Abstract
Natural killer cell leukemia is characterized by clonal expansion of CD3− NK cells and comprises both chronic and aggressive forms. Currently, no effective treatment exists, thus providing a need for identification of novel therapeutics. Lipidomic studies revealed dysregulated sphingolipid metabolism as evidenced by decreased levels of overall ceramide species and increased levels of cerebrosides in leukemic NK cells, concomitant with increased glucosylceramide synthase (GCS) expression. GCS, a key enzyme of this pathway, neutralizes pro-apoptotic ceramide by transfer of a UDP-glucose. Thus, we treated both rat and human leukemic NK cells in combination with: 1) exogenous C6-ceramide nanoliposomes in order to target mitochondria and increase physiological pro-apoptotic levels of long chain ceramide, and 2) 1-phenyl-2-palmitoylamino-3-morpholino-1-propanol (PPMP), an inhibitor of GCS. Co-administration of C6-ceramide nanoliposomes and PPMP elicited an increase in endogenous long-chain ceramide species, which led to cellular apoptosis in a synergistic manner via the mitochondrial intrinsic cell death pathway in leukemic NK cells.
Keywords: NK-LGL leukemia, ceramide, glucosylceramide, PPMP
INTRODUCTION
Large granular lymphocytes (LGL) consist of a lymphoid subset representing 10–15% of peripheral blood mononuclear cells (PBMC).1,2 LGL leukemia is a rare disorder characterized by the clonal expansion of these cytotoxic lymphocytes. In 1986, it was proposed that LGL leukemia could be subclassified into two major lineages, CD3− NK cells and CD3+ T cells.3 In addition, the WHO recently characterized the NK-LGL leukemia subtype into two distinctive groups of aggressive NK-cell LGL leukemia and as a provisional entity: chronic lymphoproliferative disorder of NK cells (also known as NK-cell lymphocytosis/chronic NK-cell leukemia).4 There is a great need to identify targeted therapeutics as there is no curative treatment for aggressive NK-cell leukemia and treatment of chronic NK-cell leukemia remains undefined.
The sphingolipid pathway, a diverse network of pro-survival and pro-apoptotic signaling lipids has been identified as dysregulated in LGL leukemia.5 Ceramide is the central molecule in sphingolipid metabolism,6 and has been implicated in activating several death-signaling pathways which include JNK/SAPK, TNFα, Fas, radiation, and certain chemotherapeutic agents.7–10 One possible therapeutic target in this pathway, glucosylceramide synthase (GCS), is an enzyme that catalyzes the conversion of ceramide to glucosylceramide by transferring a glucose residue from UDP-glucose to ceramide. Glucosylceramide is the precursor to more than 300 other lipids such as lactosylceramides and gangliosides, and signifies the first step in committed synthesis of these complex glycosphingolipids.11–14 GCS has been found to be upregulated in cancer cells and its overexpression has been associated with multidrug resistance in leukemia.15–18 A pharmacological inhibitor for GCS, 1-phenyl-2-palmitoylamino-3-morpholino-1-propanol (PPMP), has been shown to sensitize chemotherapy refractive cells to such treatment.12,16 Previously, our lab has shown that systemic i.v. delivery of nanoliposomal C6-ceramide led to complete remission in 5/14 animals in the syngeneic Fischer F344 rat model of NK-LGL leukemia.19 Therefore, we were interested in investigating whether the combination treatment of PPMP with C6-ceramide nanoliposomes would enhance the ability of exogenous short chain ceramide to cause death of leukemic NK cells.
We hypothesized that inhibiting GCS in combination with exogenous C6-ceramide nanoliposomes would cause physiological long-chain ceramide (C14–C26) levels to rise and increase apoptosis in NK cells. Our data showed that combinatorial treatment of PPMP and C6-ceramide nanoliposomes did increase apoptosis of leukemic NK LGL cells by synergistically increasing short- and long-chain ceramide species. We found that such combination treatment targeted the mitochondrial cell death pathway, resulting in decreased mitochondrial membrane potential, increased reactive oxygen species (ROS) production and decreased levels of pro-survival proteins Mcl-1 and survivin.
MATERIALS AND METHODS
Cell lines and culture conditions
Human NKL cells were obtained from American Type Culture Collection (ATCC) and grown in RPMI-1640 (Invitrogen) supplemented with 15% fetal bovine serum (Hyclone) plus 100 IU IL-2/ml. RNK-16 cells (kindly provided by Dr. Craig Reynolds at National Cancer Institute) were cultured in RPMI-1640 (Invitrogen), supplemented with 10% FBS from Hyclone (Fisher Thermo Scientific), 1% non-essential amino acids (Sigma), 1% sodium pyruvate (Sigma) and 25 µM 2-ME (Sigma). All cells were maintained in a 37°C humidified 5% CO2 atmosphere incubator.
Patient characteristics and preparation of peripheral blood mononuclear cells (PBMC)
All patients met the criteria of chronic NK leukemia with increased numbers (>80%) of CD3− CD56+ or CD3−CD16+ NK cells in the peripheral blood. Peripheral blood specimens from LGL leukemia patients were obtained and informed consents signed for sample collection according to a protocol approved by the Institutional Review Board of Penn State Hershey Cancer Institute. Normal blood samples were obtained from the Blood Bank of Milton S. Hershey Medical Center at College of Medicine, Penn State University. PBMCs were isolated from whole blood by Ficoll-Hypaque gradient separation from (Pharmacia Biotech). Cell viability was determined by trypan blue exclusion assay with more than 95% viability in all the samples.
GCS gene expression: Real-time quantitative PCR (qRT- PCR)
qRT-PCR was performed using primer sets specific for human GCS, and an internal standard, GAPDH, in an ABI PRISM 7900 sequence detector. PBMCs (5×106) from eight chronic NK leukemia patients (CD3−/CD56+ or CD3−/CD16+ NK cells >80%) and freshly purified PBMCs from the same number of age matched normal healthy male and female controls were subjected to RNA extraction using TRIzoL Reagent (Invitrogen) following the manufacturer’s instructions. In this study, 4 µg of total RNA per sample was used to synthesize the first strand cDNA using random hexamers and MMLV reverse transcription reagent (Invitrogen) in a total volume of 10 µl. A total of 1 µg cDNA was applied in a 10 µl PCR mix using a QuantiTect SYBR Green PCR kit (Qiagen). Amplification of triplicate cDNA template samples was performed with denaturation for 15 min at 95°C, followed by 45 cycles of denaturation at 94°C for 15 sec, annealing at 55°C for 30 sec, and extension at 72°C for 30 sec. A standard curve of cycle thresholds using serial dilutions of cDNA samples was established and used to calculate the relative abundance of the target gene between samples from patients and normal healthy controls. Values were normalized to GAPDH mRNA, which was obtained from a similar curve. The changes in fluorescence of SYBR green dye in every cycle was monitored and calculated by the ABI 7900 system software and the threshold cycle (Ct) for each reaction. The relative amount of PCR products generated from each primer set was determined based on the threshold cycle or Ct value. PCR analysis was performed on each cDNA in triplicate. The following specific primers for human GCS were used: sense 5’-CAA GCT CCC AGG TGT CTC TC-3’, antisense 5’-GAT TAA TGC CAA CTT TTT TAC CAC CTA-3’ and for human GAPDH: sense 5’-GAC CCC TTC ATT GAC CTC AAC TAC ATG-3’, antisense 5’-GTC CAC CAC CCT GTT GCT GTA GCC-3’. All primers were supplied by IDTDNA.
