Abstract
Desmosomal cadherins constitute the adhesive core of desmosomes. The different types of these cadherins are differentially expressed in a tissue specific as well as differentiation dependent manner. The skin and the heart are two examples of tissues whose vital functions require the ability to endure mechanical stress, and therefore, the integrity of desmosomal adhesion. When this adhesion is compromised via mutations in genes encoding desmosomal cadherins or associated plaque proteins, both tissues can suffer the consequences. Open questions revolve around whether the resulting phenotypes are solely due to physical disruption of cell adhesion, or whether these events are coupled with signaling mechanisms that influence many additional cellular processes. In this review, we focus on new developments in desmosomal adhesion with an emphasis on the skin, hair and heart.
Keywords: Desmosomes, Desmoglein, Desmocollin, Skin, Hair, Heart
Introduction
In recent years, elegant research from many different laboratories has provided definitive evidence that desmosomes, the “welding spots” of cell-cell junctions, are essential for the morphogenesis, differentiation, and maintenance of tissues that are subjected to high mechanical stress such as the skin and heart [1]. The highly electron dense and symmetric desmosomes are abundant between the keratinocytes of the skin epidermis and its appendages, as well as the myocytes of the heart, but can also be found in some specialized cells of the meninges and lymph nodes [2]. The importance of desmosomes lies in their unique ability to connect cells on the extracellular side while coupling this connection to the intermediate filament cytoskeleton on the intracellular side, for example, to keratins in keratinocytes and to desmin in myocytes (Figure 1). This extracellular-intracellular connection confers resilient mechanical properties to the whole tissue, in addition to regulating cytoskeletal organization and impacting on gene expression.
Figure 1.
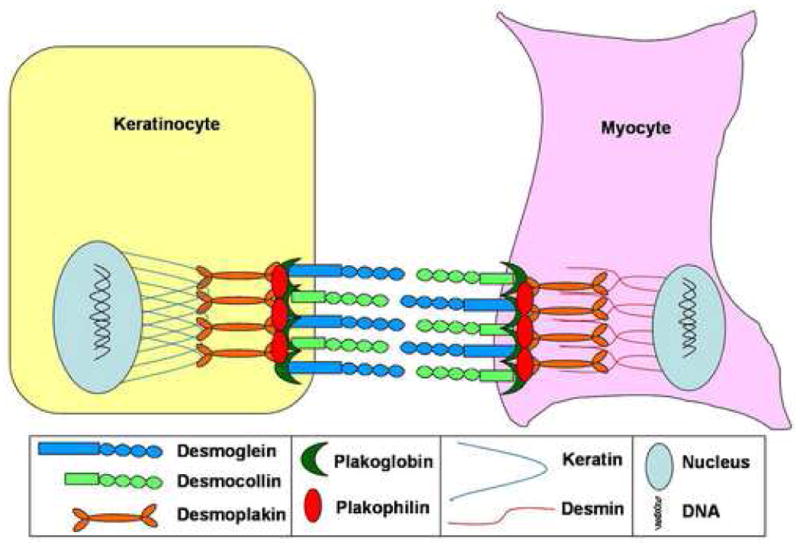
Desmosomal adhesion is essential between cell types that must withstand high mechanical stress such as skin keratinocytes and cardiac myocytes.
The desmosomal cadherins are calcium-dependent adhesive glycoproteins whose N-termini comprise the outermost extracellular junctional interface of desmosomes (Figure 1). They belong to two families, the desmogleins (DSG) and desmocollins (DSC) [3], and interact preferentially in a heterophilic manner [4]. To date, there are four known desmogleins (DSG1-4) and three desmocollins (DSC1-3) that reside in a genomic cluster on human chromosome 18q21, and the syntenic regions of chromosome 18 in the mouse and rat genomes [5]. DSG and DSC are expressed in a tissue- and differentiation-specific manner, as exemplified by their expression pattern in the epidermis and hair follicle, highlighting the unique nature of each of these desmosomal cadherins in conferring or maintaining cellular differentiation programs (see below) [6].
Desmosomal cadherins interact on the intracellular side with desmosomal plaque proteins of the armadillo and plakin families that provide the continuum with the intermediate filament cytoskeleton of the cell (Figure 1). The armadillo protein, plakoglobin (PKG, gene symbol JUP for junction plakoglobin), and the plakin protein, desmoplakin (DSP), are ubiquitously expressed in all desmosome-containing tissues, including the skin and heart. The armadillo proteins, plakophilins (PKP1-4), on the other hand, also show tissue and differentiation specific expression patterns [7].
