Abstract
Background:
Sepsis is a pro-inflammatory state caused by systemic infection. As sepsis progresses, multiple organ systems become affected with subsequent increase in mortality. Elevated red cell distribution width (RDW) has been seen with changes of other inflammatory markers and thus could potentially serve as a means of assessing sepsis severity. In this study, we examine the association of RDW with APACHE II score and in-hospital mortality.
Meterials and Methods:
We conducted a retrospective study involving a cohort of patients with sepsis. The study period spanned 2 years with a cohort of 349 patients. Data were collected to determine if RDW is associated with APACHE II scores and in-hospital mortality in this cohort.
Results:
RDW correlated weakly (rs = 0.27), but significantly (P < 0.0001) with APACHE II scores; coefficient of determination (r2 = 0.09). The odds ratios for the association of RDW with APACHE II were calculated over the RDW range 12-20% at a dichotomized level of APACHE II, i.e., <15 and ≥15. At a RDW ≥16%, multivariate analysis including all potential confounders indicated that RDW was independently associated with an APACHE II score of ≥15. Similarly, mortality was associated with RDW ≥16%.
Conclusion:
A prognostic biomarker for sepsis in the form of a routine blood test may be of considerable clinical utility. The results of our study suggest that RDW may have value in differentiating between more severe and less severe cases of sepsis. Future studies with larger samples are needed to confirm these findings.
Keywords: APACHE II score, biomarkers, mortality, red blood cell distribution width, sepsis
INTRODUCTION
Sepsis is a pro-inflammatory state due to a source of infection which, despite advances in supportive care, continues to have significant medical, social and financial impact. There are an estimated 751,000 (2.26 per 100 hospital discharges) cases of sepsis annually in the United States.[1] The incidence rate of sepsis has increased at a rate of 8.7% per year.[2] More than half of septic patients require admission to intensive care units and the cost of care is estimated at $16.7 billion annually.[1] Despite advancements, the mortality rate is still between 17.9% and 28.6%.[1,2]
Worsening sepsis is associated with increased mortality as multiple organ systems fail. The degree of severity is, most often, quantified by the APACHE II score, which can predict the severity and outcome of multiple organ failure.[3] However, calculating APACHE II scores is cumbersome[4] and it would be advantageous to identify a biomarker that would be associated with the degree of severity in patients with sepsis.
The red cell distribution width (RDW) is the coefficient of variation of red blood cell (RBC) volume and is a representation of the RBC size heterogeneity of an individual patient.[5] RDW is elevated by increased red cell destruction, nutritional deficiencies and blood transfusions.[6] Nutritional deficiencies affecting RDW include iron, vitamin B12 and folate.[5] Biomarkers of chronic inflammation, erythrocyte sedimentation rate and C-reactive protein, have been associated with elevated RDW.[7,8]
In this study, we examined a cohort of patients with sepsis to determine if RDW is associated with APACHE II scores and sepsis in these subjects.
MATERIALS AND METHODS
Settings
This study was conducted in a 650-bed, tertiary-care, teaching hospital in New Jersey, USA. The Institutional Review Board of the Hospital categorized the study as exempt.
Protocol and subjects
Data were obtained by retrospective review of adult patients admitted with the diagnosis of sepsis from January 1, 2007 to December 31, 2008. Inclusion in the cohort required age ≥ 18 years and confirmed evidence of sepsis as defined by the American College of Chest Physicians/Society of Critical Care Medicine.[9] Sepsis was defined as systemic inflammatory response syndrome and documented clinical suspicion or confirmed infection.[9] Subjects that had missing data were excluded. The final cohort of subjects studied included 349 patients.
APACHE II score was calculated using the 12 acute physiological variables within 24 h of presentation. These variables included temperature, blood pressure, heart rate, respiratory rate, arterial oxygenation, arterial pH, serum sodium, serum potassium, serum creatinine, hematocrit, white blood cell count and Glasgow Coma score. These variables were then combined with the chronic health assessment to arrive at the APACHE II score.