PPMP and C6-ceramide nanoliposome drug studies
C6-ceramide nanoliposomes were manufactured in our laboratory with the following lipids purchased from Avanti Polar Lipids: 1,2-distearoyl-sn-glycero-3-phosphocholine, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine, N-hexanoyl-D-erythro-sphingosine (C6), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy polyethylene glycol-2000], and N-octanoyl-sphingosine-1-[succinyl (methoxy polyethylene glycol-750] (PEG(750)-C8), which were combined at a molar ratio of 3.75:1.75:3:0.75:0.75, respectively. The resultant C6-ceramide nanoliposomes contained 12% PEG to enable increased circulation time. Lipids were dried under nitrogen gas and resuspended in 0.9% sterile NaCl and incubated at 60°C for 3 hr.19 Following incubation, the lipid solution was sonicated for 5 min at 60°C in a heated water bath sonicator (Avanti Polar Lipids). This sonicated mixture was immediately extruded through a 100-nm polycarbonate membrane in the Avanti Mini Extruder (Avanti Polar Lipids). The control ghost nanoliposomes were manufactured as previously described but without the N-hexanoyl-D-erythro-sphingosine (C6) and N-octanoyl-sphingosine-1-[succinyl (methoxy polyethylene glycol-750] (PEG (750)-C8). Characteristics such as particle size and zeta potential (ZP) were determined through a Malvern Zetasizer Nano ZS (Malvern). Our nanoliposomes were formed at 80±15 nm in size and contained ~30 mol% cell-permeable ceramide.19–22 Our average ZP value of ghost nanoliposomes was −10.7 ± 0.6 mV, while C6-ceramide nanoliposomes had an average ZP of −13.9 ± 0.8 mV. The C6-ceramide nanoliposomes and PPMP (Matreya) were used singly and in combination at 6.25, and 12.5 µM concentrations and 5, 10, and 15 µM respectively, on RNK-16, NKL, and patient samples to establish an IC50 and determine therapeutic efficacy. DMSO was used as a vehicle control for PPMP. Calcusyn software (Biosoft) was used to determine amount of synergistic or additive effect with combination of PPMP and C6-ceramide nanoliposomes. Nanoliposomes were stored at room temperature at a concentration of 25 mg/ml before use.
Determination of in vitro cell apoptosis
Flow cytometry (2-color) with Annexin-V (5 µl per sample; BD Pharmingen) and 7-amino-actinomycin D (7-AAD; 10 µl per sample; BD Pharmingen) was used in order to assess the degree of cellular apoptosis in RNK-16, NKL, and human NK-LGL samples. Cells were immediately analyzed by flow cytometry on BD FacsCalibur. For each sample, 5×105 cells were plated in triplicate, and the percentage specific apoptosis was calculated using the following formula: apoptosis (%) = (% Annexin-V-allophycocyanin conjugate [APC] positive in assay well - % Annexin-V-APC positive in the control well) × 100/ (100- %Annexin-V-APC positive in the control well).
Quantification of sphingolipids by mass spectroscopy
RNK-16 cells (4×106) were treated in triplicate with 6.25 µM C6-ceramide nanoliposomes singly and in combination at 5, 10, and 15 µM PPMP (Matreya) for 24 hrs. Cells were homogenized with sonication at 60°C in a 10mM Tris buffer (pH 7.2) and protein concentration measured using DC protein assay kit (BIORAD). Lipids were then extracted from samples by chloroform/methanol/water as originally described23 after internal standards had been added (C17-sphingosine-1-phosphate, C17-sphingosine, C17-sphinganine, C17-sphinganine-1-phosphate, C12-ceramide, C12-glucosylceramide). Sphingoid bases and 1-phosphates were separated as described previously.24 Sphingolipids from these cells were then chromatographically separated on an Agilent 1100 HPLC system and analyzed by electrospray ionization-tandem mass spectrometry (ESI-MS/MS) on a 4000 QTRAP (AB Sciex) based on the method described previously.25 The peak areas for different sphingolipid subspecies were quantified according to internal standards and then normalized to protein concentrations.
Measurement of Mitochondrial Membrane Potential
JC-1, a mitochondrial matrix potential (ΔΨm) indicator, was used to show the changes in ΔΨm in RNK-16 and human NK leukemia cells. JC-1 (Invitrogen), a cationic and lipophilic dye specifically permeates mitochondrial membranes and fluoresces red when it aggregates in the matrix of healthy, high potential mitochondria, whereas it fluoresces green in cells with low ΔΨm. Briefly, cells were collected after 24 hrs combination treatment with C6-ceramide nanoliposomes and PPMP, and then incubated with 2 µg/ml JC-1 dye at a density of 5×105 cells/0.5 ml. After incubation for 20 minutes at 37°C in the dark, all samples were washed twice with 1× PBS and then incubated for 15 minutes at 37°C in the dark with Annexin-V-APC. Cells were immediately analyzed by flow cytometry on BD FacsCalibur at 488-nm excitation. Data were collected at 529-nm emission for green fluorescence and 590-nm for red fluorescence. Results are expressed in arbitrary units as % of JC-1 expression.
Measurement of Reactive Oxygen Species (ROS)
The amount of cytoplasmic ROS was used to determine the level of cellular oxidative stress. RNK-16 cells were treated for 24 hr with the combination treatment of C6-ceramide nanoliposomes and PPMP, then were collected and resuspended in PBS containing freshly prepared H2-DCF-DA at 37°C in the dark. This reagent penetrates cells and emits green fluorescence on oxidation with H2O2. Cells were then incubated for 30 minutes with 2 µM H2-DCF-DA, washed twice in PBS, and analyzed immediately by cell flow cytometry at 488 nm excitation and 530 nm emission. 10,000 events were collected on a BD FACScalibur, and data are expressed as the median fluorescence in arbitrary units of three independent replicates. 1 µM H2O2 (Sigma) was used as a positive control.
Western blot analysis
Cell lysates were harvested by addition of RIPA lysis buffer containing phosphatase inhibitor cocktail and protease inhibitor (Sigma). Whole cell lysates were centrifuged (≥10,000 × g) for 10 min. at 4°C to remove cell debris. Protein concentrations were quantified using a Nanodrop Spectrophotometer 2000C (Thermo Fisher Scientific). 30 µg of protein were loaded per lane onto 10% precasted Nupage electrophoresis gels (Invitrogen). Following electrophoresis, samples were transferred to a polyvinylidene difluoride membrane (Pall Corporation). The blots were probed with antibodies according to each supplier’s recommendations: antibodies specific for caspase 3, Mcl-1, survivin, and β-actin were purchased (Cell Signalling Technology Inc.). Secondary antibodies conjugated to horseradish peroxidase were obtained (GE Healthcare UK Limited) Buckinghamshire, UK. Immunoblots were developed using the enhanced chemiluminescence reagent (Amersham Biosciences Inc.).
Statistical analysis
Analysis for lipidomics data were performed with one-way ANOVA using Tukey’s adjustment for multiple comparisons by SAS software. All other statistical analysis between two treatment groups were performed with Student’s t-test for statistical comparison among groups, and differences were considered significant when *, P<0.05, **, PP<0.005, ***, PP<0.0005.
