Abstract
Objective
To retrospectively compare treatment of hepatocellular carcinoma (HCC) with transarterial chemoembolization (TACE) using gelatin sponges or microspheres plus lipiodol-doxorubicin vs. doxorubicin-loaded drug-eluting beads (DEB).
Materials and Methods
A total of 158 patients with HCC received TACE from November 2010 to November 2011 were enrolled in this study, including 64 (40.5%) received TACE with lipiodol-doxorubicin and gelatin sponges (group A), 41 (25.9%) received TACE with lipiodol-doxorubicin and microspheres (group B), and 53 (33.5%) received TACE with doxorubicin-loaded DEB (group C). Tumor response and adverse events (AEs) were evaluated.
Results
No significant difference was found at baseline among the three groups. The doxorubicin dosage in group C was significantly (p < 0.001) higher compared to the dose used in groups A or B (median, 50 mg vs. 31 mg or 25 mg). Significantly (p < 0.001) more patients in group C achieved complete response compared to those in groups A or B (32.1% vs. 6.3% or 2.4%). Significantly (p < 0.001) less patients in group C had progressive disease compared to those in groups A or B (34.0% vs. 57.8% or 68.3%). Minor AEs were more common in groups A and B compared to group C, with rates of 54.7%, 34.1%, and 5.7%, respectively.
Conclusion
In patients with HCC, TACE with DEB offers better safety and efficacy profiles compared to either TACE with gelatin sponges or TACE with microspheres.
Keywords: Chemoembolization, Drug-eluting bead, Hepatocellular carcinoma, Doxorubicin, Microsphere
INTRODUCTION
Hepatocellular carcinoma (HCC), the most common primary malignant tumor of the liver, is one of the most lethal malignancies (1). It is the sixth most commonly diagnosed cancer worldwide and the third leading cause of cancer death (2, 3). Liver transplantation is currently the best treatment for HCC. However, the number of available donors is limited. Thus, hepatic resection remains the treatment of choice for potentially curable disease in most areas of the world (3, 4, 5). Curative therapies including liver transplantation and resection are applicable in only 30-40% of HCC patients. Therefore, most patients are suitable only for locoregional or palliative therapies (6, 7).
Transarterial chemoembolization (TACE) is one of the preferred treatments for patients with HCC who are not suitable for curative therapy (8, 9). TACE is also considered the standard of care for non-surgical patients with tumors limited to the liver because it can preserve liver function (4, 10, 11). Conventional TACE involves intra-arterial infusion of a viscous emulsion of an ethiodized oil (e.g., lipiodol) and a chemotherapeutic agent such as doxorubicin, followed by an injection of gelatin sponge particles or other agents to embolize the blood vessel (12). This embolization ensures that lipiodol is retained selectively in HCC, enhancing drug delivery to the tumor. The embolizing agent also reduces drug washout from the tumor and induces ischemic necrosis. Ideally, TACE should result in a maximum sustained concentration of chemotherapeutic agent within the tumor with minimal systemic exposure. Additionally, TACE should obstruct the tumor vessels without obstructing blood supply to the surrounding tissue (13, 14). Randomized and controlled studies have shown that TACE has survival benefits superior to those of supportive care in appropriately selected patients (4, 7, 10, 11, 15). Despite the clinical efficacy of TACE with lipiodol, there are recognized limitations. In some cases, HCC does not exhibit lipiodol retention which may result in decreased effectiveness of the treatment and increased risk of liver damage (16, 17).
To release cytotoxic drugs (e.g., epirubicin or doxorubicin) in a controlled and sustained manner, drug-eluting beads (DEBs) have been introduced to TACE for transarterial treatment of HCCs (18, 19). These microspheres allow local delivery of high concentrations of chemotherapeutic agents to the tumor, with systemic concentrations comparable to conventional chemotherapeutic regimens. The use of DEB can reduce the occurrence of common adverse events (AEs) such as abdominal pain, fever, nausea, and vomiting that typically occur with TACE when gelatin sponges or microspheres (Embosphere; Merit Medical Systems, South Jordan, UT, USA) are used (20, 21, 22). Studies highlighting the use of DEB with TACE for the treatment of HCCs have shown similar or better results compared to conventional TACE with lipiodol (14, 15, 18, 20). The goal of this study, therefore, was to compare transarterial chemoembolization using gelatin sponges or microspheres plus lipiodol-doxorubicin vs. doxorubicin-loaded beads for the treatment of hepatocellular carcinoma.