In this review, we will focus on recent developments in studying the roles of desmosomal cadherins in epidermal and hair follicle keratinocytes, as well as cardiac myocytes, through loss of function and gain of function analyses.
Lessons from Human Skin and Heart Diseases and Mutant Animals
Desmosomal Cadherins in the Skin
In 1997, Koch et al. reported the first targeted ablation of a desmosomal cadherin, Dsg3, which is allelic to the naturally occurring balding mouse [8]. Dsg3 knockout mice are runted, display hair loss due to failure of telogen club hair anchorage, and recapitulate many of the aspects of the autoimmune disease pemphigus vulgaris such as oral blistering. To date, no equivalent human mutations have been found in the DSG3 gene. On the other hand, Dsc1 knockout mice show epidermal barrier defects and ulcerating lesions and hair follicle degeneration in older mice, thus revealing a role for Dsc1 in the integrity of the differentiating layers of the epidermis and its appendages [9]. Likewise, no known human diseases have been associated with DSC1 mutations. Dominant mutations in the DSG1 gene result in haploinsufficiency which causes striate palmoplantar keratoderma in humans (OMIM# 148700 ) characterized by callous thickening of the palms and soles, areas that are under continuous mechanical stress and friction [10], however, in this case no corresponding mouse model exists. Unlike in humans and rats, the mouse genome has three Dsg1 genes (Dsg1α, Dsg1β, Dsg1γ) that show redundant expression patterns, and due to their genomic proximity, a tandem knockout might prove to be technically difficult [11].
The most recently discovered desmosomal cadherin, desmoglein 4, is essential for hair shaft integrity and its loss of function leads to localized autosomal recessive hypotrichosis in humans (LAH, OMIM# 607903) and the lanceolate hair phenotype in mice and rats [5,12]. DSG4 is highly expressed in the hair shaft cortex where the abnormal phenotype arises in LAH patients and lah rodents [6]. In recently reported cases, recessively inherited human mutations in DSG4 can also result in moniliform hairs [13–15]. Interestingly, there is some clinical overlap between monilethrix (OMIM# 158000 and # 252200) which is caused by dominant mutations in type II hair keratin genes that are also expressed in the hair cortex (Hb1, Hb3, or Hb6) and LAH. It is conceivable, therefore, that DSG4 interacts intracellularly with the hair keratin proteins, though this remains to be shown experimentally, and therefore mutations in their respective genes cause similar hair phenotypes [16]. This suggests that LAH and monilethrix are skin desmosomal diseases associated primarily with the hair shaft.
Not all desmosomal cadherin knockout mutations are born alive and exhibit a phenotype, in fact, somewhat surprisingly, some are embryonic lethal in early development. For example, Dsg2 knockout mice die around the time of implantation, revealing the importance of this desmosomal cadherin, and perhaps desmosomes in general, during embryonic development [17]. More recently, Den et al. reported that Dsc3 knockout mice die before implantation [18]. Surprisingly, Dsc3 knockout mice die even before the formation of any discernible mature desmosomes, hinting at a novel and perhaps extra-desmosomal role for desmosomal cadherins during development [18]. Similar to DSG3 and DSC1, there is no known human disease associated with mutations in DSC3 (Table 1). DSG2 mutations in humans will be discussed below.
Table 1. Mutations in the genes encoding desmosomal components affect the skin, heart or both.
LAH, Localized autosomal recessive hypotrichosis; ARVC, Arrhythmogenic right ventricular cardiomyopathy; AD, Autosomal dominant; AR, Autosomal recessive; CH, Compound heterozygous; KO, Knockout; N/A, Not applicable (not reported).
