Abstract
Objective
Arterial stenosis is a major obstacle for subsequent interventional procedures. We hypothesized that the stenosis is caused by gelatin sponge embolization and performed an experimental study in a rabbit renal model.
Materials and Methods
A total of 24 rabbits were embolized with porcine gelatin sponge particles injected into the renal arteries. Four rabbits were sacrificed on 1 day, 4 days, 1 week, 2 weeks, 3 weeks, and 4 weeks after embolization. Microscopic evaluations were performed on hematoxylin-eosin and smooth muscle actin immunohistochemical stained sections.
Results
Gelatin sponge particles were mainly observed in the segmental and interlobar arteries. Transmural inflammation of the embolized arterial wall and mild thickening of the media were observed 1 week after embolization. Resorption of the gelatin sponge and organization of thrombus accompanied by foreign body reactions, were observed from 2 to 4 weeks after embolization. Microscopic images of the 3 weeks group showed vessel lumens filled mostly with organized thrombi, resulting in severe stenosis. Additionally, vessels showed a thickened intima that contained migrating smooth muscle cells and accompanying interruption of the internal elastic lamina. The migrating smooth muscle cells were distributed around the recanalized arterial lumen.
Conclusion
Gelatin sponge embolization may induce arterial stenosis by causing organized thrombus and intimal hyperplasia, which consists of migrating smooth muscle cells and intimal collagen deposits.
Keywords: Gelatin sponge, Absorbable, Embolization, Arterial stenosis
INTRODUCTION
Gelatin sponge particles (GSPs) have been used in transarterial embolization procedures for the treatment of hepatocellular carcinoma since the late 1970s, most commonly in transcatheter arterial chemoembolization (TACE) (1, 2, 3, 4). The main goal of embolic therapy is to devitalize the target lesion while minimizing ischemia to the parenchyma of the underlying organ (5). It is important to maintain the patency of the hepatic arteries to enable effective further administration of drugs and achieve a successful outcome for procedures that require repeated sessions, such as TACE (6). The factors associated with reduced hepatic artery patency after TACE include the accumulated dose of anticancer drug per hepatic artery, the number of anticancer drugs administered, and the Child-Pugh score (7, 8).
We hypothesized that GSPs can be an independent cause of arterial stenosis. The experimental study was performed to test the hypothesis and to investigate the underlying pathological processes in a rabbit renal model.
MATERIALS AND METHODS
The study included 24 New Zealand white rabbits, aged 3 to 4 months and weighing 3 to 3.5 kg. The animals were kept under controlled conditions (12/12-hour light/dark cycles, ambient temperature of 22 ± 5℃, and relative humidity of 50 ± 10%) and fed standard food pellets that were sterilized using gamma rays, and autoclaved tap water, ad libitum. The pre and postsurgical animal care and surgical interventions followed the guidelines of the Laboratory Animals Welfare Act, the Guide for the Care and Use of Laboratory Animals, and the Guidelines and Policies for Rodent Survival Surgery provided by the Institutional Animal Care and Use Committee of the School of Medicine at our institution.
Gelatin Sponge Particles
Spongostan (Ethicon Inc., Somerville, NJ, USA), a porcine gelatin sponge, was used for embolization. Immediately before embolization, the gelatin sponge was cut into 1 × 1 × 1 mm cubes and hydrated with a 50/50 mixture of sterile normal saline and iopromide (Ultravist 300; Bayer Healthcare Korea, Seoul, Korea).
Angiography and Embolization
Angiography and embolization were performed in all rabbits by 2 interventional radiologists. The animals were anesthetized by intramuscular injection (1 mL/kg) of a 50/50 mixture of ketamine hydrochloride (Ketalar 50 mg/mL; Yuhan, Seoul, Korea) and xylazine hydrochloride (Rompun 23.32 mg/mL; Bayer, Seoul, Korea) in one of the hind legs. The skin was incised, and right inguinal dissection was performed to locate the common femoral artery. Cut-down was performed to enable direct visualization of the right common femoral artery during catheterization. The common femoral artery was punctured with an 18-gauge Teflon intravenous catheter (Sewoon Medical, Cheonan, Korea), and the needle and stylet were removed. A 2.2-Fr microcatheter (Sirabe; Piolax Medical Devices, Yokohama, Japan) with a curved tip, was inserted through the catheter and advanced to the main renal artery. Selective arteriography was performed, and the tip of the microcatheter was placed in the inferior segmental artery. The right renal artery was catheterized in most cases. The left renal artery was catheterized, if the right renal artery was difficult to catheterize. Arterial embolization was performed using the hydrated GSPs. The endpoint for embolization was defined as stasis of flow for more than 5 heartbeats, or reflux of contrast material into the main renal artery. Completion arteriography was performed to confirm occlusion of the embolized artery after embolization. The catheter was then removed, the punctured femoral artery was ligated, and the skin incision was closed.