All RDW values were taken on initial presentation to the emergency department on day 1 of the hospital stay. Collection of data on blood transfusions was deferred since RDW values were taken from the initial complete blood count (CBC) done on presentation to the emergency department prior to any intervention. Initial septic work-ups to culture the potential source of infection were also done in the emergency department.
Baseline characteristics, data used to compute the APACHE II scores for the patients were collected based on retrospective chart review as were data on survival to hospital discharge.
Determination of red cell distribution width
A Coulter® LH 700 series Hematology Analyzer was used by the laboratory during the period of this study. This instrument incorporates an algorithm to automatically reject outliers. The reference interval (RI) in our institution is 11.5-14.5%.
Statistical methods
Continuous variables were tested for fit-to-normality by the D’Agostino-Pearson omnibus normality test. Because the distributions of most variables were found to deviate significantly from normal, non-parametric tests were used throughout. Group-wise differences were examined by the Mann-Whitney test and correlation was studied by Spearman's rank method. The cut-off for age was determined through the use of receiver operator characteristic (ROC) curves. Linear regression was done using the ordinary least squares (OLS) methodology.
Categorical data were associated by cross-tabulation in contingency tables, which were tested for statistical significance with Fisher's exact test; because of the retrospective nature of the study the odds ratio (OR) with confidence intervals (CI) at the 95% level (95% CI) are provided.
For this study, α was set at 0.05; however, for baseline characteristics, a level of 5α (0.25) was established as the criterion for consideration as potential confounders. Thus, any characteristic for which P ≤ 0.25, was included in a logistic regression model. The characteristics examined are provided in Table 1. Power was assessed on a post-hoc basis at RDW ≥14.5%. We determined that based on the proportionate difference between the groups and the sample size in each group that power was 0.92 (β =0.08).
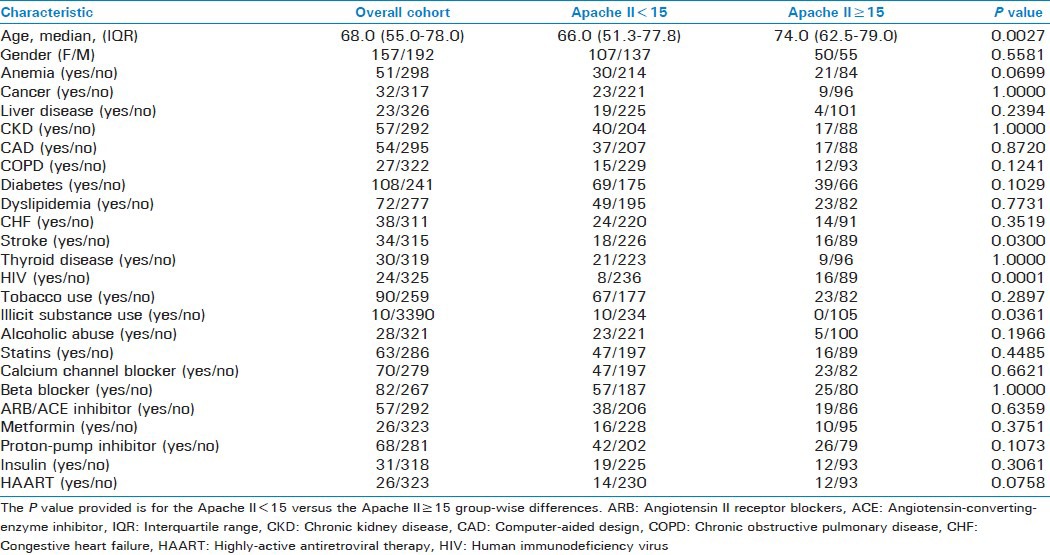
Table 1.
Baseline characteristics of subjects in the study
All statistical calculations were made using Prism® version 5.04 software (GraphPad Corp, San Diego, CA, USA) except for logistic regression, which was computed using a web-based routine (www.statpages.org/logistic.html; last accessed 2nd January, 2013).