| Tissue Affected | Gene | Human Disease | Pattern of Inheritance | Mouse Model | References |
|---|---|---|---|---|---|
| Skin and/or Hair Only | DSG1 | Striate Palmo- Plantar Keratoderma | AD | N/A | [10], [2] |
| DSG3 | N/A | N/A | balding mouse, KO | [8] | |
| DSG4 | LAH | AR | Lanceolate hair mice and rats | [5], [12], [13–16] | |
| DSC1 | N/A | N/A | KO | [9] | |
| PKP1 | Ectodermal Dysplasia/Skin Fragility | AR, CH | N/A | [35], [2] | |
|
| |||||
| Heart Only | DSG2 | ARVC | AD, CH | KO | [17], [19], [21], [22] |
| DSC2 | ARVC | AD | KO | [20] | |
| PKP2 | ARVC | AD, AR | N/A | [30–33] | |
|
| |||||
| Skin, Hair and Heart | DSP | Carvajal Syndrome, ARVC | AD, AR, CH | KO, Conditional KO | [27], [28], [36] |
| PKG | Naxos Disease | AR | KO | [26] | |
Desmosomal Caderins in the Heart
Unlike DSG1/3/4 and DSC1/3 which are predominantly expressed in the skin epidermis and its appendages, DSG2 and DSC2 are highly expressed in the myocardium of the heart. In support of the important function of these desmosomal cadherins in the heart, recent studies show that heterozygous mutations in DSC2 and DSG2 in humans cause arrhythmogenic right ventricular cardiomyopathy (ARVC, OMIM# 107970) characterized by right ventricular fibro-fatty replacement of myocardial tissue by the conversion of myocytes into adipocyes [19–22] (Table 1). As mentioned earlier, desmosomes are important in tissues that must withstand high significant mechanical stress. Accordingly, the major affected areas of the heart in ARVC are the thinnest portions of the right ventricle [23]. This explanation relies on the physical and mechanical aspects of desmosomal adhesion, but what is the mechanism of transdifferentiation of myocytes into adipocytes? A study by Garcia-Gras et al. offers an explanation that invokes a signaling role for desmosomes [24]. This study suggests that mutations in the desmosomal cadherins expressed in the heart lead to the accumulation of nuclear PKG. They provide evidence that excess PKG translocates to the nucleus and displaces ß-catenin, thus compromising Wnt signaling. These authors propose that the ensuing suppression of Wnt/ß-catenin signaling could direct the differentiation of cardiac myocytes into adipose cells [24].
Desmosomal Plaque Components: Binding Together the Skin and Heart
If mutations in the genes encoding desmosomal cadherins cause abnormal skin and heart phenotypes, it is conceivable that corresponding mutations in genes encoding desmosomal plaque components (PKG, DSP and PKP), which are also expressed in all desmosome-bearing tissues, might lead to similar phenotypes in these tissues. It is noteworthy that knockout mice for PKG and DSP are early embryonic lethal, thus highlighting the importance of desmosomes in the developing vital embryonic tissues, such as the heart [25]. However, in humans, milder recessive mutations in the genes encoding the plaque proteins PKG and DSP cause Naxos disease (OMIM# 601214) and Carvajal syndrome (OMIM# 605656), respectively, characterized by ARVC in addition to woolly hair and striate palmoplantar keratoderma (Table 1) [26–28]. There must be yet additional gene mutations underlying Naxos syndrome since DSP and PKG have been excluded as candidate genes in some families [29]. Moreover, dominantly inherited mutations in PKP2 also cause ARVC in humans, whereas Pkp2 knockout mice show reduced heart trabeculation, rupture of cardiac walls, and blood leakage into the pericardiac cavity leading to lethality at mid-gestation [30–33]. Therefore, these studies suggest that ARVC is a cardiac desmosomal disease that can be caused by mutations in the genes encoding the desmosomal cadherins DSG2 and DSC2, as well as desmosomal plaque components such as PKG, DSP and PKP2 (Table 1) [34]. The same reasoning is also true for skin and hair diseases that can arise due to mutations in either genes encoding desmosomal cadherins that are expressed in the skin or genes encoding desmosomal plaque components (e.g. DSP, PKG and PKP1) (Table 1). In addition to the above examples on DSP and PKG, it is now well established in the literature that mutations in PKP1 cause ectodermal dysplasia- skin fragility syndrome (OMIM# 604536) characterized by skin blistering, alopecia, nail dystrophy, and focal keratoderma [35]. In mice, the conditional knockout of DSP from the epidermis compromises epidermal sheet formation and integrity in vivo and in vitro [36].
Disturbing the Balance of Desmosomal Cadherins: Transgenic Mouse Models and Cancer
A key unsolved question in the field of desmosomal cadherins is why there are so many different cadherins that are so uniquely differentially expressed? The previous dogma was that desmsomes are simply a “clamp” between neighboring cells. Under this premise, a logical answer to this question maybe that different desmosomal cadherins possess different adhesive properties that are custom suited for different types of cells or differentiation programs. In this respect, different permutations are possible between different desmosomal cadherins in order to adjust the level of adhesiveness of desmosomes. However, an emerging view is that desmosomes, like the classical adherens junctions, are involved in signaling pathways that dictate or maintain the differentiation status or program of the cells [1]. Due to its functional similarity to β-catenin in desmosomes, the armadillo protein PKG, also called γ-catenin, has been at the center of investigation with ample evidence to support a signaling role besides its adhesive role [37,38]. These two possibilities of desmosome function are by no means mutually exclusive and more in vivo experiments will be required to thoroughly explore the signaling role desmosomal components.