MATERIALS AND METHODS
Study Design
This is a retrospective study of consecutive HCC patients who received TACE at a single medical center from November 2010 to November 2011. The study was approved by the Institutional Review Board of the hospital. Because of its retrospective nature, the requirement of informed patient consent was waived. All patients underwent pretreatment assessment including a medical history, physical examination, laboratory assessment, and imaging studies (contrast-enhanced computed tomography [CT] or magnetic resonance imaging [MRI]). Inclusion criteria for the study were: 1) adult ≥ 18 years old with HCC diagnosed based on noninvasive criteria (4); 2) at least one tumor that was treatment-naïve and > 1 in diameter; 3) Barcelona Clinic Liver Cancer (BCLC) criteria A or B; 4) Eastern Cooperative Oncology Group (ECOG) performance score of 0 or 1; 5) serum creatinine < 1.2 mg/dL (normal range, 0.6-1.2 mg/dL); 6) aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels < 200 IU/L (normal range, 0-40 IU/L and 0-45 IU/L, respectively); and 7) total bilirubin < 3 mg/dL (normal range, 0.1-1 mg/dL). Exclusion criteria were: 1) if the tumor invaded the portal vein, hepatic vein, or biliary duct; 2) if the tumor had an extrahepatic arterial supply (drug-eluting beads are not recommended to be used on extrahepatic feeders); 3) if they were diagnosed with atypical HCC (e.g., infiltrative).
Treatment
Treatment with chemoembolization was planned by a multidisciplinary team. Patients were treated with either conventional TACE with a gelatin sponge (group A), TACE with Embosphere microspheres (Biosphere, Roissy, France) (group B), or chemoembolization with doxorubicin-loaded DEB (DC Beads; Biocompatibles, Farnham, United Kingdom) (group C). All patients included in this study received only one cycle of TACE during the data collection period. The attending physician explained the tumor response rate and complication rate of each method to the patient based on current published literature. The patient was asked to decide on the method based on the information they were provided.
On treatment day, a thorough diagnostic angiographic evaluation of the celiac trunk, superior mesenteric artery, and hepatic artery was performed to determine the vascular anatomy and to assess portal flow (23). Super-selective angiography was subsequently performed using a microcatheter to catheterize the segmental or subsegmental arteries feeding the tumor. In patients whose right hepatic artery arose from the superior mesenteric artery, the right hepatic artery was selectively when necessary. Embolization of the cystic artery and falciform artery was carefully avoided. The phrenic artery was studied when it was suspected to supply the target tumor.
Variations in the preparation of lipiodol-drug emulsion can affect the release of the drug into the systemic circulation, thus affecting the outcome of TACE (24). In group A and B, 50 mg of doxorubicin was mixed with 10 mL of lipiodol so that a consistent concentration was achieved. In group A, the lipiodol/doxorubicin was injected into a segmental or subsegmental artery, followed by an injection of 500-700 µm gelatin sponges (Spongostan standard, Johnson & Johnson, Gargrave, Skipton, United Kingdom). In group B, the lipiodol/doxorubicin was injected into a segmental or subsegmental artery, followed by injection of 100-300 µm Embosphere microspheres. In both groups, the amount of lipiodol/doxorubicin injected was based on tumor diameter as described previously (25). In both groups, the endpoint of embolization was stasis in the second- or third-order branch of the right or left hepatic artery (25).
In group C, 2 mL of DEB at 300-500 µm in diameter were loaded with 70 mg of doxorubicin and injected (15). If "near stasis" was not achieved after the injection of the first dose of DEB, an additional volume of DEB was injected until "near stasis" occurred in the artery, i.e., when the contrast column was found clear within 2 to 5 heartbeats (14). The amount of beads injected was based on the manufacturer's recommendation using the inscribed measurements on the injection syringe.
We chose 300 to 500 µm-sized DEB because the 100 to 300 µm-sized beads have not yet been approved for use in Taiwan. After binding the drug, the 300 to 500 µm-sized beads shrunk to 80% of the original diameter (i.e., 240 to 400 µm), which was close to the size of Embospheres.
Response Evaluation and Follow-Up
According to modified Response Evaluation Criteria in Solid Tumors (mRECIST) (26), patients received triple-phase contrast-enhanced CT at 3 months following the procedure. Tumor response was assessed every 3-4 months. If residual tumor was considered a partial response (PR) or stable disease by mRECIST criteria, the follow-up was continued every 3-4 months. If the residual tumor was enlarged (progressive disease by mRECIST criteria), another treatment was given to patients according to BCLC guidelines and disease status (27). Complete response (CR) was defined as the disappearance of any intratumoral enhancement at CT. PR was defined as at least a 30% decrease in the sum of the diameters of the visible target lesions compared to the baseline measurements. We defined progression based on mRECIST criteria. Target and non-target lesions were treated at the same time. Therefore, it was unnecessary to separate target tumor response from overall tumor response.
