Abstract
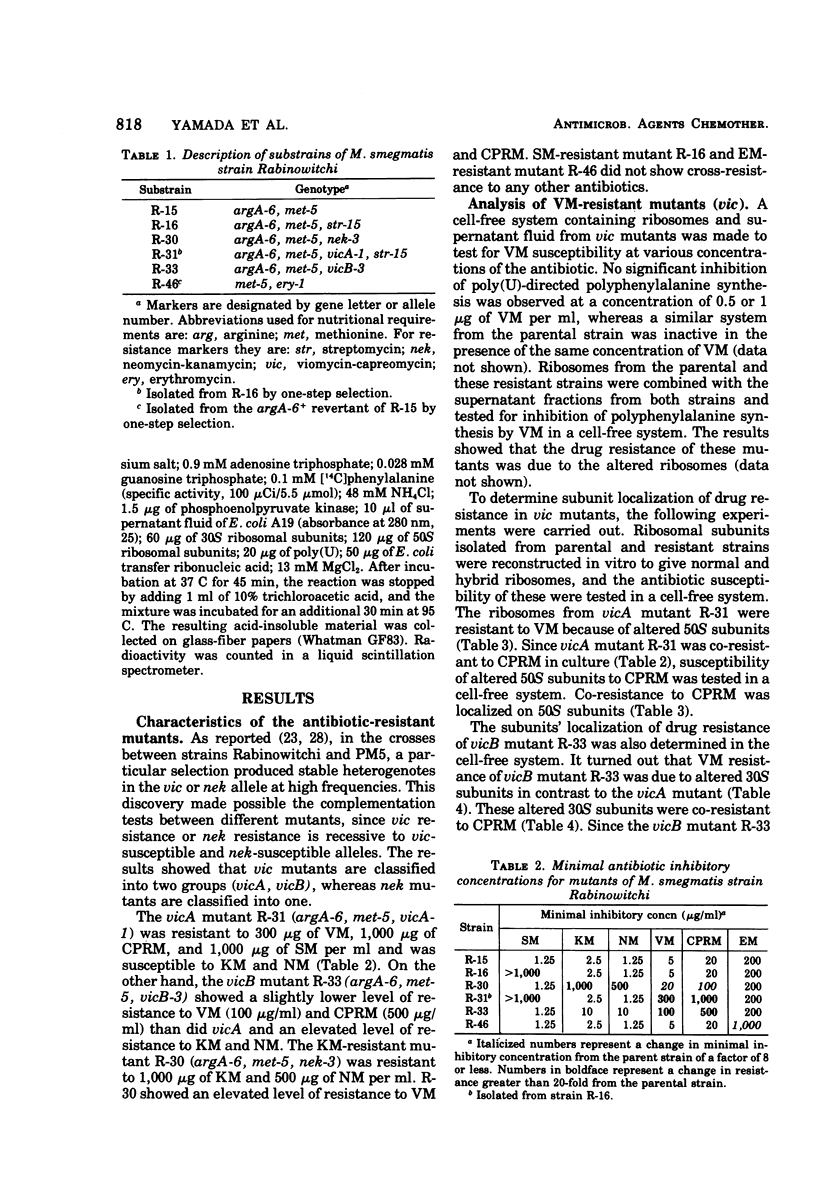
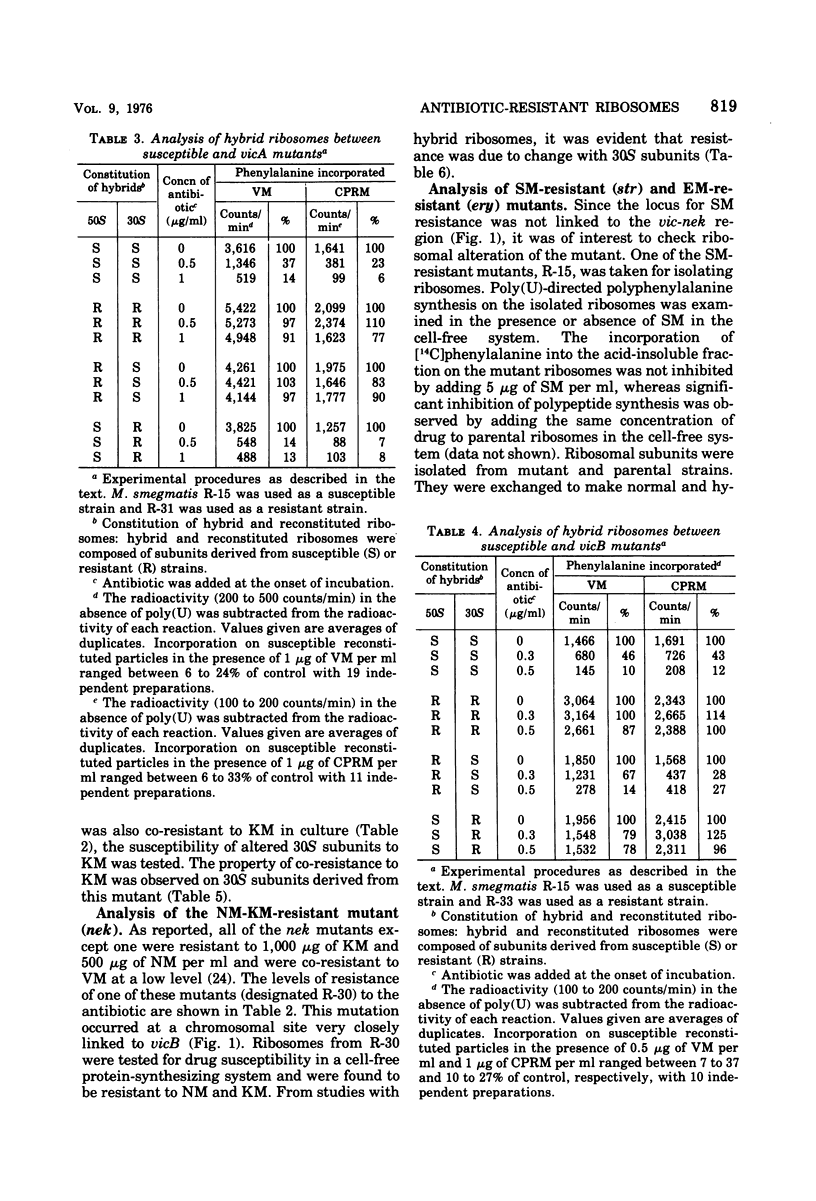
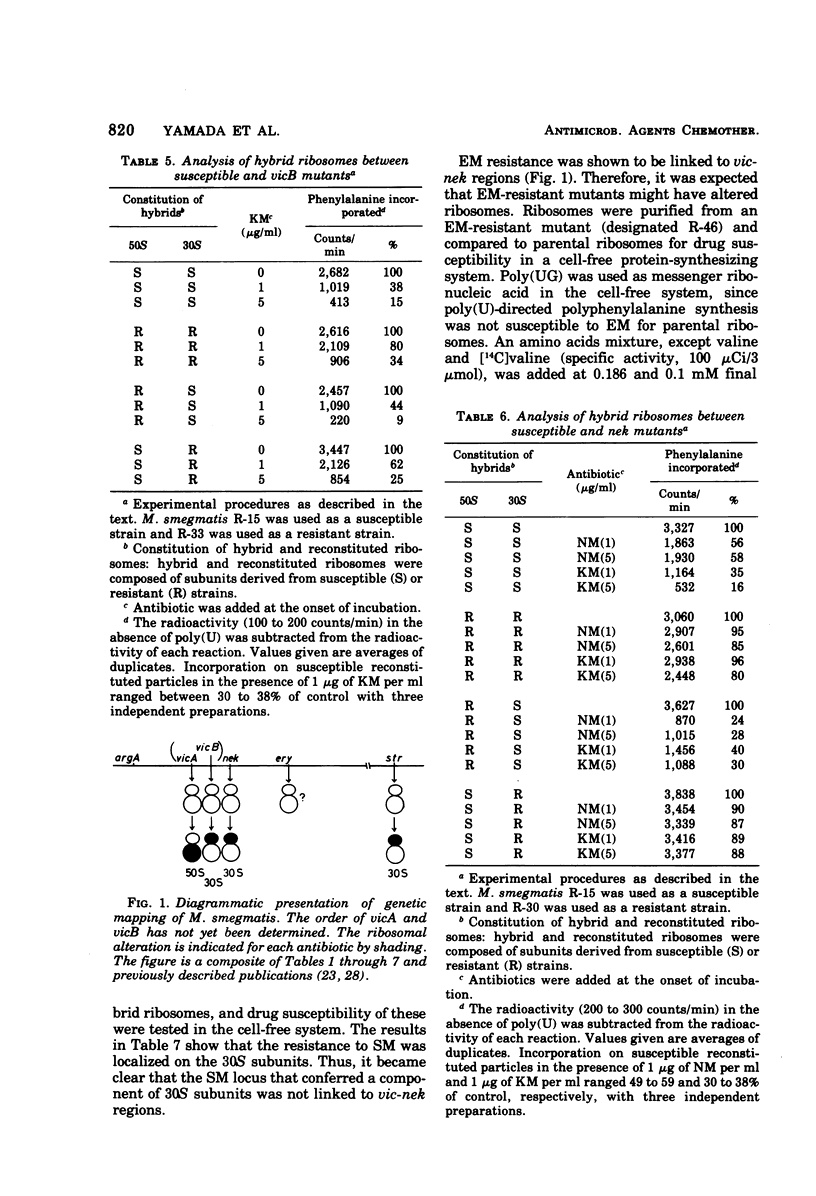
Two alleles for viomycin-capreomycin resistance (vic) in Mycobacterium smegmatis affect ribosome structures. One (vicA) affects a component of 50S subunits and the other (vicB) affects a component of 30S subunits. The locus for neomycin-kanamycin resistance (nek), which is linked to vicA and vicB, affects a component of 30S subunits. Although the erythromycin resistance locus (ery) is linked to vic and nek, no ribosomal alterations could be detected. Mutations at the streptomycin locus (str) not linked to vic and nek caused alterations of 30S subunits.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apirion D., Saltzman L. Functional interdependence of 50S and 30S ribosomal subunits. Mol Gen Genet. 1974;135(1):11–18. doi: 10.1007/BF00433896. [DOI] [PubMed] [Google Scholar]
- Apirion D., Schlessinger D. Coresistance to neomycin and kanamycin by mutations in an Escherichia coli locus that affects ribosomes. J Bacteriol. 1968 Sep;96(3):768–776. doi: 10.1128/jb.96.3.768-776.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apirion D., Schlessinger D. Functional interdependence of ribosomal components of Escherichia coli. Proc Natl Acad Sci U S A. 1969 Jul;63(3):794–799. doi: 10.1073/pnas.63.3.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apirion D., Schlessinger D., Phillips S., Sypherd P. Escherichia coli: reversion from streptomycin dependence, a mutation in a specific 30 s ribosomal protein. J Mol Biol. 1969 Jul 28;43(2):327–329. doi: 10.1016/0022-2836(69)90271-x. [DOI] [PubMed] [Google Scholar]
- Apirion D., Schlessinger D. Reversion from streptomycin dependence in Escherichia coli by a further change in the ribosome. J Bacteriol. 1967 Oct;94(4):1275–1276. doi: 10.1128/jb.94.4.1275-1276.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R., Davies J. Mechanisms of antibiotic resistance in bacteria. Annu Rev Biochem. 1973;42:471–506. doi: 10.1146/annurev.bi.42.070173.002351. [DOI] [PubMed] [Google Scholar]