Transgenic Mouse Models: Ectopic Expression of Desmosomal Cadherins in the Epidermis
Transgenic mice with ectopic gene expression are very useful tools to assess whether different desmosomal cadherins are tailored for one differentiation program or another. Moreover, the epidermis is an excellent stratified epithelium in which to test these hypotheses. Desmosomal cadherins show gradients of expression in the epidermis that are correlated with the differentiation status of keratinocytes from the proliferative basal layer to the fully differentiated cornified layer [6]. In addition, the ability to target gene expression to a particular layer using well-defined promoters, such as K5/K14 to the basal layer and K1/K10 and involucrin to the suprabasal layers, enables the ectopic expression of a particular desmosomal cadherin to a layer in which it is normally not expressed or is expressed at lower levels.
Using this strategy, Hardman et al. ectopically expressed Dsc3 under the regulation of the K1 promoter in the suprabasal layers of the epidermis where it is normally expressed at low levels [39]. The transgenic mice developed ventral alopecia in adulthood associated with a hyperproliferative epidermal phenotype. In addition, a link between ß-catenin stability and Dsc3 transgene expression was noted in this study, thus pointing once again to a connection between desmosomal cadherins and the Wnt signaling pathway [39]. This study also provided evidence for an equivalent function between the two Dsc isoforms “a” and “b” which differ in their cytoplasmic tail due to alternative splicing, the latter of which was assumed to have no function so far.
A similar and more recent study by Brennan et al. utilized the involucrin promoter to ectopically express Dsg2 in the suprabasal layers where it is not normally detected [40]. These transgenic mice developed epidermal hyperplasia that is NFκB dependent, and notably, were more susceptible to chemically induced carcinogenesis [40].
Desmosomes and Cancer
DSG2 misexpression has been associated with human squamous cell carcinomas and gastric cancers, where it is either lost or overexpressed [41–43]. Along the same lines, DSC2 and DSC3 expression is downregulated in colorectal and breast cancer, respectively [44,45]; whereas DSG3 is overexpressed in squamous cell carcinoma and head and neck cancer [43,46]. In general, whether desmosomes are involved in cancer and metastasis is very poorly understood [47]. The links between classical cadherin (such as E-cadherin) downregulation and metastasis through epithelial-mesenchymal transition are well established [48]. It remains to be determined whether a similar link between desmosomes and cancer exists and if so, which desmosomal components or which type of desmosomal cadherins.
Desmosomes have evolved to enable tissues like the heart and the skin and its appendages (e.g. hair) withstand mechanical stress. Consequently, when desmosomal adhesion is compromised through gene mutations these tissues are primarily affected. As more research is conducted on desmosomes, we are bound to further understand not only the physical nature of these junctions but also their prospective roles as signaling centers during development, homeostasis and disease.
Acknowledgments
We thank members of the Christiano laboratory for critical reading and discussions. . We apologize to our colleagues whose work we were not able to cite in detail due to space limitations. Our original studies cited herein were supported in part by NIH/NIAMS grant R01AR44924 to AMC.