RESULTS
Baseline characteristics of subjects
Table 1 provides the clinical characteristics of the subjects included in this study. Categorical variables for which differences achieved a P ≤ 0.25 and were thus considered for inclusion in the logistic regression model were: anemia, liver disease, chronic obstructive pulmonary disease, diabetes, cerebrovascular accident, human immunodeficiency virus disease, illicit substance use, alcohol abuse, proton pump inhibitor use and highly-active antiretroviral therapy. Age was also significantly different and was subjected to ROC curve analysis, which yielded a cut-off of 71 years.
Regression and correlation of red cell distribution width with APACHE II scores
RDW correlated weakly (rs = 0.27) but significantly (P < 0.0001) with APACHE II. The OLS regression analysis of RDW and APACHE II is provided in Figure 1 and demonstrates statistical significance (P < 0.0001), but with considerable as scatter and a low coefficient of determination (r2 = 0.09).
Figure 1.
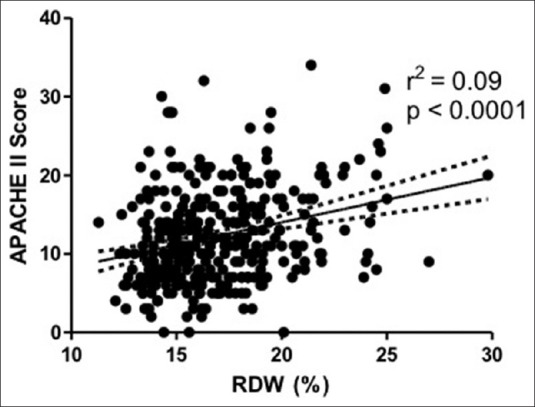
Linear regression of APACHE II score with red cell distribution width
Categorical association of red cell distribution widthand APACHE II
The ORs for the association of RDW with APACHE II were studied, stepwise, in 0.5% increments of RDW at a dichotomized level of APACHE II, i.e., <15 and ≥15. These data are shown in Figure 2. Within most of the RI for RDW, no significant association was appreciated. However, at RDW ≥14.5%, a significant association between the variables can be seen.
Figure 2.
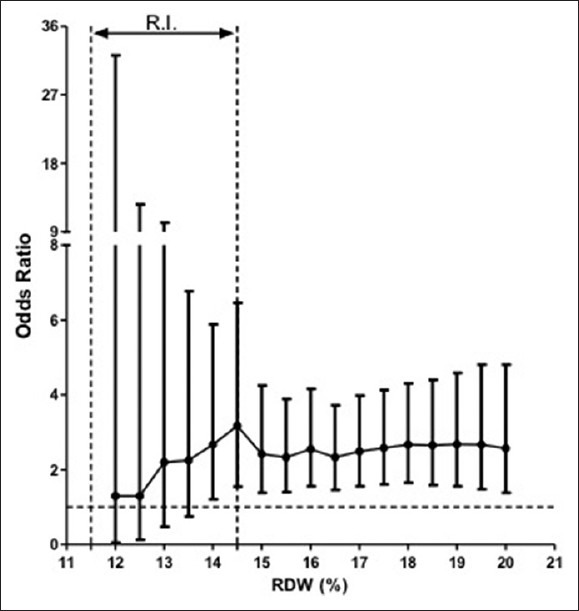
Bivariate forest plot showing odds ratios and 95% confidence interval (error bars) for APACHE II scores as a function of red cell distribution width. The dashed line delineates the reference interval
Figure 3 demonstrates the effect of the potential confounders that were identified on the OR and 95% CI at RDW = 14.5% [Figure 3a] and at 16% [Figure 3b]. At RDW = 14.5%, there was a significant interaction caused by anemia. This effect was not observed at RDW = 16%; a level at which RDW appears to be an independent predictor of increased APACHE II score.
Figure 3.

Multivariable analysis for odds ratio of red cell distribution width for APCHE II scores. Unadjusted OR and OR adjusted for potential confounders at RDW = 14.5% (a) and RDW = 16.5% (b)
Association of red cell distribution width and mortality
The ORs for the association of RDW with mortality were studied at 14.5% and 16%. The results at RDW = 14.5% were not significant (OR: 1.66 [95% CI: 0.88-3.15]; P = 0.1360). RDW at 16% was found to be an independent predictor of increased mortality [Figure 4].
