Abstract
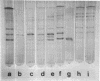
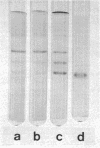
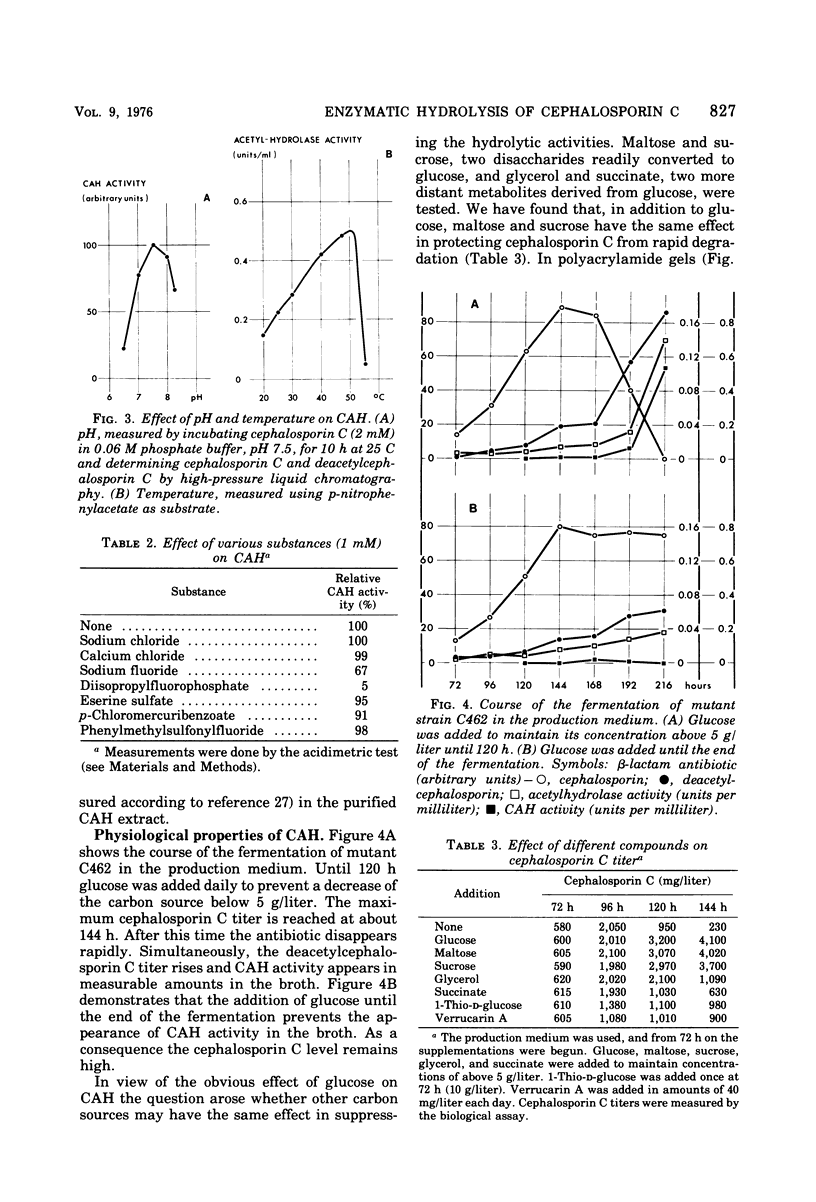
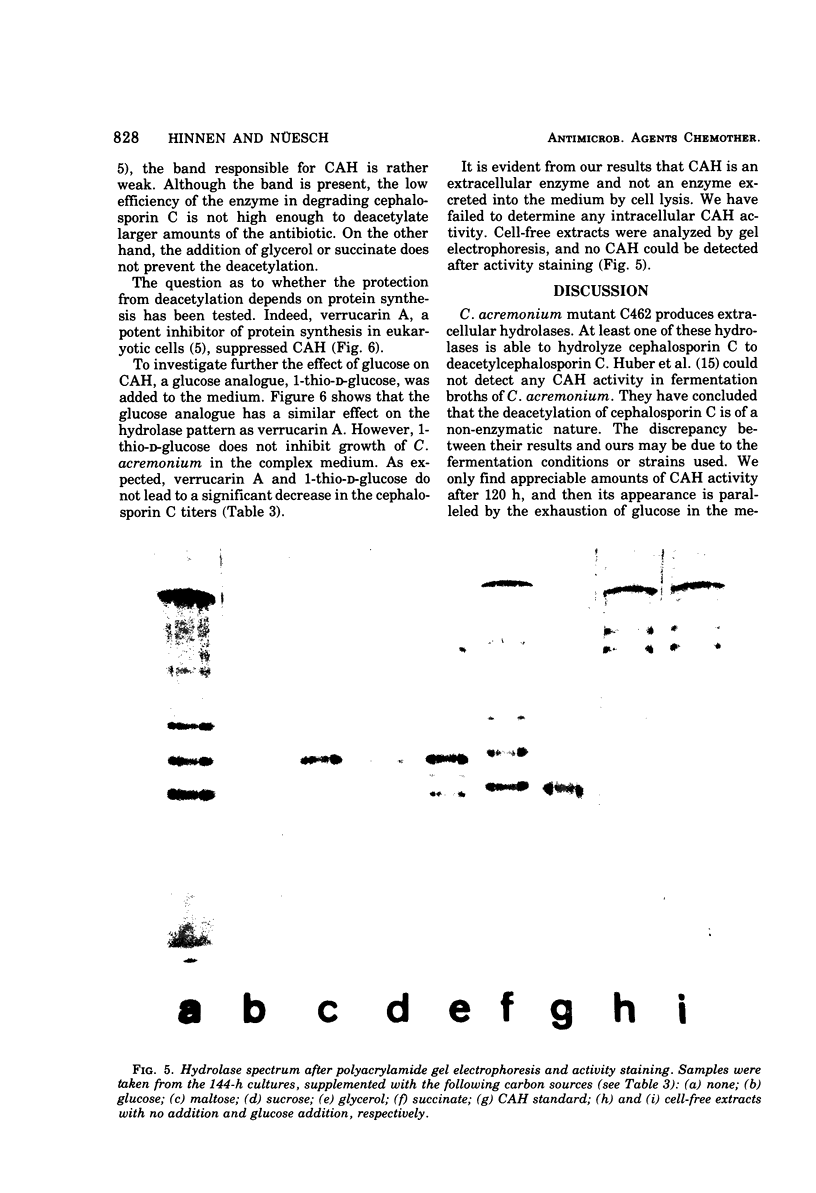
Extracellular hydrolases from Cephalosporium acremonium were analyzed according to their ability to deacetylate the β-lactam antibiotic cephalosporin C. One out of at least six hydrolases exhibits appreciable cephalosporin C acetylhydrolase (CAH) activity. This enzyme was separated from other hydrolases and purified 220-fold. The purified CAH has a relatively low affinity for cephalosporin C (Km, 20 mM) and is strongly inhibited by diisopropylfluorophosphate and less markedly affected by fluoride. Addition of glucose, maltose, and sucrose to the culture broth suppresses CAH production, whereas glycerol and succinate have no effect. Verrucarin A prevented the enzyme from appearing in the medium, which indicates the necessity of protein synthesis for CAH formation. When 1-thio-d-glucose was added to the culture medium, the results suggested that this glucose analogue is able to inhibit CAH synthesis. Our data provide evidence for a regulation of CAH synthesis similar to the catabolite repression system in bacteria.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arst H. N., Jr, MacDonald D. W. A gene cluster in Aspergillus nidulans with an internally located cis-acting regulatory region. Nature. 1975 Mar 6;254(5495):26–31. doi: 10.1038/254026a0. [DOI] [PubMed] [Google Scholar]
- Bailey C., Arst H. N., Jr Carbon catabolite repression in Aspergillos nidulans. Eur J Biochem. 1975 Feb 21;51(2):573–577. doi: 10.1111/j.1432-1033.1975.tb03958.x. [DOI] [PubMed] [Google Scholar]
- Benz F., Liersch M., Nüesch J., Treichler H. J. Methionine metabolism and cephalosporin C synthesis in Cephalosporium acremonium. D-amino acid oxidase. Eur J Biochem. 1971 May 11;20(1):81–88. doi: 10.1111/j.1432-1033.1971.tb01365.x. [DOI] [PubMed] [Google Scholar]