Progressive disease was defined as at least a 20% increase in the sum of the diameters of the visible target lesions compared to the smallest measurements recorded since the start of the treatment. Stable disease was defined as any case that did not meet the definition of either PR or progressive disease (26). Two experienced radiologists evaluated the images. Discrepancies were resolved by consensus.
Safety
Adverse events were categorized according to the clinical practice guidelines of the Society of Interventional Radiology (28). Major AEs included those needed increased level of care, major therapy, prolonged hospitalization, and those with in permanent sequelae or death. Minor AEs were defined as those required only nominal therapy or observation. The primary safety endpoint was liver toxicity defined as an increase in the levels of AST, ALT, or bilirubin at 48 hours after the procedure.
Statistical Analysis
Tumor response and complications within the three treatment groups were compared using Fisher's exact test. Normally distributed data were compared by one-way analysis of variance, with Bonferroni post-hoc tests for pair-wise groups. For data that had abnormal distribution, non-parametric Kruskal-Wallis test was used to compare the three groups. Mann-Whitney test was used for pair-wise group comparison with Bonferroni correction. Kaplan-Meier survival curves were plotted to describe the progression-free survival rates of the three groups. Log-rank test was performed to compare the Kaplan-Meier survival curves of the three groups. A two-tailed p value < 0.05 was considered statistically significant. All analyses were performed using SPSS version 20.0 software (IBM Corp., Armonk, NY, USA).
RESULTS
Patient Characteristics
A total of 158 patients with HCC who met the inclusion criteria received TACE from November 2010 to November 2011, including 64 (40.5%) who received TACE with gelatin sponges, 41 (25.9%) with Embospheres, and 53 (33.5%) with DEB. The demographic and clinical characteristics of the patients are summarized in Table 1. The mean follow-up periods for group A, B, and C were 7.3 months (range, 2-17 months), 8.0 months (range, 4-16 months), and 10.8 months (range, 4-16 months), respectively. No significant difference was observed among the three treatment groups with respect to demographic characteristics, etiology of underlying liver disease, liver function, renal function, ECOG performance status, Child-Pugh score, or tumor burden. Significantly (p = 0.039) more group C patients had tumors classified as BCLC stage B compared to group A or B (100% vs. 90.6% or 97.6%) (Table 1).
Table 1.
Patient Baseline Characteristics (n = 158)
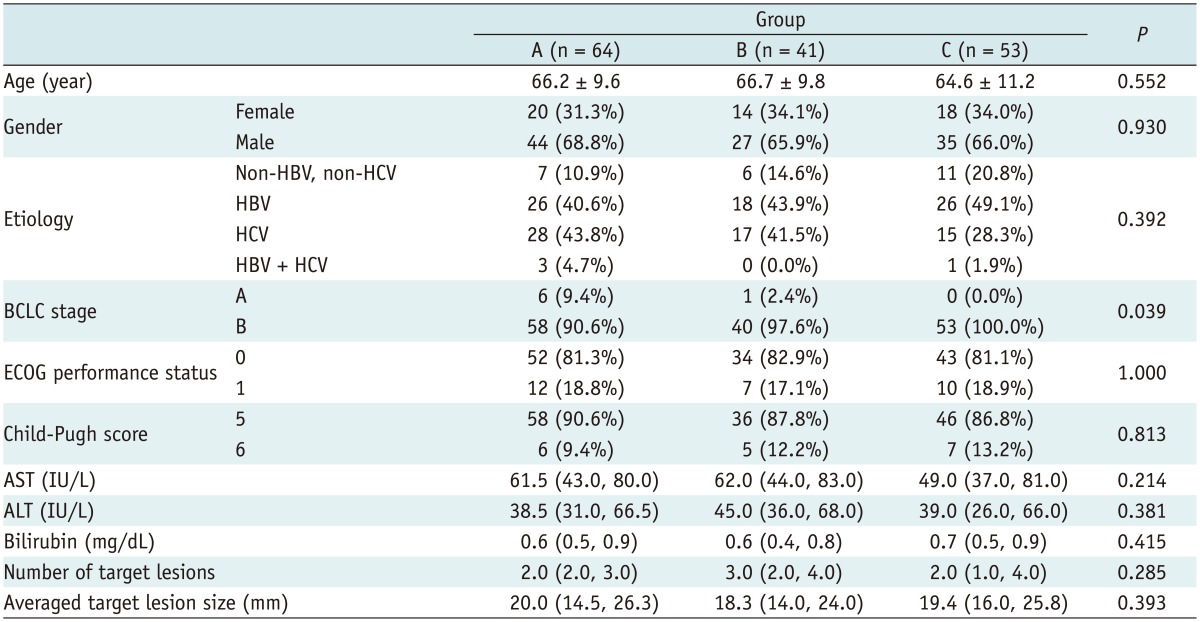
Note.- Data are presented as number (%), median (interquartile range), and mean ± standard deviation. Group A: TACE with gelatin sponge; Group B: TACE with Embosphere; Group C: TACE with DEB; all received doxorubicin as chemotherapeutic agent. ALT = alanine aminotransferase, AST = aspartate aminotransferase, BCLC = Barcelona Clinic for Liver Cancer, ECOG = Eastern Cooperative Oncology Group, HBV = hepatitis B virus, HCV = hepatitis C virus
The doxorubicin dosage, number of complications, laboratory data, and tumor response after TACE are summarized in Table 2. The doxorubicin dose used in group C (median, 50 mg) was significantly (p < 0.001) higher than that used in group A or B (31 mg and 25 mg, respectively). Significantly (p < 0.001) higher levels of AST, ALT, and total bilirubin were observed in group A compared to either group B or C at 48 hours post the procedure. However, post-treatment creatinine ratios were not significantly different among the three groups (Table 2).