RESULTS
Identification of role of GCS in dysregulated ceramide metabolism in leukemic NK cells
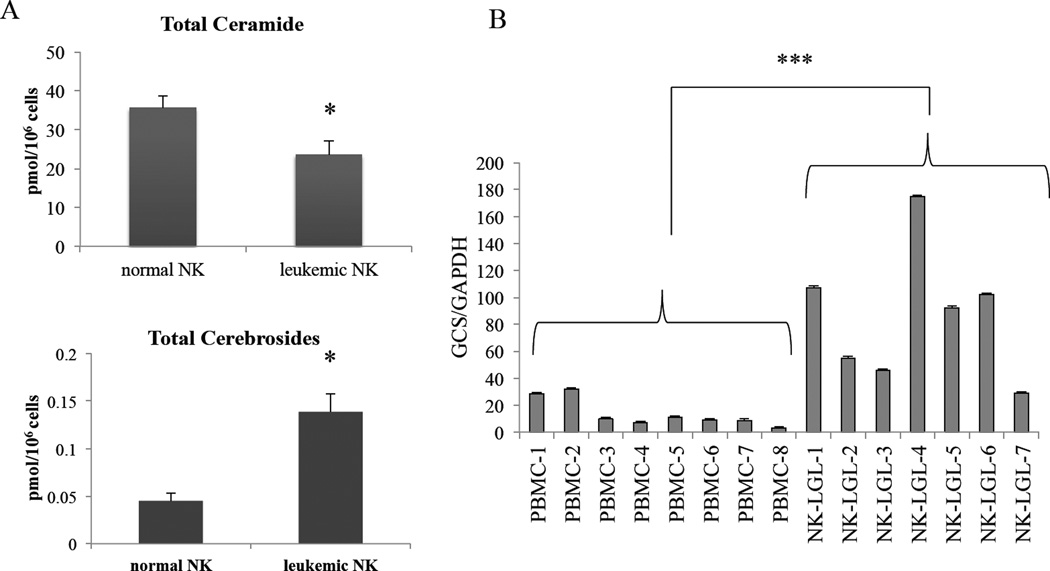
First, we investigated whether levels of pro-apoptotic ceramide were intrinsically lower in leukemic NK cells compared to normal NK cells, which might account for their persistent survival. Lipidomics studies revealed that overall ceramide species levels were significantly decreased (P<0.05) whereas cerebrosides (ceramides with a sugar added at the 1-hydroxl group) were significantly increased (P<0.05) in patient leukemic NK cells compared to normal NK cells (Figure 1A). Such a shift in ceramide metabolism could be explained by overexpression of GCS. We next examined the mRNA expression levels of GCS in leukemic NK cells. Indeed, we found that GCS transcripts were significantly overexpressed (P<0.001) in leukemic NK cells from patients with NK leukemia compared to normal PBMCs (Figure 1B).
Figure 1. GCS is overexpressed in leukemic NK cells.
(A) Lipids from normal NK cells from donors or leukemic NK cells from patients were extracted and processed via mass spectrophotometry for ceramide and cerebroside lipid levels. (B) RNA was extracted and processed into cDNA to run for qRT-PCR from PBMCs that were collected from 8 normal donors and 7 NK-LGL leukemia patients. GCS expression is significantly higher in NK-LGL leukemia patients compared to normal PBMCs, (P<0.005).
Combination therapy leads to apoptosis of NK leukemia cells
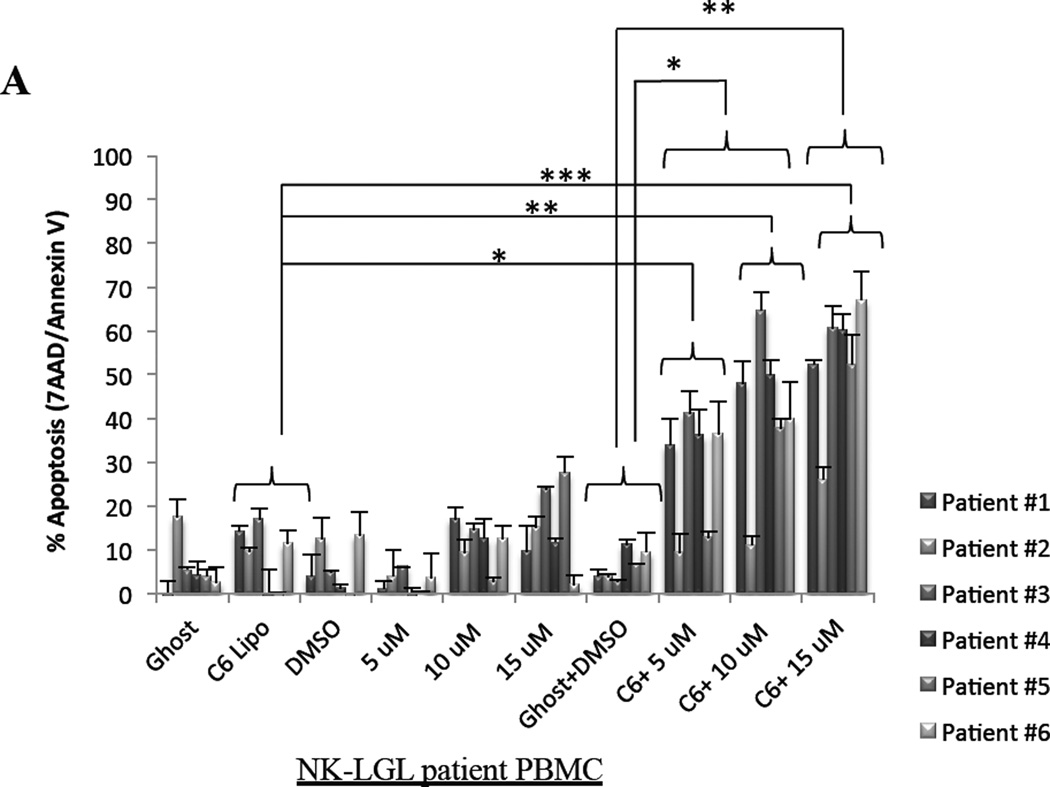
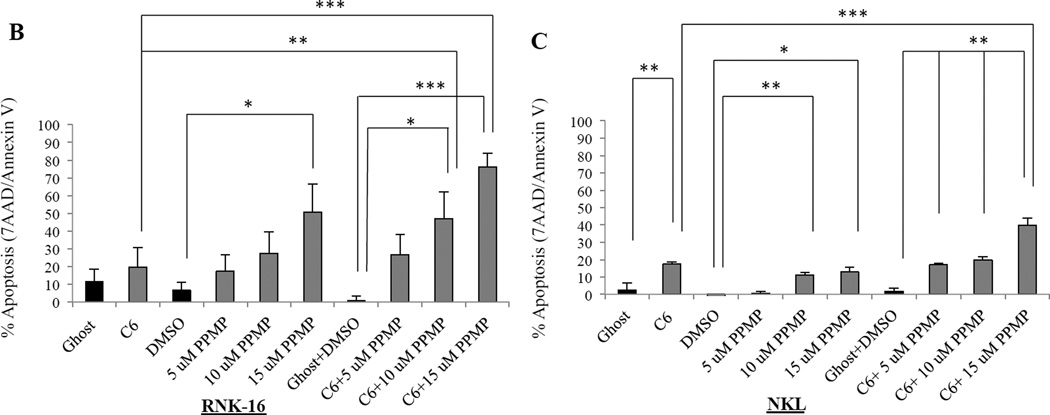
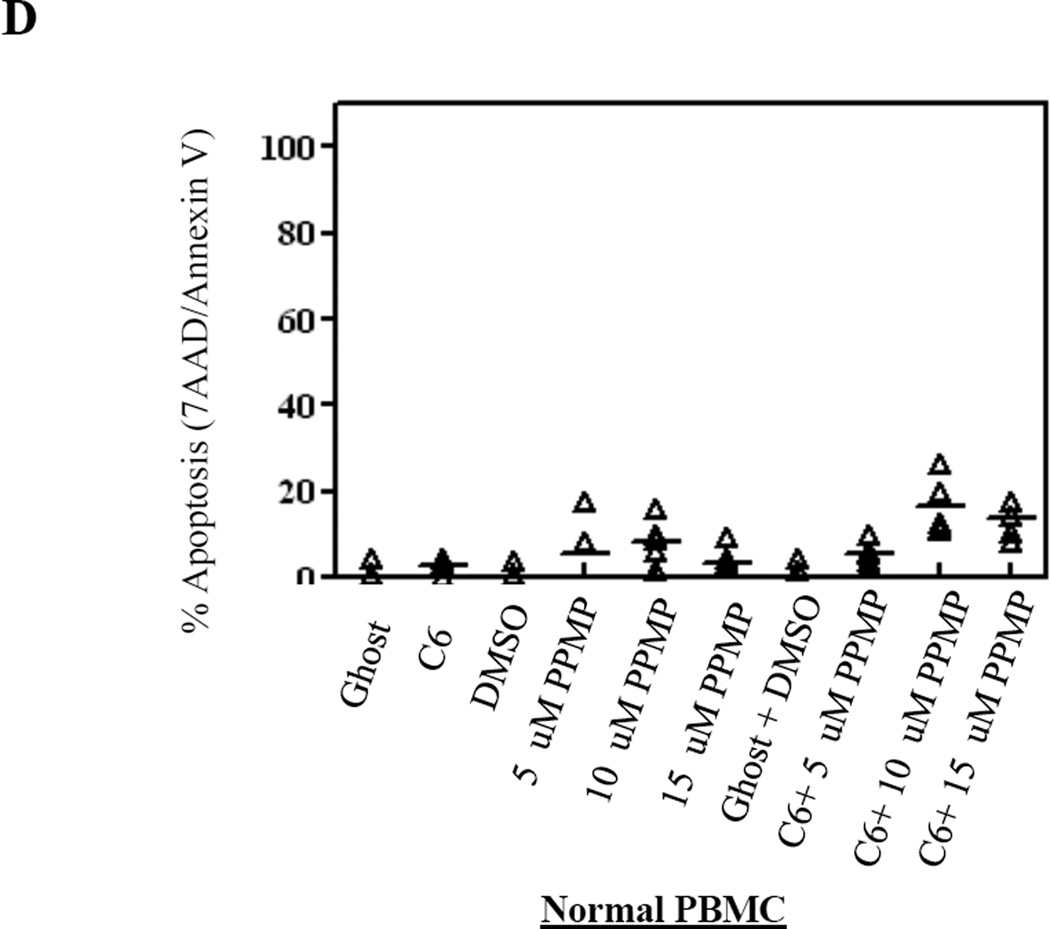
We next sought to establish the mechanism of how cell death is induced with C6-ceramide nanoliposomes and PPMP combination treatment. As previously reported by our lab, ceramide induces apoptosis in leukemic NK cells.19 Utilizing 7AAD/Annexin V staining of leukemic NK cells, we observed significant apoptosis in patient leukemic NK cells with 12.5 µM C6-ceramide nanoliposomes in combination with both 5 µM and 10 µM PPMP (P<0.05), and with 15 µM PPMP (P<0.005), when compared to DMSO and ghost combination (Figure 2A). Comparing C6-ceramide nanoliposome treatment alone to 12.5 µM C6-ceramide nanoliposomes in combination with both 5 µM (P<0.05), 10 µM PPMP (P<0.005), and with 15 µM PPMP (P<0.0005) also yielded significant results (Figure 2A). In RNK-16 cells (Figure 2B), combination treatment with 10 µM PPMP and 6.25 µM C6-ceramide nanoliposomes produced an additive effect (CI=1.062), with a significant 46% increase in apoptosis (P<0.05) compared to the DMSO and ghost combination. A synergistic effect was observed with the 15 µM PPMP dose in combination with 6.25 µM C6-ceramide (RNK-16, CI=0.591) and in combination with 12.5 µM C6-ceramide (NKL, CI=0.779) in inducing apoptosis at 76% and 40%, respectively (Figure 2B & 2C). We found minimal apoptotic induction when we utilized this combination treatment on normal human PBMCs (Figure 2D). Overall, apoptosis was selectively increased in both leukemic NK cell lines and patient cells in a dose-response manner.