Follow-Up
The rabbits were carefully observed for complications and changes in behavior after embolization. Four rabbits were randomly assigned to be sacrificed at each of the 6 time points: 1 day, 4 days, 1 week, 2 weeks, 3 weeks, and 4 weeks after embolization. Prior to sacrifice, each animal was anesthetized as described above, laparotomy was performed, and the abdominal aorta was punctured with an 18-guage needle for follow-up angiography.
Histopathological Examination
Each renal specimen was embedded in paraffin, and the block was cut into 4 pieces by transverse and coronal sectioning along the renal hilum. Two 4-µm-thick sections were then cut from each piece and stained with hematoxylin and eosin and immunohistochemical staining for smooth muscle actin. Pathological examination included evaluation of arterial occlusion levels, changes in the arterial walls, proliferation and migration of smooth muscle cells, inflammatory changes in the adjacent tissues, thrombosis, and degeneration of GSPs. The histopathological findings were analyzed by 2 radiologists and 1 pathologist by consensus.
RESULTS
All embolization procedures were completed without procedure-related vascular complications, such as dissection or spasm. No accidental embolization of non-target vessels was observed. None of the animals had post-procedural changes in behavior or sensation.
Light microscopy on day 1 post-procedure showed GSPs mainly in the renal segmental and interlobar arteries, with completely occluded vascular lumens and some thrombi. There were some inflammatory changes in the tissues adjacent to the embolized vessels, but the 3 layers of the arterial walls were intact. On day 4, the 3 layers of the arterial walls were still intact, and there was progression of arterial thrombi composed of fibrin and platelets. The thrombi were partially organized and associated with aggregates of macrophages and polymorphonuclear leukocytes in the lumens of vessels that contained GSPs (Fig. 1A). After 1 week, there was focal intimal destruction with transmural inflammation of the arterial wall. Polymorphonuclear leukocytes were the main inflammatory cells, and were located exclusively in the intima. The medial and adventitial layers were mildly thickened with proliferation of smooth muscle cells; however the surfaces of these layers were intact and the internal elastic lamina was well preserved (Fig. 1B). Angiograms performed immediately before sacrifice at 1 day, 4 days, and 1 week after the procedure showed persistence of the wedge-shaped defect and complete occlusion of the embolized arteries in all rabbits.
Fig. 1.
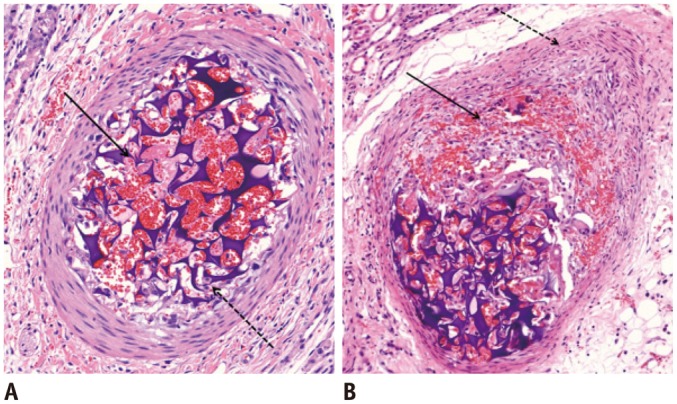
Microscopic images of segmental arteries within 1 week after embolization with GSPs.