- Birge E. A., Kurland C. G. Reversion of a streptomycin-dependent strain of Escherichia coli. Mol Gen Genet. 1970;109(4):356–369. doi: 10.1007/BF00267704. [DOI] [PubMed] [Google Scholar]
- Bollen A., Faelen M., Lecocq J. P., Herzog A., Zengel J., Kahan L., Nomura M. The structural gene for the ribosomal protein S18 in Escherichia coli. I. Genetic studies on a mutant having an alteration in the protein S18. J Mol Biol. 1973 Jun 5;76(4):463–472. doi: 10.1016/0022-2836(73)90485-3. [DOI] [PubMed] [Google Scholar]
- Brownstein B. L., Lewandowski L. J. A mutation suppressing streptomycin dependence. I. An effect on ribosome function. J Mol Biol. 1967 Apr 14;25(1):99–109. doi: 10.1016/0022-2836(67)90281-1. [DOI] [PubMed] [Google Scholar]
- Davies J., Nomura M. The genetics of bacterial ribosomes. Annu Rev Genet. 1972;6:203–234. doi: 10.1146/annurev.ge.06.120172.001223. [DOI] [PubMed] [Google Scholar]
- Deusser E., Stöffler G., Wittmann H. G. Ribosomal proteins. XVI. Altered S4 proteins in Escherichia coli revertants from streptomycin dependence to independence. Mol Gen Genet. 1970;109(4):298–302. doi: 10.1007/BF00267699. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Smith I., Marmur J. Gene conservation in Bacillus species. II. The location of genes concerned with the synthesis of ribosomal components and soluble RNA. Proc Natl Acad Sci U S A. 1965 Sep;54(3):724–730. doi: 10.1073/pnas.54.3.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaks J. G., Leboy P. S., Birge E. A., Kurland C. G. Mutations and genetics concerned with the ribosome. Cold Spring Harb Symp Quant Biol. 1966;31:623–631. doi: 10.1101/sqb.1966.031.01.081. [DOI] [PubMed] [Google Scholar]
- Friesen J. D., Fiil N. P., Parker J. M., Haseltine W. A. A new relaxed mutant of Escherichia coli with an altered 50S ribosomal subunit. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3465–3469. doi: 10.1073/pnas.71.9.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldthwaite C., Smith I. Physiological characterization of antibiotic resistant mutants of Bacillus subtilis. Mol Gen Genet. 1972;114(3):190–204. doi: 10.1007/BF01788888. [DOI] [PubMed] [Google Scholar]