Figure 2. Cellular apoptosis is induced after combination treatment.
(A) Patient leukemic NK cells from 6 patients were treated with 12.5 µM C6-Ceramide and 5, 10, or 15 µM PPMP for 24 hrs then stained for 7AAD//7-AAD then assayed for apoptosis by flow cytometry. (B) RNK-16 cells were treated with 6.25 µM C6-Ceramide nanoliposomes and 5, 10, or 15 µM of PPMP (n=3 independent experiments), then apoptosis assay was performed. (C) NKL were treated with 12.5 µM C6-Ceramide and 5, 10, or 15 µM PPMP (n=3 independent experiments), then apoptosis assay was performed. (D) Normal PBMCs were treated with 12.5 µM C6-Ceramide nanoliposomes and 5, 10, or 15 µM PPMP (n=8), then apoptosis assay was performed. *, P<0.05, **, P<0.005, ***, P<0.0005 indicate significant differences of C6-Ceramide nanoliposomes+PPMP versus control treated cells (Student’s t-test).
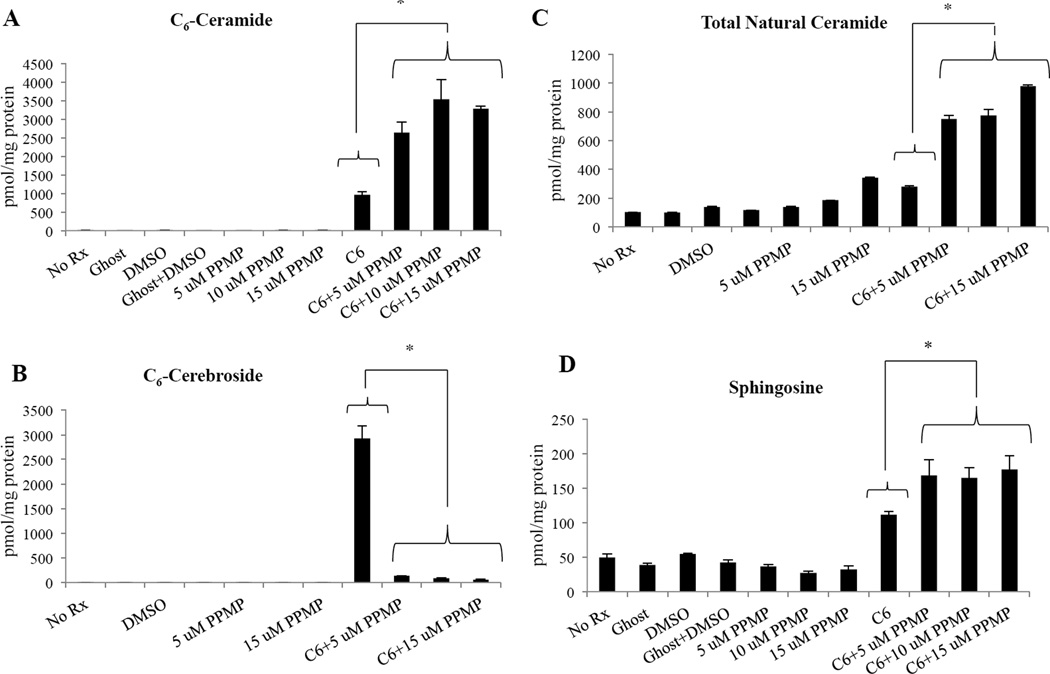
Lipidomics reveal that combinatorial treatment of C6-ceramide nanoliposomes and GCS inhibition decreases leukemic NK cell viability through increase of short-and long-chain ceramide species
We wanted to confirm the specificity of PPMP, the GCS inhibitor utilized in these experiments. Similar to the natural endogenously occurring form of ceramide, these short-chain C6 formulations are also metabolized by various enzymes in the sphingolipid pathway such as GCS14 and ceramidases.26,27 Most commonly, metabolism of short-chain ceramide results in formation of short-chain cerebrosides and short-chain sphingomyelin, which neutralizes the pro-apoptotic aspect of ceramide.27 The combination treatment showed a significant increase in C6-ceramide levels (P<0.05) (Figure 3A) and a significant decrease of C6-cerebrosides levels when compared to C6-ceramide nanoliposome treatment alone (P<0.05) (Figure 3B), indicating that the addition of PPMP to C6-ceramide nanoliposomes induced an increase in levels of ceramide species while selectively inhibiting cerebroside levels. In addition to C6-ceramide, total long-chain natural ceramide species (C14–26), were also increased with the combination treatment when compared to C6-ceramide nanoliposome treatment alone (P<0.05) (Figure 3C). Levels of sphingosine, associated with induction of apoptosis in leukemic NK cells,28 were also significantly increased by the combination treatment when compared to C6-ceramide nanoliposome treatment alone (P<0.05) (Figure 3D). These results showing that C6-ceramide levels are increased while C6-cerebrosides are decreased, demonstrate the specificity of PPMP to target GCS, thus preventing the neutralization of ceramide. Moreover, the increase of total natural ceramides with the combinatorial treatment suggests that these long-chain ceramide species contribute to cell death mechanisms.