A. Four days after embolization. There is partially organized thrombus (arrow), and aggregation of macrophages and polymorphonuclear leukocytes between GSPs (dotted arrow). Three layers of vessel wall are preserved. B. One week after embolization. There is focal intimal destruction with transmural inflammation (arrow). Medial and adventitial layers are slightly thickened with proliferation of smooth muscle cells (dotted arrow). Internal elastic lamina is intact (hematoxylin and eosin staining, original magnification × 20). GSPs = gelatin sponge particles
Most embolized arteries showed partial resorption of GSPs and a foreign body reaction with giant cells, after 2 weeks. There was focal dehiscence with propagation of transmural inflammation to the internal elastic lamina. In some vessels, there was destruction of all 3 layers of the wall due to severe transmural inflammation (Fig. 2). Angiography showed partial recanalization of the larger sections of the target arteries, with mild luminal irregularities. The GSPs were completely resorbed after 3 weeks. The vessel lumens were mostly filled with organized thrombi, and the recanalized arterial lumens were lined with migrating smooth muscle cells. Some small intraluminal collaterals had developed in the organized thrombi. Immunohistochemical staining for smooth muscle actin showed proliferation of smooth muscle cells in the media, thickened intima with migration of smooth muscle cells from the media, and interruption of the internal elastic lamina. The arteries with intimal thickening and severe organized thrombi had decreased luminal diameters. The migrating smooth muscle cells were mainly distributed around the recanalized arterial lumens. Angiography of the recanalized arteries showed diffuse luminal narrowing and multifocal stenosis (Fig. 3). The inflammation had subsided, and there were completely organized thrombi attached to the vessel wall after 4 weeks. There was multifocal loss of continuity of the internal elastic lamina, and the proliferation of smooth muscle cells had decreased (Fig. 4). The histological findings were shown in Table 1.
Fig. 2.
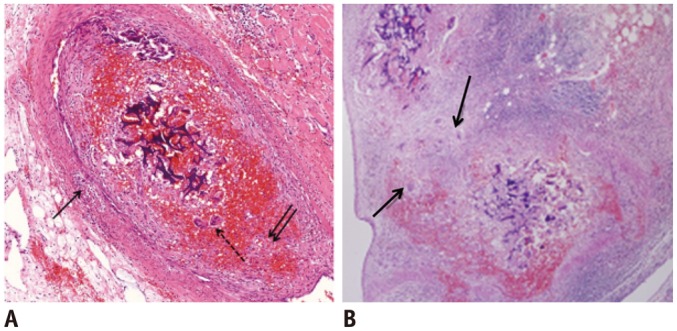
Microscopic images of interlobar arteries 2 weeks after embolization with GSPs.
A. There is partial resorption of GSPs. Note focal dehiscence of internal elastic lamina with propagation of transmural inflammation (arrows). There are some giant cells, indicating foreign body reaction (dotted arrow) (hematoxylin and eosin staining, original magnification × 20). B. There is destruction of all 3 layers in some arteries, resulting from extravasation of GSPs (arrows) (hematoxylin and eosin staining, original magnification × 10). GSPs = gelatin sponge particles
Fig. 3.
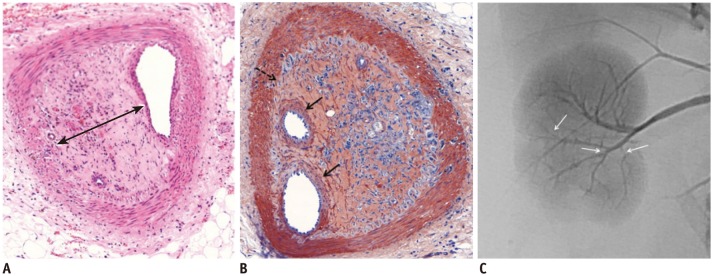
Microscopic images of interlobar arteries 3 weeks after embolization with GSPs.
A. Embolized GSPs have completely disappeared, resulting in recanalization. Original lumen is mainly filled with thick organized thrombi (arrow). B. Immunohistochemical staining for smooth muscle actin, showing proliferation of smooth muscle cells in media and thickened intima with migration of smooth muscle cells from media, and interruption of internal elastic lamina (dotted arrow). Migrating smooth muscle cells are mainly distributed around recanalized arterial lumen (arrows). C. Completion angiography, showing diffuse luminal narrowing and multifocal stenosis (arrows) in recanalized arteries of lower pole, compared with non-embolized arteries of upper pole. GSPs = gelatin sponge particles
Fig. 4.