Abbreviations
- DSG
desmoglein
- DSC
desmocollin
- DSP
desmoplakin
- PKG
plakoglobin
- PKP
plakophilin
- LAH
localized autosomal recessive hypotrichosis
- ARVC
arrhythmogenic right ventricular cardiomyopathy. Genes are italicized and non-human genes/proteins with only the first letter capitalized
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dusek RL, Godsel LM, Green KJ. Discriminating roles of desmosomal cadherins: beyond desmosomal adhesion. J Dermatol Sci. 2007;45:7–21. doi: 10.1016/j.jdermsci.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 2.McGrath JA. Inherited disorders of desmosomes. Australas J Dermatol. 2005;46:221–229. doi: 10.1111/j.1440-0960.2005.00188.x. [DOI] [PubMed] [Google Scholar]
- 3.Koch PJ, Goldschmidt MD, Zimbelmann R, Troyanovsky R, Franke WW. Complexity and expression patterns of the desmosomal cadherins. Proc Natl Acad Sci U S A. 1992;89:353–357. doi: 10.1073/pnas.89.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Syed SE, Trinnaman B, Martin S, Major S, Hutchinson J, Magee AI. Molecular interactions between desmosomal cadherins. Biochem J. 2002;362:317–327. doi: 10.1042/0264-6021:3620317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kljuic A, Bazzi H, Sundberg JP, Martinez-Mir A, O'Shaughnessy R, Mahoney MG, Levy M, Montagutelli X, Ahmad W, Aita VM, et al. Desmoglein 4 in hair follicle differentiation and epidermal adhesion: evidence from inherited hypotrichosis and acquired pemphigus vulgaris. Cell. 2003;113:249–260. doi: 10.1016/s0092-8674(03)00273-3. This report highlights the power of comparative genomics between humans and mice affected with a similar phenotype of hypotrichosis. It is the first report of Dsg4 mutations causing LAH in humans and lah hair in mice. [DOI] [PubMed] [Google Scholar]
- 6.Bazzi H, Getz A, Mahoney MG, Ishida-Yamamoto A, Langbein L, Wahl JK, 3rd, Christiano AM. Desmoglein 4 is expressed in highly differentiated keratinocytes and trichocytes in human epidermis and hair follicle. Differentiation. 2006;74:129–140. doi: 10.1111/j.1432-0436.2006.00061.x. [DOI] [PubMed] [Google Scholar]
- 7.Kottke MD, Delva E, Kowalczyk AP. The desmosome: cell science lessons from human diseases. J Cell Sci. 2006;119:797–806. doi: 10.1242/jcs.02888. [DOI] [PubMed] [Google Scholar]
- 8.Koch PJ, Mahoney MG, Ishikawa H, Pulkkinen L, Uitto J, Shultz L, Murphy GF, Whitaker-Menezes D, Stanley JR. Targeted disruption of the pemphigus vulgaris antigen (desmoglein 3) gene in mice causes loss of keratinocyte cell adhesion with a phenotype similar to pemphigus vulgaris. J Cell Biol. 1997;137:1091–1102. doi: 10.1083/jcb.137.5.1091. This represents the first report of a desmosomal cadherin genetic knockout in mice. Dsg3 knockout not only causes hair loss during the telogen phase of the hair cycle, but also recapitulates the phenotype of the autoimmune blistering disease, pumphigus vulgaris, in which Dsg3 is an autoantigen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chidgey M, Brakebusch C, Gustafsson E, Cruchley A, Hail C, Kirk S, Merritt A, North A, Tselepis C, Hewitt J, et al. Mice lacking desmocollin 1 show epidermal fragility accompanied by barrier defects and abnormal differentiation. J Cell Biol. 2001;155:821–832. doi: 10.1083/jcb.200105009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rickman L, Simrak D, Stevens HP, Hunt DM, King IA, Bryant SP, Eady RA, Leigh IM, Arnemann J, Magee AI, et al. N-terminal deletion in a desmosomal cadherin causes the autosomal dominant skin disease striate palmoplantar keratoderma. Hum Mol Genet. 1999;8:971–976. doi: 10.1093/hmg/8.6.971. [DOI] [PubMed] [Google Scholar]
- 11.Brennan D, Hu Y, Kljuic A, Choi Y, Joubeh S, Bashkin M, Wahl J, Fertala A, Pulkkinen L, Uitto J, et al. Differential structural properties and expression patterns suggest functional significance for multiple mouse desmoglein 1 isoforms. Differentiation. 2004;72:434–449. doi: 10.1111/j.1432-0436.2004.07208009.x. [DOI] [PubMed] [Google Scholar]