Figure 3. Sphingolipid levels are altered after combination treatment.
RNK-16 (4×106) cells were treated for 24 hours with 6.25 µM C6-ceramide and 5, 10, and 15 µM PPMP. Cells were harvested and lipids were extracted and analyzed with LC-MS/MS. Lipid levels relative to total cell protein levels were determined for: (A) C6-ceramide, (B) C6-cerebroside, (C) total natural ceramide, (D) sphingosine. *, P<0.05, indicates differences of C6-Ceramide nanoliposomes alone vs. C6-Ceramide nanoliposomes+PPMP combinations (one-way ANOVA)..
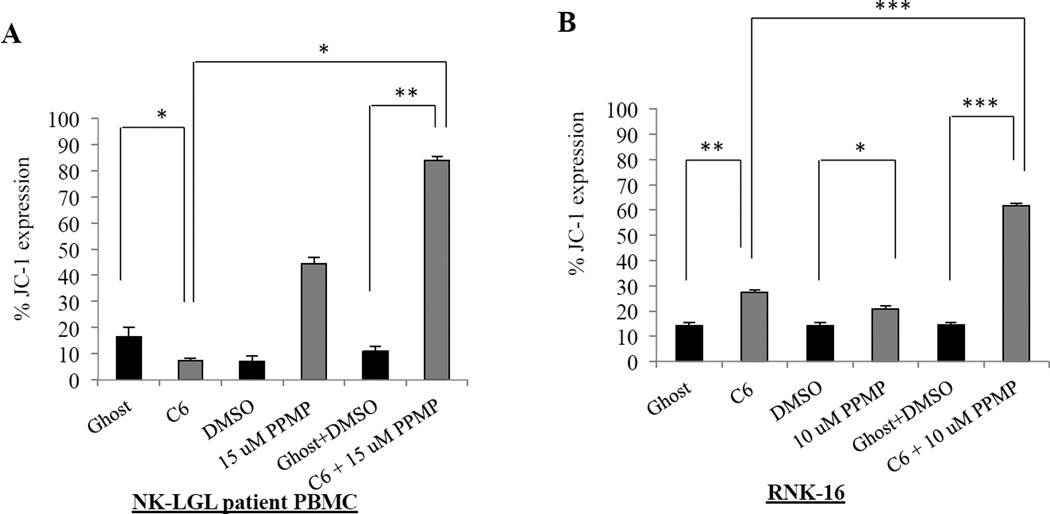
Mitochondrial membrane depolarization in leukemic NK cells after combination treatment
A significant hallmark of mitochondrial dependent cellular apoptosis is the increase of mitochondrial depolarization. We used a cationic and lipophilic dye, JC-1, which exhibits a potential dependent accumulation in healthy mitochondria, indicated by a fluorescence shift from green (529 nm) to red (590 nm). Depolarization occurs as cells are dying and the shift can be detected in the fluorescence by a decrease in the red/green ratio as the dye is no longer able to accumulate and form aggregates in the mitochondria. We saw significant depolarization occurring in both patient leukemic NK cells (Figure 4A) by 84% (P<0.005) and RNK-16 cells (Figure 4B) by 62% (P<0.0005) that were treated with the combination of PPMP and C6-ceramide nanoliposomes when compared to DMSO and ghost combination control treatment.
Figure 4. Mitochondrial disruption occurs in leukemic NK cells after combination treatment.
(A) Patient NK cells (12.5 µM C6-Ceramide nanoliposomes+PPMP), and RNK-16 cells (6.25 µM C6-Ceramide nanoliposomes+PPMP) were treated for 24 hrs then stained with JC-1 and assayed for mitochondrial depolarization via cell flow cytometry. *, P<0.05, **, P<0.005, ***, P<0.0005 indicate significant differences of C6-Ceramide nanoliposomes+PPMP versus control treated cells (Student’s t-test).
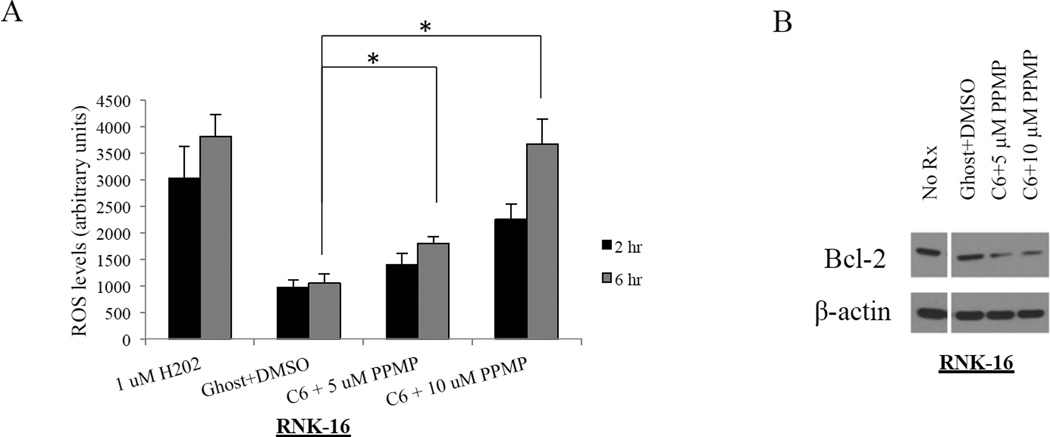
ROS production is increased while mitochondrial membrane depolarization is decreased in leukemic NK cells after combination treatment
We further investigated if ROS contributes to mitochondrial dysfunction in leukemic NK cells, before and after treatment, in order to determine if the mitochondrial intrinsic cell death pathway was a mechanism responsible for cell death. Increased levels of ceramide have been linked to generation of ROS due to its ability to uncouple the electron transport chain and cause oxidative cell damage.6,29,30 We observed statistically significant increased ROS production at 6 hr for RNK-16 cells that were treated with 5 and 10 µM PPMP and 6.25 µM C6-ceramide nanoliposomes compared to the vehicle control combination (P<0.05) (Figure 5A). H2O2 was used as a positive control. ROS has also been shown to dysregulate expression of anti-apoptotic Bcl-2 and it has been hypothesized that Bcl-2 may interact indirectly to increase antioxidant levels.31,32 Therefore, it is believed that ROS acts to down-regulate endogenous Bcl-2 levels in order to sensitize cells to apoptosis. One study has demonstrated that Bcl-2 levels were restored and apoptosis prevented when activated T-cells were grown in the presence of antioxidants.31 We showed that Bcl-2 levels were decreased in RNK-16 cells treated with the two drug combination (Figure 5B). In addition, we also saw a decrease in ROS expression with both single treatments of 10 µM PPMP and C6-ceramide nanoliposomes (data not shown).
Figure 5. Increase in ROS level and decrease of Bcl-2 after combination treatment.
(A) RNK-16 cells were treated for 24 hours with 6.25 µM C6-ceramide nanoliposomes and 5 or 10 µM PPMP and then assayed for ROS levels via cell flow cytometry. *, P<0.05, indicates significant differences of C6-Ceramide nanoliposomes+PPMP versus control treated cells C6-Ceramide nanoliposomes alone (Student’s t-test). (B) Bcl-2 production decreases with increasing ROS levels (n=3 independent experiments).
Overall, our findings of increased ROS production and increased depolarization of the mitochondrial membranes in the leukemic NK cells provide evidence for cell death through the mitochondrial intrinsic cell death pathway.