Table 2.
Doxorubicin Dosage, Complications, Laboratory Data, and Tumor Response after TACE
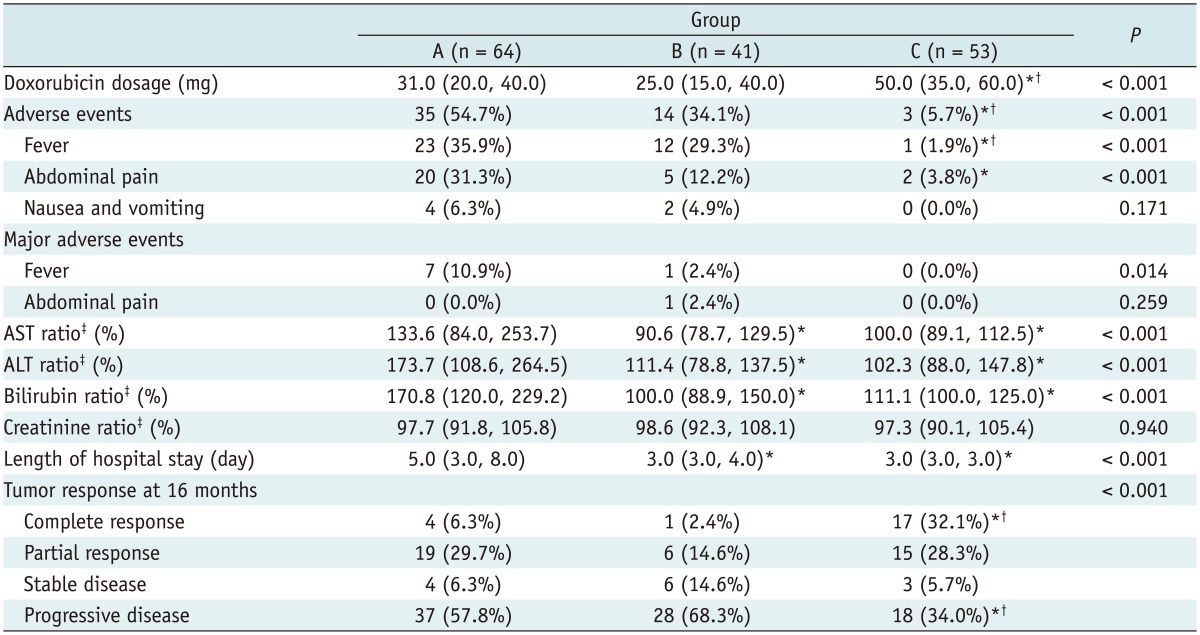
Note.- Data are presented as number (%) and median (interquartile range). *Significant difference compared to group A, †Significant difference compared to group B, ‡Ratio calculated as baseline/post-treatment level × 100. ALT = alanine aminotransferase, AST = aspartate aminotransferase, TACE = transarterial chemoembolization
Adverse Events
No major AE was observed in group C patients. Minor AEs were significantly (p < 0.001) more common in groups A and B patients compared to group C patients, with rates of 54.7%, 34.1%, and 5.7%, respectively (Table 2). Significantly (p < 0.001) more instances of fever and abdominal pain were observed in group A patients compared to that in group C patients (35.9% vs. 1.9%; 31.3% vs. 3.8%, respectively) (Table 2).