- Harford N., Sueoka N. Chromosomal location of antibiotic resistance markers in Bacillus subtilis. J Mol Biol. 1970 Jul 28;51(2):267–286. doi: 10.1016/0022-2836(70)90142-7. [DOI] [PubMed] [Google Scholar]
- Hasenbank R., Guthrie C., Stöffler G., Wittmann H. G., Rosen L., Apirion D. Electrophoretic and immunological studies on ribosomal proteins of 100 Escherichia coli revertants from streptomycin dependence. Mol Gen Genet. 1973 Dec 14;127(1):1–18. doi: 10.1007/BF00267778. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. Streptomycin Resistance in Escherichia Coli Analyzed by Transduction. Genetics. 1960 Jan;45(1):49–62. doi: 10.1093/genetics/45.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreider G., Brownstein B. L. Ribosomal proteins involved in the suppression of streptomycin dependence in Escherichia coli. J Bacteriol. 1972 Feb;109(2):780–785. doi: 10.1128/jb.109.2.780-783.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzatto L., Schlessinger D., Apirion D. Escherichia coli: high resistance or dependence on streptomycin produced by the same allele. Science. 1968 Aug 2;161(3840):478–479. doi: 10.1126/science.161.3840.478. [DOI] [PubMed] [Google Scholar]
- Mizuguchi Y., Suga K., Masuda K., Yamada T. Genetic and biochemical studies on drug-resistant mutants in Mycobacterium smegmatis. Jpn J Microbiol. 1974 Nov;18(6):457–462. doi: 10.1111/j.1348-0421.1974.tb00834.x. [DOI] [PubMed] [Google Scholar]
- Mizuguchi Y., Tokunaga T. Recombination between Mycobacterium smegmatis strains Jucho and Lacticola. Jpn J Microbiol. 1971 Jul;15(4):359–366. doi: 10.1111/j.1348-0421.1971.tb00592.x. [DOI] [PubMed] [Google Scholar]
- Nashimoto H., Nomura M. Structure and function of bacterial ribosomes. XI. Dependence of 50S ribosomal assembly on simultaneous assembly of 30S subunits. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1440–1447. doi: 10.1073/pnas.67.3.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S. Inhibitors of ribosome functions. Annu Rev Microbiol. 1971;25:487–562. doi: 10.1146/annurev.mi.25.100171.002415. [DOI] [PubMed] [Google Scholar]