Figure 4.
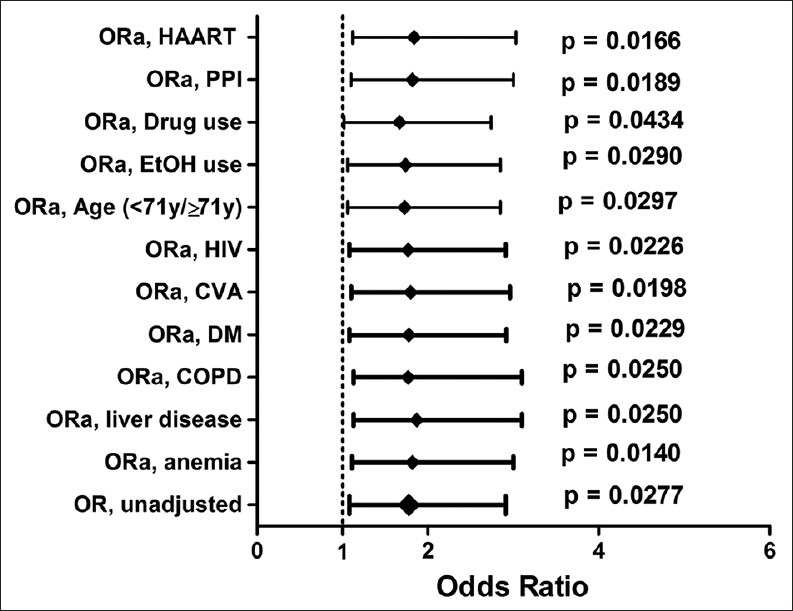
Multivariable analysis for odds ratio of red cell distribution width for APCHE II scores at RDW = 16%
DISCUSSION
RDW has been described as a prognostic marker in several conditions including coronary artery disease,[10] congestive heart failure,[11] cerebrovascular accidents,[12] pulmonary hypertension[13] and peripheral artery disease.[14] RDW has also been associated with poor lung function.[15] RDW has been described as a possible marker for all-cause mortality.[16,17] RDW has also been shown to be predictive of mortality in the critically ill-patients[18,19] and in patients with septic shock.[20] Prior studies have investigated the association of RDW with mortality in critically ill-septic patients[18] or with patients with more severe stage sepsis.[20,21] This is the first study to examine the relationship of RDW with worsening severity of illness in all septic patients.
Pro-inflammatory states play a major role in insufficient erythropoiesis leading to structural and functional alteration of RBCs.[22] Plasma cytokines such as tumor necrosis factor-α, interferon-γ, interleukin 1β and interleukin six have been shown to effect RBC survival and production.[22,23,24] Erythroid progenitor cell activity is inversely related to the amount of circulating cytokines.[25] The pro-inflammatory state of sepsis can negatively impacts RBCs leading to elevated RDW.
RDW elevation may be caused by increased turnover of erythrocytes, nutritional deficiencies and blood transfusions.[6] Baseline characteristics of subjects from Table 1 were inclusive of disease conditions and either potential medication that can affect the RDW or commonly prescribed medications. The effect of these potential confounders was analyzed and RDW appears to be an independently associated with increased APACHE II score and mortality.
The RBCs in the acute critical setting is afflicted by significant stress. The erythrocyte survival time is shortened in response to cytokines tumor necrosis factor-α and interleukin-1. The structural changes to the RBC related to inflammation leads to more rapid clearing of RBCs.[22] The combination of these processes lead to shorter RBC survival time and ultimately more morphological variations in cell sizes.
Assessing the severity of illness with APACHE II scores help guide therapeutic decisions and mortality risk. Several studies have indicated that an APACHE II score of greater than 15 is associated with significantly increased mortality[26,27,28] and benefit from more aggressive management.[29] In this study, we divided the cohort into two groups based on an APACHE II score of ≥15 or <15.
Prognostic markers in patients with sepsis are of great interest. The ability to provide prognostication based on a routinely available marker on a CBC would be greatly valuable in assessing the severity of illness.