- 12.Jahoda CA, Kljuic A, O'Shaughnessy R, Crossley N, Whitehouse CJ, Robinson M, Reynolds AJ, Demarchez M, Porter RM, Shapiro L, et al. The lanceolate hair rat phenotype results from a missense mutation in a calcium coordinating site of the desmoglein 4 gene. Genomics. 2004;83:747–756. doi: 10.1016/j.ygeno.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Shimomura Y, Sakamoto F, Kariya N, Matsunaga K, Ito M. Mutations in the desmoglein 4 gene are associated with monilethrix-like congenital hypotrichosis. J Invest Dermatol. 2006;126:1281–1285. doi: 10.1038/sj.jid.5700113. The above three reports highlight the similarity in clinical presentation between LAH and monilethrix that are caused by mutations in desmosomal cadherins (DSG4 for LAH) or hair keratins (monilethrix), respectively. [DOI] [PubMed] [Google Scholar]
- 14.Zlotogorski A, Marek D, Horev L, Abu A, Ben-Amitai D, Gerad L, Ingber A, Frydman M, Reznik-Wolf H, Vardy DA, et al. An autosomal recessive form of monilethrix is caused by mutations in DSG4: clinical overlap with localized autosomal recessive hypotrichosis. J Invest Dermatol. 2006;126:1292–1296. doi: 10.1038/sj.jid.5700251. The above three reports highlight the similarity in clinical presentation between LAH and monilethrix that are caused by mutations in desmosomal cadherins (DSG4 for LAH) or hair keratins (monilethrix), respectively. [DOI] [PubMed] [Google Scholar]
- 15.Schaffer JV, Bazzi H, Vitebsky A, Witkiewicz A, Kovich OI, Kamino H, Shapiro LS, Amin SP, Orlow SJ, Christiano AM. Mutations in the desmoglein 4 gene underlie localized autosomal recessive hypotrichosis with monilethrix hairs and congenital scalp erosions. J Invest Dermatol. 2006;126:1286–1291. doi: 10.1038/sj.jid.5700237. The above three reports highlight the similarity in clinical presentation between LAH and monilethrix that are caused by mutations in desmosomal cadherins (DSG4 for LAH) or hair keratins (monilethrix), respectively. [DOI] [PubMed] [Google Scholar]
- 16.Schweizer J. More than one gene involved in monilethrix: intracellular but also extracellular players. J Invest Dermatol. 2006;126:1216–1219. doi: 10.1038/sj.jid.5700266. [DOI] [PubMed] [Google Scholar]
- 17.Eshkind L, Tian Q, Schmidt A, Franke WW, Windoffer R, Leube RE. Loss of desmoglein 2 suggests essential functions for early embryonic development and proliferation of embryonal stem cells. Eur J Cell Biol. 2002;81:592–598. doi: 10.1078/0171-9335-00278. [DOI] [PubMed] [Google Scholar]
- 18.Den Z, Cheng X, Merched-Sauvage M, Koch PJ. Desmocollin 3 is required for pre-implantation development of the mouse embryo. J Cell Sci. 2006;119:482–489. doi: 10.1242/jcs.02769. This work has an interesting implication that Dsc3 may be involved in extradesmosomal functions, since the phenotype of the knockout mice arises even before the formation of mature desmosomes. [DOI] [PubMed] [Google Scholar]
- 19.Awad MM, Dalal D, Cho E, Amat-Alarcon N, James C, Tichnell C, Tucker A, Russell SD, Bluemke DA, Dietz HC, et al. DSG2 mutations contribute to arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am J Hum Genet. 2006;79:136–142. doi: 10.1086/504393. The above reports demonstrate ARVC is a cardiac desmosomal disease, irrespectiveof the desmosomal component involved. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heuser A, Plovie ER, Ellinor PT, Grossmann KS, Shin JT, Wichter T, Basson CT, Lerman BB, Sasse-Klaassen S, Thierfelder L, et al. Mutant desmocollin-2 causes arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet. 2006;79:1081–1088. doi: 10.1086/509044. The above reports demonstrate ARVC is a cardiac desmosomal disease, irrespectiveof the desmosomal component involved. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pilichou K, Nava A, Basso C, Beffagna G, Bauce B, Lorenzon A, Frigo G, Vettori A, Valente M, Towbin J, et al. Mutations in desmoglein-2 gene are associated with arrhythmogenic right ventricular cardiomyopathy. Circulation. 2006;113:1171–1179. doi: 10.1161/CIRCULATIONAHA.105.583674. The above reports demonstrate ARVC is a cardiac desmosomal disease, irrespectiveof the desmosomal component involved. [DOI] [PubMed] [Google Scholar]