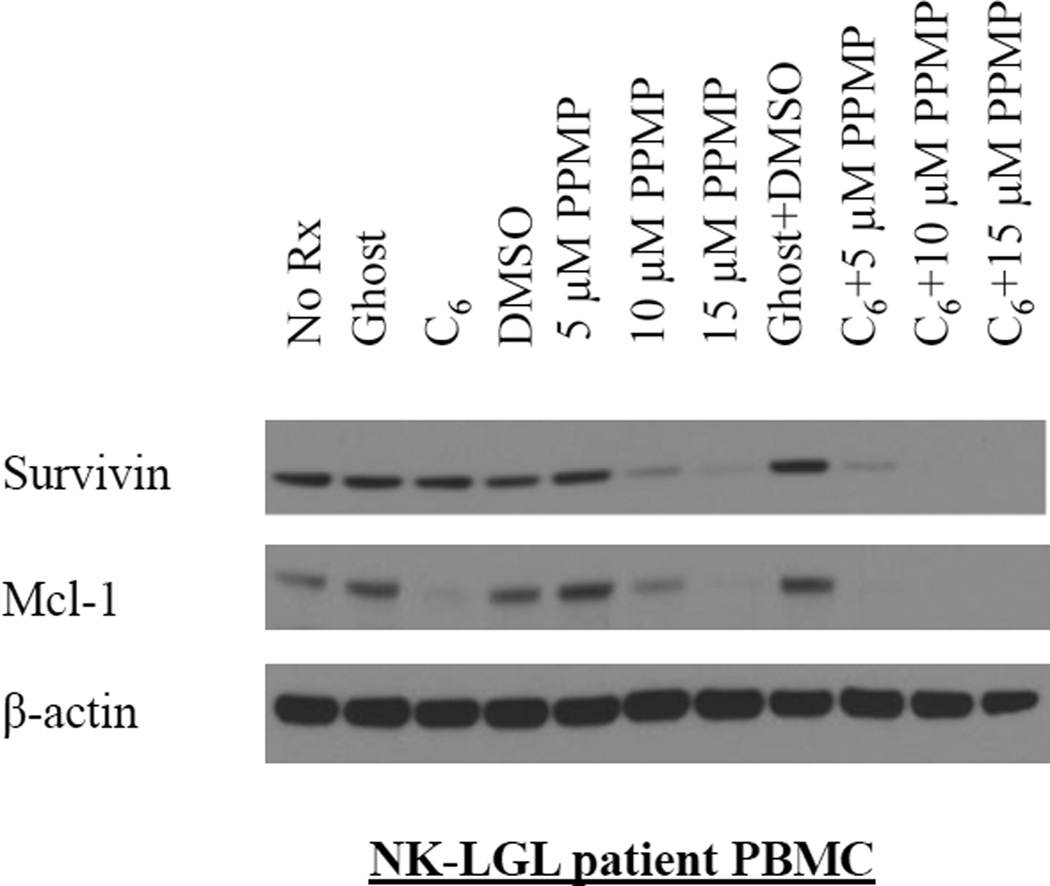
Mcl-1 and survivin signaling are decreased in leukemic NK cells in a dose-dependent manner after combination treatment
In our previous work, we demonstrated that survivin, a member of the inhibitor of apoptosis protein (IAP) family, was highly upregulated and plays an important role in regulating leukemic NK cell survival.19 We found that C6-ceramide nanoliposomes would abolish survivin expression in a time- and dose- dependent manner in RNK-16 and NKL cells, due to its ability to localize in the mitochondria and target ERK, an upstream activator of survivin.19 In that same study we found that cationic nanoliposomes complexed with siRNA targeted toward survivin, resulted in greater than 80% cell death in NKL cells. Therefore, in the present work we sought to examine how survivin expression was affected with our combination treatment. We treated a set of patient cells (Figure 6) for 24 hr, both singly and in combination with PPMP and C6-ceramide nanoliposomes. We saw a significant decrease in survivin with 10 or 15 µM PPMP in combination with 12.5 µM C6-ceramide nanoliposomes in leukemic NK cells in a dose-dependent fashion. 10 µM and 15 µM PPMP alone also had a significant effect on survivin expression (Figure 6).
Figure 6. Mcl-1 and Survivin expression is decreased in a dose-dependent manner.
Western blot analysis was performed for Mcl-1 and Survivin, after 24 hrs treatment of PBMC from a chronic NK leukemia patient with 12.5 µM C6-Ceramide nanoliposome and 5, 10 and 15 µM PPMP (n=3 independent experiments). β-actin served as a loading control.
In addition, we also probed for Mcl-1 protein expression, an important member of the Bcl-2 family.33 Mcl-1 functions by localizing to the mitochondria and interacting with and antagonizing apoptotic members of the Bcl-2 family.34,35 Mcl-1 has been documented to be upregulated in both TLGL36 and NK leukemia.19 Previously we showed that shRNA-mediated knockdown of Mcl-1 resulted in a 5.5-fold increase of apoptosis in our human NKL cells.24 These data together with the knowledge that Mcl-1 is associated with the mitochondria provided the rationale to examine Mcl-1 protein expression after combination treatment with C6-ceramide nanoliposomes and PPMP. We found that Mcl-1 protein expression levels were significantly downregulated in patient leukemic NK cells with all three combination treatments (Figure 6). Single treatment with C6-ceramide nanoliposomes, 10 µM PPMP, and 15 µM PPMP also had a significant effect on Mcl-1 expression. These data show that combination treatment targets the pro-survival molecules survivin and Mcl-1. Since both survivin and Mcl-1 are localized to the mitochondria, these data provide further support that cell death may be occurring through the mitochondrial intrinsic cell death pathway.
DISCUSSION
Our results show that leukemic NK cells can be characterized by dysregulated ceramide metabolism and that targeting this pathway may be an attractive therapeutic approach. We clearly demonstrate that endogenous ceramide species are low in leukemic NK cells suggesting a role for exogenous short-chain ceramide treatment to raise physiological ceramide levels and therefore induce cell death in leukemic NK cells. However, there are several physicochemical properties of ceramide, such as poor bioavailability and lack of an appropriate solvent, that inhibit effective delivery of ceramide as a drug.21,22 As a result of these issues, ceramide has been incorporated into nanoliposomal formulations by us and several other groups to enhance its solubility through the incorporation into the lipid bilayer.22,37–39 Previously, we showed that nanoliposomal C6-ceramide produced remission in some but not all rats in an aggressive rat NK-LGL leukemia model.19 The full therapeutic affect for the addition of exogenous short chain C6-ceramide may be hindered as there are many enzymes that target ceramide for degradation into other metabolites. Indeed, our lipidomic analyses showing elevated levels of cerebrosides and decreased levels of ceramide in leukemic NK cells suggest increased GCS activity in this disease. In particular, we demonstrated that GCS mRNA levels were increased in leukemic NK cells. GCS presents an excellent therapeutic target, due to its role in glycosylating ceramide and thereby neutralizing its apoptotic capacity. We found that utilization of PPMP to inhibit GCS allowed a 4-fold decrease in effective dose of C6-ceramide nanoliposomes to treat NK-LGL leukemia then previously administered (Figure 2A).19 Modulation of GCS activity has been shown to significantly affect the ability of ceramide to induce apoptosis.11 Altered levels of GCS have been linked with cancer, in particular its upregulation allows for cellular protection and proliferation.14 GCS overexpression has also been linked to multidrug resistance.14 In addition, it has been demonstrated that P-gp inhibitors, such as Tamoxifen are able to inhibit the production of glucosylceramide.40 Likewise, treatment of MCF-7-Adriamycin-resistant breast cancer cells with PPMP sensitized them to Adriamycin.40 Thus, targeting GCS may increase effectiveness of C6-ceramide nanoliposomes either by inhibiting neutralization or by increasing the re-acylation of this shortchain therapeutic into longer chain physiological ceramides. We hypothesize that C6-ceramide can be metabolized by the cooperative actions of GCS and ceramidases and sphingomyelin synthase. Thus, inhibiting GCS with PPMP will increase the deacylation of C6-ceramide and/or increase C6-sphingomyelin by mass action. We have no evidence that PPMP increases specific ceramide synthases through off-target effects. GCS is the preferential pathway available to C6-ceramide. If GCS is blocked, then the accumulating C6-ceramide could then be preferentially metabolized by other enzymes, including ceramidases. In particular ceramidases cleave the hexanoyl (C6) moiety and ceramide synthase produces a longer fatty acid.26 This is consistent with the upregulation of sphingosine (Figure 3D), a substrate for long-chain ceramides. Alternately, PPMP may increase expression of specific ceramide synthases that would also form long-chain ceramide species.