Progression-Free Survival and Tumor Response Over 16 Months
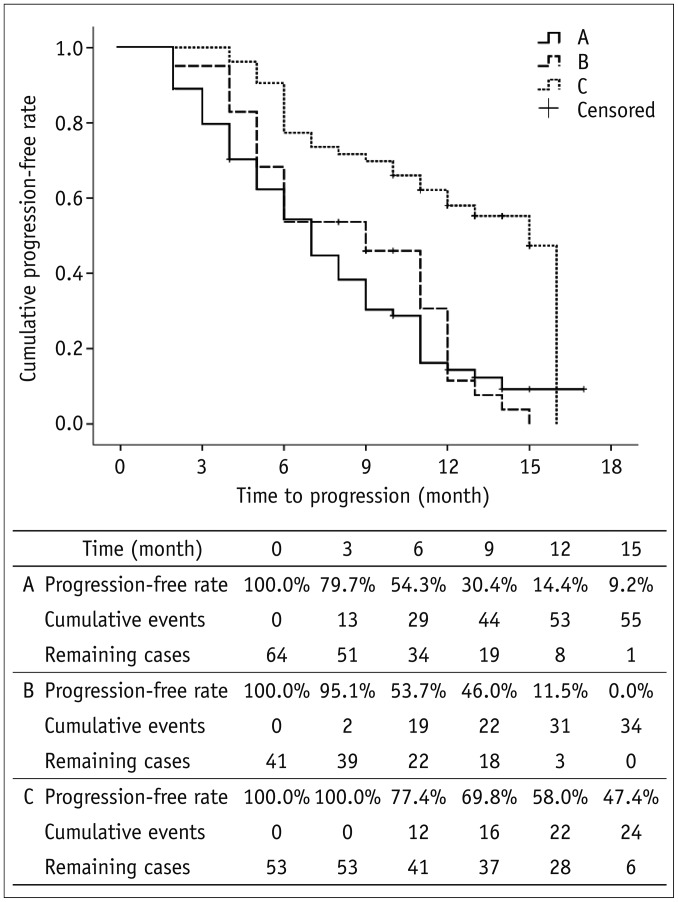
Significantly (p < 0.001) more patients in group C had a CR compared to group A or B (32.1% vs. 6.3% or 2.4%) (Table 2). The Kaplan-Meier survival curves were plotted to describe the progression-free survival rates of the three groups (Fig. 1). The median time to progression for groups A, B, and C were 7, 9, and 15 months, respectively. The 3- and 6-month progression-free rates for groups A, B, and C were 79.7% and 54.3% for group A, 95.1% and 53.7%, and 100% and 77.4%, respectively. Log-rank test showed that group C patients had significantly (p < 0.001) higher progression-free survival rates compared to group A or B.
Fig. 1.
Kaplan-Meier survival curves for three groups. Last progression event occurred in 16th month. Therefore, progression-free rate was not available after 16 months. Log-rank test shows that group C patients had significantly (p < 0.001) higher progression-free rate compared to group A or group B. Group A: TACE with gelatin sponge; Group B: TACE with Embosphere; Group C: TACE with DEB. DEB = drug-eluting bead, TACE = transarterial chemoembolization
DISCUSSION
This study demonstrated that TACE with DEB exhibited a better safety profile (fewer AEs) than conventional chemoembolization with either gelatin sponges or Embospheres. In addition, significantly more patients treated with DEB had CR compared to the other two groups of patients. The log-rank test showed that group C patients had significantly higher progression-free survival rates compared to group A or group B patients. Chemoembolization has been used for the treatment of HCC for decades. Its outcomes have been improved by advances in interventional techniques and refinement in patient selection (1). Commonly used embolic agents include gelatin sponges, polyvinyl alcohol particles, and microspheres. Gelatin sponges, the most widely used embolic agents, can be prepared in various forms such as particles, pellets, or fragments. The use of a gelatin sponge alone for embolization results in temporary occlusion of an artery with recanalization taking place within 2 weeks.