In our cohort of patients admitted with sepsis, a RDW ≥16% was independently associated with an APACHE II score of ≥15. This suggests that septic patients with a RDW ≥16% may have a higher severity of illness. In addition, we found RDW ≥16% was independently associated with mortality. Thus, RDW ≥16% in septic patients also suggests higher in-hospital mortality.
Sepsis is an inflammatory condition with markedly increased oxidative stress. APACHE II is a measure of increasing severity of illness and worsening severity of illness leads to increasing inflammation. The negative impact to RBC morphology and erythroid cell activity results in anisocytosis and a measurable impact on RDW.
The limitations of the study are that it was conducted in a single center and was a retrospective design. However, the cohort was adequate and generalizable and the results appear to have adequate effect size to suggest a reasonable strength of evidence. The underlying mechanism responsible for this relationship remains a matter of continued research. Future studies with larger samples are needed to confirm these findings.
In conclusion, RDW may be a useful in differentiating severity of illness in septic patients.
Footnotes
Source of Support: Pulmonary Division Fund, St. Joseph's Regional Medical Center
Conflict of Interest: No.
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Qiao Q, Lu G, Li M, Shen Y, Xu D. Prediction of outcome in critically ill elderly patients using APACHE II and SOFA scores. J Int Med Res. 2012;40:1114–21. doi: 10.1177/147323001204000331. [DOI] [PubMed] [Google Scholar]
- 4.Del Prete M, Castiglia D, Meli M, Perri S, Nicita A, Dalla Torre A, et al. Prognostic value of C reactive protein in acute pancreatitis. Chir Ital. 2001;53:33–8. [PubMed] [Google Scholar]
- 5.Evans TC, Jehle D. The red blood cell distribution width. J Emerg Med. 1991;9(Suppl 1):71–4. doi: 10.1016/0736-4679(91)90592-4. [DOI] [PubMed] [Google Scholar]
- 6.Fukuta H, Ohte N, Mukai S, Saeki T, Asada K, Wakami K, et al. Elevated plasma levels of B-type natriuretic peptide but not C-reactive protein are associated with higher red cell distribution width in patients with coronary artery disease. Int Heart J. 2009;50:301–12. doi: 10.1536/ihj.50.301. [DOI] [PubMed] [Google Scholar]
- 7.Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. 2009;133:628–32. doi: 10.5858/133.4.628. [DOI] [PubMed] [Google Scholar]
- 8.Perlstein TS, Weuve J, Pfeffer MA, Beckman JA. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med. 2009;169:588–94. doi: 10.1001/archinternmed.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Chest. Vol. 136. American college of chest physicians/society of critical care medicine. 1992; 2009. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM consensus conference committee; p. e28. [DOI] [PubMed] [Google Scholar]
- 10.Tonelli M, Sacks F, Arnold M, Moye L, Davis B, Pfeffer M, et al. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation. 2008;117:163–8. doi: 10.1161/CIRCULATIONAHA.107.727545. [DOI] [PubMed] [Google Scholar]
- 11.Al-Najjar Y, Goode KM, Zhang J, Cleland JG, Clark AL. Red cell distribution width: An inexpensive and powerful prognostic marker in heart failure. Eur J Heart Fail. 2009;11:1155–62. doi: 10.1093/eurjhf/hfp147. [DOI] [PubMed] [Google Scholar]