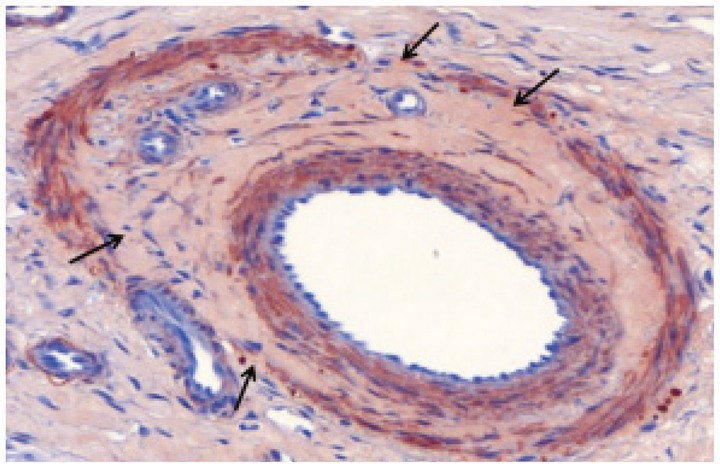
Microscopic image of interlobar artery 4 weeks after embolization with gelatin sponge particles. There are no inflammatory cells. Completely organized thrombus attached to vessel wall, and decreased proliferation of smooth muscle cells in media is observed. Internal elastic lamina has multifocal loss of continuity (arrows) (immunohistochemical staining for smooth muscle actin, original magnification × 20).
Table 1.
Summary of Histologic Findings

Note.- ++++ = very high intensity, +++ = moderate intensity, ++ = low intensity, + = very low intensity, - = absence. GSP = gelatin sponge particle (+ = exist, ± = partial resorption, - = complete resorption)
DISCUSSION
Gelatin sponge particles have frequently been used in transarterial embolization, since their identification as effective material for temporary arterial embolization (1, 2, 3, 4). Embolization with GSPs results in recanalization of the embolized artery within a few weeks, which contributes to the preservation of normal tissue in the embolized area and enables further transarterial embolization to control tumor growth (9, 10). Our experience with TACE showed that the embolized arteries were recanalized but frequently had areas of stenosis or occlusion; and the extent of stenosis/occlusion increased with the number of TACE sessions. Hepatic arterial stenosis or occlusion increases the difficulty of repeat catheterization, which can decrease the therapeutic effectiveness of further TACE procedures. We focused on the likely causative factors of vascular changes, and designed this study to investigate whether GSPs could independently induce arterial stenosis.
Histopathological examination showed significant stenoses in the recanalized arteries, mainly caused by organized thrombi and intimal hyperplasia. Intimal hyperplasia is defined as abnormal proliferation and migration of vascular smooth muscle cells with associated deposition of extracellular connective tissue matrices (11). Intimal hyperplasia has been regarded as a significant pathophysiological cause of the high restenosis rate in the current interventional environment (12, 13), ever since it was first described by Carrel and Guthries (14). Factors that are frequently associated with intimal hyperplasia after endovascular intervention, include angioplasty, stent insertion, and mechanical trauma from long-term venous catheterization (15). However, several studies reported that intimal hyperplasia could be induced by infusion of chemotherapeutic agents into arteries, even without mechanical injury. Maeda et al. (7) found that the accumulated dose of epirubicin during TACE was a significant predictor of hepatic arterial damage. Chemoembolization may result in as much or more damage to the arterial wall endothelium, as angioplasty (16). Chemotherapeutic agents such as doxorubicin and epirubicin are cytotoxic, and therefore reduce the ability of the hepatic arterial endothelium to recover, which triggers intimal hyperplasia in addition to the effects of catheterization trauma (17).
Erinjeri et al. (5) found that the main factor associated with vascular occlusion after chemoembolization was stasis of chemotherapeutic agents in large vessels; and that repeated hepatic artery bland embolization did not result in significant changes to the arterial vasculature. Their findings differ from the results of our study, which found that some of the recanalized arterial lumens were almost completely occluded after 3 weeks (Fig. 3). This difference may have resulted from the use of different embolic agents in the 2 studies. Erinjeri et al. (5) used spherical embolic agents that were 300 µm or smaller (Embosphere; Biosphere Medical, Rockland, MA, USA) for bland embolization. Spherical embolic agents form less aggregates and travel more distally into smaller vessels because there is little variability in particle size (5), resulting in fewer effects on the larger and more proximal arteries. Furthermore, spherical embolic agents cause fewer inflammatory reactions than GSPs, resulting in less intimal hyperplasia and organized thrombus (18, 19).