We specifically targeted GCS using the well studied inhibitor, PPMP.41 Indeed, we observed synergistic effects on apoptotic leukemic NK cell death using the combination therapy of C6-ceramide liposomes and PPMP. We demonstrated selective targeting of GCS by PPMP in our lipidomics analysis by showing decreased C6-cerebroside levels and increased levels of C6-ceramide, endogenous long chain (C14–C26) ceramides, and sphingosine. The synergistic pro-apoptotic effects of these lipids may occur through a mitochondrial-dependent pathway. It has been previously demonstrated that nanoliposomal C6-ceramide is able to deliver C6-ceramide to the mitochondria, thus initiating antiproliferative and proapoptotic effects,6 resulting in mitochondrial dysfunction and ultimate cell death.20 Studies have shown that physiological levels of long-chain ceramides and sphingosine may also result in mitochondrial intrinsic cell death pathway.42 Through inhibition of GCS, we show that increased ceramide levels led to apoptosis and produced depolarization of the mitochondrial membrane in both the RNK-16 cell line and leukemic NK cells from patients.
It is also well documented that ceramide disrupts the electron transport chain in mitochondria, which leads to generation of ROS, thus resulting in direct oxidative damage of cells.6,29,30 Targeting GCS with PPMP and treating with exogenous C6-ceramide resulted in decreased expression of the antiapoptotic molecules survivin and Mcl-1, therefore triggering apoptosis. We have previously shown that both survivin and Mcl-1 are important survival factors in NK leukemia and that targeting them will induce apoptosis.19,28 Survivin, a member of the IAP family, works to inhibit caspases and therefore apoptosis.43 There is a mitochondrial pool of survivin that is necessary for tumorigenesis by inhibition of mitochondria-mediated apoptosis.19 Mcl-1 has been identified as a mitochondrial anti-apoptosis gene that is overexpressed in cancer.44 Ceramide has been shown to activate JNK, which then goes on to phosphorylate and inactivate Mcl-1.45 Therefore, the combination of C6-ceramide nanoliposomes and GCS inhibition aid in restoring long-chain ceramide levels in NK cells, thus contributing to Mcl-1 and survivin dependent mediated cell death.
Our findings support targeted therapeutics centered on ceramide metabolism. Delivery of exogenous short-chain ceramide in nanoliposomal formulation in combination with PPMP leads to increased total ceramide levels resulting in apoptosis of leukemic NK cells through the activation of the mitochondrial intrinsic cell death pathway.
Acknowledgments
We thank Nate Sheaffer, David Stanford, and Vanessa Andes of the Cell Science/Flow Cytometry Core Facility, Robert Brucklacher of the Functional Genomics Core Facility, the Biostatistics Core, and Wade Edris of Microscopy & Histology Core Facility at Penn State Hershey Cancer Institute/Milton S. Hershey Medical Center for their technical assistances.
Grant Support:
This study was supported by National Institutes of Health Grants CA098472 and CA133525 (to T.P.L), Penn State Hershey Cancer Institute Startup Fund (to X.L), and Tobacco Settlement funds (to M.K.).
Footnotes
Authorship Contribution:
R.J.W., T.P.L., and X.L. designed and organized the experiments; R.J.W performed real time RT-PCR, Western blot experiments, ROS assays, and JC-1 assays. J.E.C. assisted R.J.W. with the apoptosis studies. K.B. and R.J.W isolated NK cells from normal donors or PBMCs from patients; T.E.F. and R.J.W. performed lipid extractions and MS/LCS analysis; R.J.W. and S.S. manufactured ghost and C6-ceramide nanoliposomes for drug studies. J.L. assisted with statistical analysis. M.K. and M.C. advised on manuscript; R.J.W., T.P.L., and X.L. wrote manuscript.
Conflict-of-interest disclosure:
M.K. Penn State Research Foundation (PSRF), licensed ceramide nanoliposome to Keystone Nano, State College, PA. M.K. is Chief Medical Officer of Keystone Nano Inc, State College, PA. The remaining authors declare no competing financial interests.
REFERENCES
- 1.Alekshun TJ, Sokol L. Diseases of large granular lymphocytes. Cancer Control. 2007;14(2):141–150. doi: 10.1177/107327480701400207. [DOI] [PubMed] [Google Scholar]
- 2.Zhang R, Shah MV, Loughran TP., Jr The root of many evils: indolent large granular lymphocyte leukaemia and associated disorders. Hematological oncology. 2010;28(3):105–117. doi: 10.1002/hon.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan WC, Link S, Mawle A, Check I, Brynes RK, Winton EF. Heterogeneity of large granular lymphocyte proliferations: delineation of two major subtypes. Blood. 1986;68(5):1142–1153. [PubMed] [Google Scholar]
- 4.Lamy T, Loughran TP., Jr How I treat LGL leukemia. Blood. 2011;117(10):2764–2774. doi: 10.1182/blood-2010-07-296962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah MV, Zhang R, Irby R, et al. Molecular profiling of LGL leukemia reveals role of sphingolipid signaling in survival of cytotoxic lymphocytes. Blood. 2008;112(3):770–781. doi: 10.1182/blood-2007-11-121871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birbes H, El Bawab S, Obeid LM, Hannun YA. Mitochondria and ceramide: intertwined roles in regulation of apoptosis. Adv Enzyme Regul. 2002;42:113–129. doi: 10.1016/s0065-2571(01)00026-7. [DOI] [PubMed] [Google Scholar]
- 7.Bourbon NA, Yun J, Kester M. Ceramide directly activates protein kinase C zeta to regulate a stress-activated protein kinase signaling complex. The Journal of biological chemistry. 2000;275(45):35617–35623. doi: 10.1074/jbc.M007346200. [DOI] [PubMed] [Google Scholar]
- 8.Hofmann K, Dixit VM. Ceramide in apoptosis--does it really matter? Trends Biochem Sci. 1998;23(10):374–377. doi: 10.1016/s0968-0004(98)01289-4. [DOI] [PubMed] [Google Scholar]
- 9.Kolesnick RN, Kronke M. Regulation of ceramide production and apoptosis. Annu Rev Physiol. 1998;60:643–665. doi: 10.1146/annurev.physiol.60.1.643. [DOI] [PubMed] [Google Scholar]
- 10.Lin X, Fuks Z, Kolesnick R. Ceramide mediates radiation-induced death of endothelium. Crit Care Med. 2000;28(4 Suppl):N87–N93. doi: 10.1097/00003246-200004001-00010. [DOI] [PubMed] [Google Scholar]
- 11.Bleicher RJ, Cabot MC. Glucosylceramide synthase and apoptosis. Biochim Biophys Acta. 2002;1585(2–3):172–178. doi: 10.1016/s1388-1981(02)00338-4. [DOI] [PubMed] [Google Scholar]
- 12.Abe A, Inokuchi J, Jimbo M, et al. Improved inhibitors of glucosylceramide synthase. J Biochem. 1992;111(2):191–196. doi: 10.1093/oxfordjournals.jbchem.a123736. [DOI] [PubMed] [Google Scholar]
- 13.Kester M, Kolesnick R. Sphingolipids as therapeutics. Pharmacol Res. 2003;47(5):365–371. doi: 10.1016/s1043-6618(03)00048-3. [DOI] [PubMed] [Google Scholar]
- 14.Messner MC, Cabot MC. Glucosylceramide in humans. Advances in experimental medicine and biology. 2010;688:156–164. doi: 10.1007/978-1-4419-6741-1_11. [DOI] [PubMed] [Google Scholar]