- 22.Syrris P, Ward D, Asimaki A, Evans A, Sen-Chowdhry S, Hughes SE, McKenna WJ. Desmoglein-2 mutations in arrhythmogenic right ventricular cardiomyopathy: a genotype-phenotype characterization of familial disease. Eur Heart J. 2007;28:581–588. doi: 10.1093/eurheartj/ehl380. The above reports demonstrate ARVC is a cardiac desmosomal disease, irrespectiveof the desmosomal component involved. [DOI] [PubMed] [Google Scholar]
- 23.van Tintelen JP, Hofstra RM, Wiesfeld AC, van den Berg MP, Hauer RN, Jongbloed JD. Molecular genetics of arrhythmogenic right ventricular cardiomyopathy: emerging horizon? Curr Opin Cardiol. 2007;22:185–192. doi: 10.1097/HCO.0b013e3280d942c4. The above reports demonstrate ARVC is a cardiac desmosomal disease, irrespectiveof the desmosomal component involved. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Gras E, Lombardi R, Giocondo MJ, Willerson JT, Schneider MD, Khoury DS, Marian AJ. Suppression of canonical Wnt/beta-catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J Clin Invest. 2006;116:2012–2021. doi: 10.1172/JCI27751. This is a very interesting study on the role of PKG in signaling cascades. By targeting DSP using RNA interference, the authors suggest that PKG is liberated to enter the nucleus and displace β-catenin, thereby suppressing Wnt signaling. This model could explain the abnormal myogenic to adipogenic differentiation of myocytes in ARVC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garrod DR, Merritt AJ, Nie Z. Desmosomal cadherins. Curr Opin Cell Biol. 2002;14:537–545. doi: 10.1016/s0955-0674(02)00366-6. [DOI] [PubMed] [Google Scholar]
- 26.McKoy G, Protonotarios N, Crosby A, Tsatsopoulou A, Anastasakis A, Coonar A, Norman M, Baboonian C, Jeffery S, McKenna WJ. Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease) Lancet. 2000;355:2119–2124. doi: 10.1016/S0140-6736(00)02379-5. [DOI] [PubMed] [Google Scholar]
- 27.Norgett EE, Hatsell SJ, Carvajal-Huerta L, Cabezas JC, Common J, Purkis PE, Whittock N, Leigh IM, Stevens HP, Kelsell DSP. Recessive mutation in desmoplakin disrupts desmoplakin-intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma. Hum Mol Genet. 2000;9:2761–2766. doi: 10.1093/hmg/9.18.2761. [DOI] [PubMed] [Google Scholar]
- 28.Yang Z, Bowles NE, Scherer SE, Taylor MD, Kearney DL, Ge S, Nadvoretskiy VV, DeFreitas G, Carabello B, Brandon LI, et al. Desmosomal dysfunction due to mutations in desmoplakin causes arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Res. 2006;99:646–655. doi: 10.1161/01.RES.0000241482.19382.c6. This study presents a paradox of how different DSP mutations can cause right or left ventricular myopathies. [DOI] [PubMed] [Google Scholar]
- 29.Djabali K, Martinez-Mir A, Horev L, Christiano AM, Zlotogorski A. Evidence for extensive locus heterogeneity in Naxos disease. J Invest Dermatol. 2002;118:557–560. doi: 10.1046/j.0022-202x.2001.01627.x. [DOI] [PubMed] [Google Scholar]
- 30.Gerull B, Heuser A, Wichter T, Paul M, Basson CT, McDermott DA, Lerman BB, Markowitz SM, Ellinor PT, MacRae CA, et al. Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat Genet. 2004;36:1162–1164. doi: 10.1038/ng1461. [DOI] [PubMed] [Google Scholar]
- 31.Grossmann KS, Grund C, Huelsken J, Behrend M, Erdmann B, Franke WW, Birchmeier W. Requirement of plakophilin 2 for heart morphogenesis and cardiac junction formation. J Cell Biol. 2004;167:149–160. doi: 10.1083/jcb.200402096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Syrris P, Ward D, Asimaki A, Sen-Chowdhry S, Ebrahim HY, Evans A, Hitomi N, Norman M, Pantazis A, Shaw AL, et al. Clinical expression of plakophilin-2 mutations in familial arrhythmogenic right ventricular cardiomyopathy. Circulation. 2006;113:356–364. doi: 10.1161/CIRCULATIONAHA.105.561654. [DOI] [PubMed] [Google Scholar]
