,” Proc. Natl. Acad. Sci. U.S.A., 100(10), pp. 5597–5600. 10.1073/pnas.0631609100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66]. Bonderer, L. J. , Studart, A. R. , and Gauckler, L. J. , 2008, “Bioinspired Design and Assembly of Platelet Reinforced Polymer Films,” Science, 319(5866), pp. 1069–1073. 10.1126/science.1148726 [DOI] [PubMed] [Google Scholar]
- [67]. Yao, H. M. , Dao, M. , Carnelli, D. , Tai, K. S. , and Ortiz, C. , 2011, “Size-Dependent Heterogeneity Benefits the Mechanical Performance of Bone,” J. Mech. Phys. Solids, 59(1), pp. 64–74. 10.1016/j.jmps.2010.09.012 [DOI] [Google Scholar]
- [68]. Wang, X. , Zauel, R. R. , Rao, D. S. , and Fyhrie, D. P. , 2008, “Cancellous Bone Lamellae Strongly Affect Microcrack Propagation and Apparent Mechanical Properties: Separation of Patients With Osteoporotic Fracture From Normal Controls Using a 2D Nonlinear Finite Element Method (Biomechanical Stereology),” Bone, 42(6), pp. 1184–1192. 10.1016/j.bone.2008.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69]. Mcnamara, L. M. , Van Der Linden, J. C. , Weinans, H. , and Prendergast, P. J. , 2006, “Stress-Concentrating Effect of Resorption Lacunae in Trabecular Bone,” J. Biomech., 39(4), pp. 734–741. 10.1016/j.jbiomech.2004.12.027 [DOI] [PubMed] [Google Scholar]
- [70]. Smit, T. H. , and Burger, E. H. , 2000, “Is BMU-Coupling a Strain-Regulated Phenomenon? A Finite Element Analysis,” J. Bone Miner. Res., 15(2), pp. 301–307. 10.1359/jbmr.2000.15.2.301 [DOI] [PubMed] [Google Scholar]
- [71]. Slyfield, C. R. , Tkachenko, E. V. , Fischer, S. E. , Ehlert, K. M. , Yi, I. H. , Jekir, M. G. , O'brien, R. G. , Keaveny, T. M. , and Hernandez, C. J. , 2012, “Mechanical Failure Begins Preferentially Near Resorption Cavities in Human Vertebral Cancellous Bone Under Compression,” Bone, 50(6), pp. 1281–1287. 10.1016/j.bone.2012.02.636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72]. Hernandez, C. J. , Gupta, A. , and Keaveny, T. M. , 2006, “A Biomechanical Analysis of the Effects of Resorption Cavities on Cancellous Bone Strength,” J. Bone Miner. Res., 21(8), pp. 1248–1255. 10.1359/jbmr.060514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73]. Van Rietbergen, B. , Weinans, H. , Huiskes, R. , and Odgaard, A. , 1995, “A New Method to Determine Trabecular Bone Elastic Properties and Loading Using Micromechanical Finite-Element Models,” J. Biomech., 28(1), pp. 69–81. 10.1016/0021-9290(95)80008-5 [DOI] [PubMed] [Google Scholar]
- [74]. Gross, T. , Pahr, D. H. , Peyrin, F. , and Zysset, P. K. , 2012, “Mineral Heterogeneity Has a Minor Influence on the Apparent Elastic Properties of Human Cancellous Bone: A SRμCT-Based Finite Element Study,” Comput. Methods Biomech. Biomed. Eng., 15(11), pp. 1137–1144. 10.1080/10255842.2011.581236 [DOI] [PubMed] [Google Scholar]
- [75]. Renders, G. A. , Mulder, L. , Langenbach, G. E. , Van Ruijven, L. J. , and Van Eijden, T. M. , 2008, “Biomechanical Effect of Mineral Heterogeneity in Trabecular Bone,” J. Biomech., 41(13), pp. 2793–2798. 10.1016/j.jbiomech.2008.07.009 [DOI] [PubMed] [Google Scholar]
- [76]. Renders, G. A. , Mulder, L. , Van Ruijven, L. J. , Langenbach, G. E. , and Van Eijden, T. M. , 2011, “Mineral Heterogeneity Affects Predictions of Intratrabecular Stress and Strain,” J. Biomech., 44(3), pp. 402–407. 10.1016/j.jbiomech.2010.10.004 [DOI] [PubMed] [Google Scholar]
- [77]. Chevalier, Y. , Pahr, D. , Allmer, H. , Charlebois, M. , and Zysset, P. , 2007, “Validation of a Voxel-Based Fe Method for Prediction of the Uniaxial Apparent Modulus of Human Trabecular Bone Using Macroscopic Mechanical Tests and Nanoindentation,” J. Biomech., 40(15), pp. 3333–3340. 10.1016/j.jbiomech.2007.05.004 [DOI] [PubMed] [Google Scholar]
- [78]. Currey, J. D. , 2003, “How Well Are Bones Designed to Resist Fracture?,” J. Bone Miner. Res., 18(4), pp. 591–598. 10.1359/jbmr.2003.18.4.591 [DOI] [PubMed] [Google Scholar]
- [79]. Yang, Q. D. , Cox, B. N. , Nalla, R. K. , and Ritchie, R. O. , 2006, “Fracture Length Scales in Human Cortical Bone: The Necessity of Nonlinear Fracture Models,” Biomaterials, 27(9), pp. 2095–2113. 10.1016/j.biomaterials.2005.09.040 [DOI] [PubMed] [Google Scholar]
- [80]. Buchanan, D. , and Ural, A. , 2010, “Finite Element Modeling of the Influence of Hand Position and Bone Properties on the Colles' Fracture Load During a Fall,” ASME J. Biomech. Eng., 132(8), p. 081007. 10.1115/1.4001681 [DOI] [PubMed] [Google Scholar]
- [81]. Ural, A. , and Vashishth, D. , 2006, “Cohesive Finite Element Modeling of Age-Related Toughness Loss in Human Cortical Bone,” J. Biomech., 39(16), pp. 2974–2982. 10.1016/j.jbiomech.2005.10.018 [DOI] [PubMed] [Google Scholar]
- [82]. Ural, A. , 2009, “Prediction of Colles' Fracture Load in Human Radius Using Cohesive Finite Element Modeling,” J. Biomech., 42(1), pp. 22–28. 10.1016/j.jbiomech.2008.10.011 [DOI] [PubMed] [Google Scholar]
- [83]. Ural, A. , and Mischinski, S. , 2013, “Multiscale Modeling of Bone Fracture Using Cohesive Finite Elements,” Eng. Fract. Mech., 103(1), pp. 141–152. 10.1016/j.engfracmech.2012.05.008 [DOI] [Google Scholar]
- [84]. Bruno, P. , Waldron, J. , and Ural, A. , 2014, “Influence of Reduced Compositional Heterogeneity on Fracture Resistance in Cortical Bone,” Trans. Orthop. Res. Soc., 39, p. 1496. [Google Scholar]