- Caltrider P. G., Niss H. F. Role of methionine in cephalosporin synthesis. Appl Microbiol. 1966 Sep;14(5):746–753. doi: 10.1128/am.14.5.746-753.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundliffe E., Cannon M., Davies J. Mechanism of inhibition of eukaryotic protein synthesis by trichothecene fungal toxins. Proc Natl Acad Sci U S A. 1974 Jan;71(1):30–34. doi: 10.1073/pnas.71.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMAIN A. L., NEWKIRK J. F., HENDLIN D. Effect of methionine, norleucine, and lysine derivatives on cephalosporin C formation in chemically defined media. J Bacteriol. 1963 Feb;85:339–344. doi: 10.1128/jb.85.2.339-344.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Crombrugghe B., Perlman R. L., Varmus H. E., Pastan I. Regulation of inducible enzyme synthesis in Escherichia coli by cyclic adenosine 3', 5'-monophosphate. J Biol Chem. 1969 Nov 10;244(21):5828–5835. [PubMed] [Google Scholar]
- Epstein W., Rothman-Denes L. B., Hesse J. Adenosine 3':5'-cyclic monophosphate as mediator of catabolite repression in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2300–2304. doi: 10.1073/pnas.72.6.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa Y., Shirafugi H., Kida M., Nara K., Yoneda M., Kanzaki T. New findings on cephalosporin C biosynthesis. Nat New Biol. 1973 Dec 5;246(153):154–155. doi: 10.1038/newbio246154a0. [DOI] [PubMed] [Google Scholar]
- Hayes M. B., Wellner D. Microheterogeneity of L-amino acid oxidase. Separation of multiple components by polyacrylamide gel electrofucusing. J Biol Chem. 1969 Dec 25;244(24):6636–6644. [PubMed] [Google Scholar]
- Holzer H., Duntze W. Metabolic regulation by chemical modification of enzymes. Annu Rev Biochem. 1971;40:345–374. doi: 10.1146/annurev.bi.40.070171.002021. [DOI] [PubMed] [Google Scholar]