Lipiodol is an iodinated ethyl ester of poppy seed oil that selectively remains in tumor nodules from several weeks to over a year (9). A lipiodol-drug emulsion is injected into a vessel supplying the tumor. The anticancer slowly released from lipiodol remains at high concentrations within the tumor for a prolonged period (29). DEB is a drug delivery and embolization system composed of biocompatible, nonresorbable hydrogel beads that can be loaded with chemotherapeutic drugs. Studies have shown that TACE with DEB results in higher tumor drug concentrations and lower toxicity compared to intra-arterial doxorubicin and conventional TACE (18, 20, 30). Song et al. (31) recently reported that TACE with DEB was effective for HCC refractory to conventional TACE, with tolerable adverse effects. Kalva et al. (32) performed TACE with DEB loaded with doxorubicin in 80 patients with advanced stage HCC. They reported that the procedure was safe. ECOG performance status ≤ 1 and > 2 DEB-TACE procedures were associated with better overall survival. Our study represented a unique 3-way comparison among TACE with gelfoam, microspheres, and DEB in the treatment of HCC. Only two randomized prospective studies have previously compared the conventional TACE with DEB-TACE (21, 33). Only a few retrospective studies have attempted to evaluate the effectiveness of DEB-TACE vs. conventional TACE (15, 18, 20, 30, 31, 34). Lammer et al. (21) found that their DEB group showed higher rates of CR, objective response, and disease control compared to conventional TACE. Sacco et al. (33), on the other hand, reported no difference between DEB-TACE and conventional TACE groups in time to recurrence or local recurrence, radiologic progression, or survival. Our results were in consistent with the findings of Song et al. (15) who found that TACE with DEB loaded with doxorubicin offered a distinct advantage in objective tumor response rate compared to conventional lipiodol-based TACE in Asian patients with HCC. Dhanasekaran et al. (34) found a distinct survival advantage of DEB-TACE over conventional TACE in patients with unresectable HCC.
In this study, compared to patients who underwent TACE with gelatin sponges or Embospheres, patients treated with TACE using DEB received a higher dosage of doxorubicin. Although a higher dosage of doxorubicin was used in the DEB-TACE group, the degree of liver toxicity and the number of drug-related AEs were both reduced. A sustained concentration of doxorubicin within the tumor as a result of DEB delivery system might explain the better treatment effect, because minimal systemic efflux of doxorubicin is believed to reduce liver toxicity and drug-related AEs (19). Our results are consistent with many reports in the literature (18, 20, 21, 35, 36). These benefits may result in shorter length of hospital stay due to the improved efficacy and safety profiles of high-dose doxorubicin used in patients undergoing TACE with DEB, regardless of patients' baseline characteristics (15).
The most common complication of TACE is post-embolization syndrome. This syndrome consisting of transient abdominal pain and fever occurs in 60-80% of patients after TACE. Post-embolization syndrome is typically accompanied by an elevation of hepatic transaminase (37). It is unclear whether post-embolization syndrome is a result of damage to the normal liver tissue or tumor necrosis. Post-embolization syndrome is self-limiting within 3-4 days. However, hospitalization may be required for pain control and observation (37).
Based on the results of this study, TACE with DEB appeared to be better tolerated compared to conventional TACE using gelatin sponges with respect to liver enzyme elevation and drug-related AEs. These findings are in consistent with those of previous reports by Recchia et al. (38) and other studies (18, 20, 21, 39). Drug-related AEs were rare in our group C patients, although the dosage of doxorubicin in group C was greater than group A or group B. Embosphere and gelatin sponge had few drug delivery effects. Therefore, more AEs might have occurred if higher dosages were given to patients in groups A and B. A prior study by López-Benítez et al. (40) could support such theory to some degree. Despite the improved tolerability profile of TACE with DEB, interventional radiologists should be aware of potential risks of procedure-associated AEs. They should have sufficient knowledge to manage these complications appropriately (30).
There are several limitations in this study, including its retrospective nature. Previously, only two reports of randomized prospective studies compared lipiodol TACE vs. DEB-TACE (21, 33). All other studies were retrospective series. In addition, our sample size was limited and our follow-up period was too short to determine the potential long-term benefit of chemoembolization with DEB. Future prospective studies involving larger cohorts and longer follow-up periods are needed to confirm our findings.
In conclusion, in patients with HCC, TACE with DEB offered better safety and efficacy profiles compared to TACE using gelatin sponges or TACE with microspheres. Further investigations featuring long-term use of TACE with DEB are merited.
Footnotes
This study was funded by a grant from National Cheng-Kung University Hospital (NCKUH-10102053).