Gelatin sponge particles were mostly located in the renal segmental and interlobar arteries, causing proximal occlusion, in our study. A severe inflammatory reaction to the GSPs was observed for 2 weeks, after which the GSPs were partially resorbed. The vessels were completely recanalized at 3 weeks. However, the recanalized arterial lumens had significant stenoses because of the organized thrombi and intimal hyperplasia. The intimal hyperplasia was mainly caused by migration of smooth muscle cells from the media, in addition to some collagen deposition. Massive organized thrombi and intimal hyperplasia induced by bland embolization with GSPs, therefore lead to arterial stenosis. The massive organized thrombi appeared to be induced by acute inflammatory and foreign body reactions, and the intimal hyperplasia was caused by arterial wall damage from transmural inflammation and migration of smooth muscle cells after partial loss of continuity of the internal elastic lamina. Based on these results, more distal and highly selective embolization with the use of smaller embolic particles is essential for preventing stenosis of the proximal artery. As transmural inflammation is the main cause of intimal hyperplasia, it is possible to prevent intimal hyperplasia by using embolic materials that result in a less severe inflammatory reaction, or by administering anti-inflammatory agents or agents that inhibit migration of smooth muscle cells. A recent study found that the use of 2-day-soluble GSP was associated with a lower incidence of overt stenosis and occlusion (20). Further studies are needed to clarify this issue.
Our experimental study had the following limitations. Firstly, the renal artery was selected because it is easy to cannulate and has a diameter suitable for the experiment. However, transarterial embolization of the renal artery differs from hepatic arterial embolization for tumors, because the renal artery is an end-artery with no collateral circulation. Ideally, evaluation of intimal hyperplasia and arterial patency should be performed using hepatic or internal iliac arteries, which are not end-arteries. However, it is technically difficult to perform angiography and embolization of the hepatic and internal iliac arteries in the rabbit. Additional studies using larger animals are warranted. Secondly, the cross-linkage of GSP varies according to the degree of heating during the manufacturing process, and the resorption time may therefore differ among gelatin sponge products (21), resulting in different pathological changes to the vessels. Comparison studies using various gelatin sponge products are need to evaluate the different reactions.
In conclusion, GSPs are effective for temporary arterial embolization. The target arteries may develop severe transmural inflammation during the first 2 weeks after embolization, with recanalization by week 3. However, embolization with GSPs may induce significant arterial stenosis as a result of intimal hyperplasia with migration of smooth muscle cells, and formation of organized thrombus.
Footnotes
This work was supported by grant No. RTI04-01-04 from the Regional Technology Innovation Program of the Ministry of Commerce, Industry, and Energy (MOCIE) of Republic of Korea.
References
- 1.Doyon D, Mouzon A, Jourde AM, Regensberg C, Frileux C. [Hepatic, arterial embolization in patients with malignant liver tumours (author's transl)] Ann Radiol (Paris) 1974;17:593–603. [PubMed] [Google Scholar]
- 2.Goldstein HM, Wallace S, Anderson JH, Bree RL, Gianturco C. Transcatheter occlusion of abdominal tumors. Radiology. 1976;120:539–545. doi: 10.1148/120.3.539. [DOI] [PubMed] [Google Scholar]
- 3.Katsumori T, Kasahara T. The size of gelatin sponge particles: differences with preparation method. Cardiovasc Intervent Radiol. 2006;29:1077–1083. doi: 10.1007/s00270-006-0059-y. [DOI] [PubMed] [Google Scholar]
- 4.Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983;148:397–401. doi: 10.1148/radiology.148.2.6306721. [DOI] [PubMed] [Google Scholar]