- Stöffler G., Deusser E., Wittmann H. G., Apirion D. Ribosomal proteins. XIX. Altered S5 ribosomal protein in an Escherichia coli revertant from strptomycin dependence to independence. Mol Gen Genet. 1971;111(4):334–341. doi: 10.1007/BF00569785. [DOI] [PubMed] [Google Scholar]
- Suga K., Mizuguchi Y. Mapping of antibiotic resistance markers in Mycobacterium smegmatis. Jpn J Microbiol. 1974 Mar;18(2):139–147. doi: 10.1111/j.1348-0421.1974.tb00802.x. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teraoka H., Tanaka K. Influence of the 50S ribosomal subunit on the ability of the 30S ribosomal subunit from Escherichia coli to bind dihydrostreptomycin. Biochem Biophys Res Commun. 1972 Jan 14;46(1):93–98. doi: 10.1016/0006-291x(72)90634-1. [DOI] [PubMed] [Google Scholar]
- Tokunaga T., Mizuguchi Y., Suga K. Genetic recombination in mycobacteria. J Bacteriol. 1973 Mar;113(3):1104–1111. doi: 10.1128/jb.113.3.1104-1111.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. J., Parker J., Fiil N. P., Flaks J. G., Friesen J. D. New chromosomal location for structural genes of ribosomal proteins. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2765–2769. doi: 10.1073/pnas.72.7.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Masuda K., Shoji K., Hori M. Analysis of ribosomes from viomycin-sensitive and -resistant strains of Mycobacterium smegmatis. J Bacteriol. 1972 Oct;112(1):1–6. doi: 10.1128/jb.112.1.1-6.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Masuda K., Shoji K., Hori M. Pleiotropic antibiotic resistance mutations associated with ribosomes and ribosomal subunits in Mycobacterium smegmatis. Antimicrob Agents Chemother. 1974 Jul;6(1):46–53. doi: 10.1128/aac.6.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa M., Okuyama A., Tanaka N. A third kasugamycin resistance locus, ksgC, affecting ribosomal protein S2 in Escherichia coli K-12. J Bacteriol. 1975 May;122(2):796–797. doi: 10.1128/jb.122.2.796-797.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