References
- 1.Lewandowski RJ, Mulcahy MF, Kulik LM, Riaz A, Ryu RK, Baker TB, et al. Chemoembolization for hepatocellular carcinoma: comprehensive imaging and survival analysis in a 172-patient cohort. Radiology. 2010;255:955–965. doi: 10.1148/radiol.10091473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Fan ST, Mau Lo C, Poon RT, Yeung C, Leung Liu C, Yuen WK, et al. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20-year experience. Ann Surg. 2011;253:745–758. doi: 10.1097/SLA.0b013e3182111195. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 5.Wu KT, Wang CC, Lu LG, Zhang WD, Zhang FJ, Shi F, et al. Hepatocellular carcinoma: clinical study of long-term survival and choice of treatment modalities. World J Gastroenterol. 2013;19:3649–3657. doi: 10.3748/wjg.v19.i23.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 7.Marelli L, Stigliano R, Triantos C, Senzolo M, Cholongitas E, Davies N, et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol. 2007;30:6–25. doi: 10.1007/s00270-006-0062-3. [DOI] [PubMed] [Google Scholar]
- 8.Thomas MB, Jaffe D, Choti MM, Belghiti J, Curley S, Fong Y, et al. Hepatocellular carcinoma: consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2010;28:3994–4005. doi: 10.1200/JCO.2010.28.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramsey DE, Kernagis LY, Soulen MC, Geschwind JF. Chemoembolization of hepatocellular carcinoma. J Vasc Interv Radiol. 2002;13(9 Pt 2):S211–S221. doi: 10.1016/s1051-0443(07)61789-8. [DOI] [PubMed] [Google Scholar]
- 10.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 11.Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 12.Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010;52:762–773. doi: 10.1002/hep.23725. [DOI] [PubMed] [Google Scholar]
- 13.Brown DB, Gould JE, Gervais DA, Goldberg SN, Murthy R, Millward SF, et al. Transcatheter therapy for hepatic malignancy: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009;20(7 Suppl):S425–S434. doi: 10.1016/j.jvir.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Lencioni R, de Baere T, Burrel M, Caridi JG, Lammer J, Malagari K, et al. Transcatheter treatment of hepatocellular carcinoma with Doxorubicin-loaded DC Bead (DEBDOX): technical recommendations. Cardiovasc Intervent Radiol. 2012;35:980–985. doi: 10.1007/s00270-011-0287-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song MJ, Park CH, Kim JD, Kim HY, Bae SH, Choi JY, et al. Drug-eluting bead loaded with doxorubicin versus conventional Lipiodol-based transarterial chemoembolization in the treatment of hepatocellular carcinoma: a case-control study of Asian patients. Eur J Gastroenterol Hepatol. 2011;23:521–527. doi: 10.1097/MEG.0b013e328346d505. [DOI] [PubMed] [Google Scholar]
- 16.Poyanli A, Rozaneş I, Acunaş B, Sencer S. Palliative treatment of hepatocellular carcinoma by chemoembolization. Acta Radiol. 2001;42:602–607. doi: 10.1080/028418501127347278. [DOI] [PubMed] [Google Scholar]
- 17.Lee JK, Chung YH, Song BC, Shin JW, Choi WB, Yang SH, et al. Recurrences of hepatocellular carcinoma following initial remission by transcatheter arterial chemoembolization. J Gastroenterol Hepatol. 2002;17:52–58. doi: 10.1046/j.1440-1746.2002.02664.x. [DOI] [PubMed] [Google Scholar]
- 18.Poon RT, Tso WK, Pang RW, Ng KK, Woo R, Tai KS, et al. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol. 2007;5:1100–1108. doi: 10.1016/j.cgh.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 19.Nicolini A, Martinetti L, Crespi S, Maggioni M, Sangiovanni A. Transarterial chemoembolization with epirubicin-eluting beads versus transarterial embolization before liver transplantation for hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21:327–332. doi: 10.1016/j.jvir.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 20.Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474–481. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 21.Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Granberg D, Eriksson LG, Welin S, Kindmark H, Janson ET, Skogseid B, et al. Liver embolization with trisacryl gelatin microspheres (embosphere) in patients with neuroendocrine tumors. Acta Radiol. 2007;48:180–185. doi: 10.1080/02841850601080440. [DOI] [PubMed] [Google Scholar]
- 23.Covey AM, Brody LA, Maluccio MA, Getrajdman GI, Brown KT. Variant hepatic arterial anatomy revisited: digital subtraction angiography performed in 600 patients. Radiology. 2002;224:542–547. doi: 10.1148/radiol.2242011283. [DOI] [PubMed] [Google Scholar]