- Huber F. M., Baltz R. H., Caltrider P. G. Formation of desacetylcephalosporin C in cephalosporin C fermentation. Appl Microbiol. 1968 Jul;16(7):1011–1014. doi: 10.1128/am.16.7.1011-1014.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme M. A., Stranks D. W. Induction and the regulation of production of cellulase by fungi. Nature. 1970 May 2;226(5244):469–470. doi: 10.1038/226469a0. [DOI] [PubMed] [Google Scholar]
- Hynes M. J. Mutants with altered glucose repression of amidase enzymes in Aspergillus nidulans. J Bacteriol. 1972 Sep;111(3):717–722. doi: 10.1128/jb.111.3.717-722.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konecny J., Felber E., Gruner J. Kinetics of the hydrolysis of cephalosporin C. J Antibiot (Tokyo) 1973 Mar;26(3):135–141. doi: 10.7164/antibiotics.26.135. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- NEWTON G. G., ABRAHAM E. P. Cephalosporin C, a new antibiotic containing sulphur and D-alpha-aminoadipic acid. Nature. 1955 Mar 26;175(4456):548–548. doi: 10.1038/175548a0. [DOI] [PubMed] [Google Scholar]
- Pastan I., Perlman R. Cyclic adenosine monophosphate in bacteria. Science. 1970 Jul 24;169(3943):339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- Pocker Y., Stone J. T. The catalytic versatility of erythrocyte carbonic anhydrase. 3. Kinetic studies of the enzyme-catalyzed hydrolysis of p-nitrophenyl acetate. Biochemistry. 1967 Mar;6(3):668–678. doi: 10.1021/bi00855a005. [DOI] [PubMed] [Google Scholar]
- Rubin F. A., Smith D. H. Characterization of R factor beta-lactamases by the acidimetric method. Antimicrob Agents Chemother. 1973 Jan;3(1):68–73. doi: 10.1128/aac.3.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B., Warren S. C., Newton G. G., Abraham E. P. Biosynthesis of penicillin N and cephalosporin C. Antibiotic production and other features of the metabolism of Cephalosporium sp. Biochem J. 1967 Jun;103(3):877–890. doi: 10.1042/bj1030877. [DOI] [PMC free article] [PubMed] [Google Scholar]