- 33.van Tintelen JP, Entius MM, Bhuiyan ZA, Jongbloed R, Wiesfeld AC, Wilde AA, van der Smagt J, Boven LG, Mannens MM, van Langen IM, et al. Plakophilin-2 mutations are the major determinant of familial arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation. 2006;113:1650–1658. doi: 10.1161/CIRCULATIONAHA.105.609719. [DOI] [PubMed] [Google Scholar]
- 34.Duffy HS. How do myocytes tell right from left? Circ Res. 2006;99:563–564. doi: 10.1161/01.RES.0000243582.08718.01. [DOI] [PubMed] [Google Scholar]
- 35.Ersoy-Evans S, Erkin G, Fassihi H, Chan I, Paller AS, Surucu S, McGrath JA. Ectodermal dysplasia-skin fragility syndrome resulting from a new homozygous mutation, 888delC, in the desmosomal protein plakophilin 1. J Am Acad Dermatol. 2006;55:157–161. doi: 10.1016/j.jaad.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Vasioukhin V, Bowers E, Bauer C, Degenstein L, Fuchs E. Desmoplakin is essential in epidermal sheet formation. Nat Cell Biol. 2001;3:1076–1085. doi: 10.1038/ncb1201-1076. [DOI] [PubMed] [Google Scholar]
- 37.Maeda O, Usami N, Kondo M, Takahashi M, Goto H, Shimokata K, Kusugami K, Sekido Y. Plakoglobin (gamma-catenin) has TCF/LEF family-dependent transcriptional activity in beta-catenin-deficient cell line. Oncogene. 2004;23:964–972. doi: 10.1038/sj.onc.1207254. [DOI] [PubMed] [Google Scholar]
- 38.Yin T, Getsios S, Caldelari R, Kowalczyk AP, Muller EJ, Jones JC, Green KJ. Plakoglobin suppresses keratinocyte motility through both cell-cell adhesion-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 2005;102:5420–5425. doi: 10.1073/pnas.0501676102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hardman MJ, Liu K, Avilion AA, Merritt A, Brennan K, Garrod DR, Byrne C. Desmosomal cadherin misexpression alters beta-catenin stability and epidermal differentiation. Mol Cell Biol. 2005;25:969–978. doi: 10.1128/MCB.25.3.969-978.2005. This study presents in vivo and in vitro evidence for desmosomal aberrations affecting Wnt signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brennan D, Hu Y, Joubeh S, Choi YW, Whitaker-Menezes D, O'Brien T, Uitto J, Rodeck U, Mahoney MG. Suprabasal Dsg2 expression in transgenic mouse skin confers a hyperproliferative and apoptosis-resistant phenotype to keratinocytes. J Cell Sci. 2007;120:758–771. doi: 10.1242/jcs.03392. [DOI] [PubMed] [Google Scholar]
- 41.Biedermann K, Vogelsang H, Becker I, Plaschke S, Siewert JR, Hofler H, Keller G. Desmoglein 2 is expressed abnormally rather than mutated in familial and sporadic gastric cancer. J Pathol. 2005;207:199–206. doi: 10.1002/path.1821. [DOI] [PubMed] [Google Scholar]
- 42.Yashiro M, Nishioka N, Hirakawa K. Decreased expression of the adhesion molecule desmoglein-2 is associated with diffuse-type gastric carcinoma. Eur J Cancer. 2006;42:2397–2403. doi: 10.1016/j.ejca.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 43.Kurzen H, Munzing I, Hartschuh W. Expression of desmosomal proteins in squamous cell carcinomas of the skin. J Cutan Pathol. 2003;30:621–630. doi: 10.1034/j.1600-0560.2003.00122.x. [DOI] [PubMed] [Google Scholar]
- 44.Khan K, Hardy R, Haq A, Ogunbiyi O, Morton D, Chidgey M. Desmocollin switching in colorectal cancer. Br J Cancer. 2006;95:1367–1370. doi: 10.1038/sj.bjc.6603453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oshiro MM, Kim CJ, Wozniak RJ, Junk DJ, Munoz-Rodriguez JL, Burr JA, Fitzgerald M, Pawar SC, Cress AE, Domann FE, et al. Epigenetic silencing of DSC3 is a common event in human breast cancer. Breast Cancer Res. 2005;7:R669–680. doi: 10.1186/bcr1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen YJ, Chang JT, Lee L, Wang HM, Liao CT, Chiu CC, Chen PJ, Cheng AJ. DSG3 is overexpressed in head neck cancer and is a potential molecular target for inhibition of oncogenesis. Oncogene. 2007;26:467–476. doi: 10.1038/sj.onc.1209802. [DOI] [PubMed] [Google Scholar]
- 47.Chidgey M, Dawson C. Desmosomes: a role in cancer? Br J Cancer. 2007 doi: 10.1038/sj.bjc.6603808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]