- 15.Chapman JV, Gouaze-Andersson V, Messner MC, et al. Metabolism of short-chain ceramide by human cancer cells--implications for therapeutic approaches. Biochemical pharmacology. 2010;80(3):308–315. doi: 10.1016/j.bcp.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie P, Shen YF, Shi YP, et al. Overexpression of glucosylceramide synthase in associated with multidrug resistance of leukemia cells. Leuk Res. 2008;32(3):475–480. doi: 10.1016/j.leukres.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Barth BM, Cabot MC, Kester M. Ceramide-based therapeutics for the treatment of cancer. Anti-cancer agents in medicinal chemistry. 2011;11(9):911–919. doi: 10.2174/187152011797655177. [DOI] [PubMed] [Google Scholar]
- 18.Gouaze-Andersson V, Cabot MC. Sphingolipid metabolism and drug resistance in hematological malignancies. Anti-cancer agents in medicinal chemistry. 2011;11(9):891–903. doi: 10.2174/187152011797655069. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Ryland L, Yang J, et al. Targeting of survivin by nanoliposomal ceramide induces complete remission in a rat model of NK-LGL leukemia. Blood. 2010;116(20):4192–4201. doi: 10.1182/blood-2010-02-271080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stover TC, Sharma A, Robertson GP, Kester M. Systemic delivery of liposomal short-chain ceramide limits solid tumor growth in murine models of breast adenocarcinoma. Clin Cancer Res. 2005;11(9):3465–3474. doi: 10.1158/1078-0432.CCR-04-1770. [DOI] [PubMed] [Google Scholar]
- 21.Tran MA, Smith CD, Kester M, Robertson GP. Combining nanoliposomal ceramide with sorafenib synergistically inhibits melanoma and breast cancer cell survival to decrease tumor development. Clin Cancer Res. 2008;14(11):3571–3581. doi: 10.1158/1078-0432.CCR-07-4881. [DOI] [PubMed] [Google Scholar]
- 22.Stover T, Kester M. Liposomal delivery enhances short-chain ceramide-induced apoptosis of breast cancer cells. J Pharmacol Exp Ther. 2003;307(2):468–475. doi: 10.1124/jpet.103.054056. [DOI] [PubMed] [Google Scholar]
- 23.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Canadian journal of biochemistry and physiology. 1959;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 24.Liao A, Broeg K, Fox T, et al. Therapeutic efficacy of FTY720 in a rat model of NK-cell leukemia. Blood. 2011;118(10):2793–2800. doi: 10.1182/blood-2011-01-331447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox TE, Bewley MC, Unrath KA, et al. Circulating sphingolipid biomarkers in models of type 1 diabetes. Journal of lipid research. 2011;52(3):509–517. doi: 10.1194/jlr.M010595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryland LK, Fox TE, Liu X, Loughran TP, Kester M. Dysregulation of sphingolipid metabolism in cancer. Cancer Biol Ther. 2011;11(2):138–149. doi: 10.4161/cbt.11.2.14624. [DOI] [PubMed] [Google Scholar]
- 27.Jiang Y, DiVittore NA, Kaiser JM, et al. Combinatorial therapies improve the therapeutic efficacy of nanoliposomal ceramide for pancreatic cancer. Cancer biology & therapy. 2011;12(7):574–585. doi: 10.4161/cbt.12.7.15971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao A, Broeg K, Fox T, et al. Therapeutic efficacy of FTY720 in a rat model of natural killer cell leukemia. Blood. 2011 doi: 10.1182/blood-2011-01-331447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Paola M, Cocco T, Lorusso M. Ceramide interaction with the respiratory chain of heart mitochondria. Biochemistry. 2000;39(22):6660–6668. doi: 10.1021/bi9924415. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Ruiz C, Colell A, Mari M, Morales A, Fernandez-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J Biol Chem. 1997;272(17):11369–11377. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- 31.Hildeman DA, Mitchell T, Aronow B, Wojciechowski S, Kappler J, Marrack P. Control of Bcl-2 expression by reactive oxygen species. Proc Natl Acad Sci U S A. 2003;100(25):15035–15040. doi: 10.1073/pnas.1936213100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li D, Ueta E, Kimura T, Yamamoto T, Osaki T. Reactive oxygen species (ROS) control the expression of Bcl-2 family proteins by regulating their phosphorylation and ubiquitination. Cancer science. 2004;95(8):644–650. doi: 10.1111/j.1349-7006.2004.tb03323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozopas KM, Yang T, Buchan HL, Zhou P, Craig RW. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc Natl Acad Sci U S A. 1993;90(8):3516–3520. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato T, Hanada M, Bodrug S, et al. Interactions among members of the Bcl-2 protein family analyzed with a yeast two-hybrid system. Proc Natl Acad Sci U S A. 1994;91(20):9238–9242. doi: 10.1073/pnas.91.20.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang T, Kozopas KM, Craig RW. The intracellular distribution and pattern of expression of Mcl-1 overlap with, but are not identical to, those of Bcl-2. J Cell Biol. 1995;128(6):1173–1184. doi: 10.1083/jcb.128.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Epling-Burnette PK, Liu JH, Catlett-Falcone R, et al. Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J Clin Invest. 2001;107(3):351–362. doi: 10.1172/JCI9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shabbits JA, Mayer LD. Intracellular delivery of ceramide lipids via liposomes enhances apoptosis in vitro. Biochim Biophys Acta. 2003;1612(1):98–106. doi: 10.1016/s0005-2736(03)00108-1. [DOI] [PubMed] [Google Scholar]
- 38.Khazanov E, Priev A, Shillemans JP, Barenholz Y. Physicochemical and biological characterization of ceramide-containing liposomes: paving the way to ceramide therapeutic application. Langmuir : the ACS journal of surfaces and colloids. 2008;24(13):6965–6980. doi: 10.1021/la800207z. [DOI] [PubMed] [Google Scholar]
- 39.Tokudome Y, Saito Y, Sato F, Kikuchi M, Hinokitani T, Goto K. Preparation and characterization of ceramide-based liposomes with high fusion activity and high membrane fluidity. Colloids and surfaces B, Biointerfaces. 2009;73(1):92–96. doi: 10.1016/j.colsurfb.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Lavie Y, Cao H, Volner A, et al. Agents that reverse multidrug resistance, tamoxifen, verapamil, and cyclosporin A, block glycosphingolipid metabolism by inhibiting ceramide glycosylation in human cancer cells. The Journal of biological chemistry. 1997;272(3):1682–1687. doi: 10.1074/jbc.272.3.1682. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Xie KM, Yang GQ, et al. GCS induces multidrug resistance by regulating apoptosis-related genes in K562/AO2 cell line. Cancer chemotherapy and pharmacology. 2010;66(3):433–439. doi: 10.1007/s00280-009-1177-4. [DOI] [PubMed] [Google Scholar]
- 42.Novgorodov SA, Wu BX, Gudz TI, et al. Novel pathway of ceramide production in mitochondria: thioesterase and neutral ceramidase produce ceramide from sphingosine and acyl-CoA. The Journal of biological chemistry. 2011;286(28):25352–25362. doi: 10.1074/jbc.M110.214866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 44.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiological reviews. 2007;87(1):99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 45.Nica AF, Tsao CC, Watt JC, et al. Ceramide promotes apoptosis in chronic myelogenous leukemia-derived K562 cells by a mechanism involving caspase-8 and JNK. Cell cycle. 2008;7(21):3362–3370. doi: 10.4161/cc.7.21.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]