- 5.Erinjeri JP, Salhab HM, Covey AM, Getrajdman GI, Brown KT. Arterial patency after repeated hepatic artery bland particle embolization. J Vasc Interv Radiol. 2010;21:522–526. doi: 10.1016/j.jvir.2009.12.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sueyoshi E, Hayashida T, Sakamoto I, Uetani M. Vascular complications of hepatic artery after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. AJR Am J Roentgenol. 2010;195:245–251. doi: 10.2214/AJR.08.2301. [DOI] [PubMed] [Google Scholar]
- 7.Maeda N, Osuga K, Mikami K, Higashihara H, Onishi H, Nakaya Y, et al. Angiographic evaluation of hepatic arterial damage after transarterial chemoembolization for hepatocellular carcinoma. Radiat Med. 2008;26:206–212. doi: 10.1007/s11604-007-0216-5. [DOI] [PubMed] [Google Scholar]
- 8.Sahara S, Kawai N, Sato M, Tanaka T, Ikoma A, Nakata K, et al. Prospective evaluation of transcatheter arterial chemoembolization (TACE) with multiple anti-cancer drugs (epirubicin, cisplatin, mitomycin c, 5-fluorouracil) compared with TACE with epirubicin for treatment of hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2012;35:1363–1371. doi: 10.1007/s00270-012-0352-x. [DOI] [PubMed] [Google Scholar]
- 9.Brown DB, Pilgram TK, Darcy MD, Fundakowski CE, Lisker-Melman M, Chapman WC, et al. Hepatic arterial chemoembolization for hepatocellular carcinoma: comparison of survival rates with different embolic agents. J Vasc Interv Radiol. 2005;16:1661–1666. doi: 10.1097/01.RVI.0000182160.26798.A2. [DOI] [PubMed] [Google Scholar]
- 10.Katsumori T, Nakajima K, Mihara T, Tokuhiro M. Uterine artery embolization using gelatin sponge particles alone for symptomatic uterine fibroids: midterm results. AJR Am J Roentgenol. 2002;178:135–139. doi: 10.2214/ajr.178.1.1780135. [DOI] [PubMed] [Google Scholar]
- 11.Davies MG, Hagen PO. Pathobiology of intimal hyperplasia. Br J Surg. 1994;81:1254–1269. doi: 10.1002/bjs.1800810904. [DOI] [PubMed] [Google Scholar]
- 12.Clowes AW. Intimal hyperplasia and graft failure. Cardiovasc Pathol. 1993;2:179S–186S. [Google Scholar]
- 13.Liu MW, Roubin GS, King SB., 3rd Restenosis after coronary angioplasty. Potential biologic determinants and role of intimal hyperplasia. Circulation. 1989;79:1374–1387. doi: 10.1161/01.cir.79.6.1374. [DOI] [PubMed] [Google Scholar]
- 14.Carrel A, Guthrie CC. Anastomosis of blood vessels by the patching method and transplantation of the kidney. 1906 [classical article] Yale J Biol Med. 2001;74:243–247. [PMC free article] [PubMed] [Google Scholar]
- 15.Waltham M, Harris J. Intimal hyperplasia: the nemesis of cardiovascular intervention. ANZ J Surg. 2004;74:719–720. doi: 10.1111/j.1445-1433.2004.03174.x. [DOI] [PubMed] [Google Scholar]
- 16.Strauss BH, Wilson RA, van Houten R, van Suylen RJ, Murphy ES, Escaned J, et al. Late effects of locally delivered mitomycin C on formation of neointima and on vasomotor response to acetylcholine. Coron Artery Dis. 1994;5:633–641. doi: 10.1097/00019501-199407000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Richard HM, 3rd, Silberzweig JE, Mitty HA, Lou WY, Ahn J, Cooper JM. Hepatic arterial complications in liver transplant recipients treated with pretransplantation chemoembolization for hepatocellular carcinoma. Radiology. 2000;214:775–779. doi: 10.1148/radiology.214.3.r00mr31775. [DOI] [PubMed] [Google Scholar]
- 18.Siskin GP, Dowling K, Virmani R, Jones R, Todd D. Pathologic evaluation of a spherical polyvinyl alcohol embolic agent in a porcine renal model. J Vasc Interv Radiol. 2003;14:89–98. doi: 10.1097/01.rvi.0000052296.26939.4c. [DOI] [PubMed] [Google Scholar]
- 19.Stampfl S, Bellemann N, Stampfl U, Radeleff B, Lopez-Benitez R, Sommer CM, et al. Inflammation and recanalization of four different spherical embolization agents in the porcine kidney model. J Vasc Interv Radiol. 2008;19:577–586. doi: 10.1016/j.jvir.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Kawai N, Sato M, Minamiguchi H, Ikoma A, Sanda H, Nakata K, et al. Clinical evaluation of transcatheter arterial chemoembolization with 2-day-soluble gelatin sponge particles for hepatocellular carcinoma-comparison with insoluble gelatin sponge particles. J Vasc Interv Radiol. 2013;24:1383–1390. doi: 10.1016/j.jvir.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Takasaka I, Kawai N, Sato M, Sahara S, Minamiguchi H, Nakai M, et al. A new soluble gelatin sponge for transcatheter hepatic arterial embolization. Cardiovasc Intervent Radiol. 2010;33:1198–1204. doi: 10.1007/s00270-010-9866-2. [DOI] [PubMed] [Google Scholar]