- 24.de Baere T, Zhang X, Aubert B, Harry G, Lagrange C, Ropers J, et al. Quantification of tumor uptake of iodized oils and emulsions of iodized oils: experimental study. Radiology. 1996;201:731–735. doi: 10.1148/radiology.201.3.8939223. [DOI] [PubMed] [Google Scholar]
- 25.Idée JM, Guiu B. Use of Lipiodol as a drug-delivery system for transcatheter arterial chemoembolization of hepatocellular carcinoma: a review. Crit Rev Oncol Hematol. 2013;88:530–549. doi: 10.1016/j.critrevonc.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graf D, Vallböhmer D, Knoefel WT, Kröpil P, Antoch G, Sagir A, et al. Multimodal treatment of hepatocellular carcinoma. Eur J Intern Med. 2014;25:430–437. doi: 10.1016/j.ejim.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Cardella JF, Kundu S, Miller DL, Millward SF, Sacks D Society of Interventional Radiology. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2009;20(7 Suppl):S189–S191. doi: 10.1016/j.jvir.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura H, Hashimoto T, Oi H, Sawada S. Transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology. 1989;170(3 Pt 1):783–786. doi: 10.1148/radiology.170.3.2536946. [DOI] [PubMed] [Google Scholar]
- 30.Malagari K, Alexopoulou E, Chatzimichail K, Hall B, Koskinas J, Ryan S, et al. Transcatheter chemoembolization in the treatment of HCC in patients not eligible for curative treatments: midterm results of doxorubicin-loaded DC bead. Abdom Imaging. 2008;33:512–519. doi: 10.1007/s00261-007-9334-x. [DOI] [PubMed] [Google Scholar]
- 31.Song DS, Choi JY, Yoo SH, Kim HY, Song MJ, Bae SH, et al. DC bead transarterial chemoembolization is effective in hepatocellular carcinoma refractory to conventional transarteral chemoembolization: a pilot study. Gut Liver. 2013;7:89–95. doi: 10.5009/gnl.2013.7.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalva SP, Pectasides M, Liu R, Rachamreddy N, Surakanti S, Yeddula K, et al. Safety and effectiveness of chemoembolization with drug-eluting beads for advanced-stage hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2014;37:381–387. doi: 10.1007/s00270-013-0654-7. [DOI] [PubMed] [Google Scholar]
- 33.Sacco R, Bargellini I, Bertini M, Bozzi E, Romano A, Petruzzi P, et al. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2011;22:1545–1552. doi: 10.1016/j.jvir.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Dhanasekaran R, Kooby DA, Staley CA, Kauh JS, Khanna V, Kim HS. Comparison of conventional transarterial chemoembolization (TACE) and chemoembolization with doxorubicin drug eluting beads (DEB) for unresectable hepatocelluar carcinoma (HCC) J Surg Oncol. 2010;101:476–480. doi: 10.1002/jso.21522. [DOI] [PubMed] [Google Scholar]
- 35.Malagari K, Pomoni M, Spyridopoulos TN, Moschouris H, Kelekis A, Dourakis S, et al. Safety profile of sequential transcatheter chemoembolization with DC Bead™: results of 237 hepatocellular carcinoma (HCC) patients. Cardiovasc Intervent Radiol. 2011;34:774–785. doi: 10.1007/s00270-010-0044-3. [DOI] [PubMed] [Google Scholar]
- 36.Hong K, Khwaja A, Liapi E, Torbenson MS, Georgiades CS, Geschwind JF. New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res. 2006;12:2563–2567. doi: 10.1158/1078-0432.CCR-05-2225. [DOI] [PubMed] [Google Scholar]
- 37.Wigmore SJ, Redhead DN, Thomson BN, Currie EJ, Parks RW, Madhavan KK, et al. Postchemoembolisation syndrome--tumour necrosis or hepatocyte injury? Br J Cancer. 2003;89:1423–1427. doi: 10.1038/sj.bjc.6601329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Recchia F, Passalacqua G, Filauri P, Doddi M, Boscarato P, Candeloro G, et al. Chemoembolization of unresectable hepatocellular carcinoma: decreased toxicity with slow-release doxorubicineluting beads compared with lipiodol. Oncol Rep. 2012;27:1377–1383. doi: 10.3892/or.2012.1651. [DOI] [PubMed] [Google Scholar]
- 39.Vogl TJ, Lammer J, Lencioni R, Malagari K, Watkinson A, Pilleul F, et al. Liver, gastrointestinal, and cardiac toxicity in intermediate hepatocellular carcinoma treated with PRECISION TACE with drug-eluting beads: results from the PRECISION V randomized trial. AJR Am J Roentgenol. 2011;197:W562–W570. doi: 10.2214/AJR.10.4379. [DOI] [PubMed] [Google Scholar]
- 40.López-Benítez R, Radeleff BA, Barragán-Campos HM, Noeldge G, Grenacher L, Richter GM, et al. Acute pancreatitis after embolization of liver tumors: frequency and associated risk factors. Pancreatology. 2007;7:53–62. doi: 10.1159/000101878. [DOI] [PubMed] [Google Scholar]