- 12.Ani C, Ovbiagele B. Elevated red blood cell distribution width predicts mortality in persons with known stroke. J Neurol Sci. 2009;277:103–8. doi: 10.1016/j.jns.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 13.Hampole CV, Mehrotra AK, Thenappan T, Gomberg-Maitland M, Shah SJ. Usefulness of red cell distribution width as a prognostic marker in pulmonary hypertension. Am J Cardiol. 2009;104:868–72. doi: 10.1016/j.amjcard.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Ye Z, Smith C, Kullo IJ. Usefulness of red cell distribution width to predict mortality in patients with peripheral artery disease. Am J Cardiol. 2011;107:1241–5. doi: 10.1016/j.amjcard.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grant BJ, Kudalkar DP, Muti P, McCann SE, Trevisan M, Freudenheim JL, et al. Relation between lung function and RBC distribution width in a population-based study. Chest. 2003;124:494–500. doi: 10.1378/chest.124.2.494. [DOI] [PubMed] [Google Scholar]
- 16.Cavusoglu E, Chopra V, Gupta A, Battala VR, Poludasu S, Eng C, et al. Relation between red blood cell distribution width (RDW) and all-cause mortality at two years in an unselected population referred for coronary angiography. Int J Cardiol. 2010;141:141–6. doi: 10.1016/j.ijcard.2008.11.187. [DOI] [PubMed] [Google Scholar]
- 17.Patel KV, Ferrucci L, Ershler WB, Longo DL, Guralnik JM. Red blood cell distribution width and the risk of death in middle-aged and older adults. Arch Intern Med. 2009;169:515–23. doi: 10.1001/archinternmed.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang F, Pan W, Pan S, Ge J, Wang S, Chen M. Red cell distribution width as a novel predictor of mortality in ICU patients. Ann Med. 2011;43:40–6. doi: 10.3109/07853890.2010.521766. [DOI] [PubMed] [Google Scholar]
- 19.Bazick HS, Chang D, Mahadevappa K, Gibbons FK, Christopher KB. Red cell distribution width and all-cause mortality in critically ill patients. Crit Care Med. 2011;39:1913–21. doi: 10.1097/CCM.0b013e31821b85c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadaka F, O’Brien J, Prakash S. Red cell distribution width and outcome in patients with septic shock. J Intensive Care Med. 2013;28:307–13. doi: 10.1177/0885066612452838. [DOI] [PubMed] [Google Scholar]
- 21.Jo YH, Kim K, Lee JH, Kang C, Kim T, Park HM, et al. Red cell distribution width is a prognostic factor in severe sepsis and septic shock. Am J Emerg Med. 2013;31:545–8. doi: 10.1016/j.ajem.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Scharte M, Fink MP. Red blood cell physiology in critical illness. Crit Care Med. 2003;31:S651–7. doi: 10.1097/01.CCM.0000098036.90796.ED. [DOI] [PubMed] [Google Scholar]
- 23.Pierce CN, Larson DF. Inflammatory cytokine inhibition of erythropoiesis in patients implanted with a mechanical circulatory assist device. Perfusion. 2005;20:83–90. doi: 10.1191/0267659105pf793oa. [DOI] [PubMed] [Google Scholar]
- 24.Cooper AC, Mikhail A, Lethbridge MW, Kemeny DM, Macdougall IC. Increased expression of erythropoiesis inhibiting cytokines (IFN-gamma, TNF-alpha, IL-10, and IL-13) by T cells in patients exhibiting a poor response to erythropoietin therapy. J Am Soc Nephrol. 2003;14:1776–84. doi: 10.1097/01.asn.0000071514.36428.61. [DOI] [PubMed] [Google Scholar]
- 25.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–23. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 26.Suvarna R, Pallipady A, Bhandary N, Hanumanthappa The clinical prognostic indicators of acute pancreatitis by APACHE II scoring. J Clin Diagn Res. 2011;5:459–63. [Google Scholar]
- 27.Wang BW, Mok KT, Chang HT, Liu SI, Chou NH, Tsai CC, et al. APACHE II score: A useful tool for risk assessment and an aid to decision-making in emergency operation for bleeding gastric ulcer. J Am Coll Surg. 1998;187:287–94. doi: 10.1016/s1072-7515(98)00158-6. [DOI] [PubMed] [Google Scholar]
- 28.Muckart DJ, Bhagwanjee S, Neijenhuis PA. Prediction of the risk of death by APACHE II scoring in critically ill trauma patients without head injury. Br J Surg. 1996;83:1123–7. doi: 10.1002/bjs.1800830829. [DOI] [PubMed] [Google Scholar]
- 29.Hsieh HF, Chen TW, Yu CY, Wang NC, Chu HC, Shih ML, et al. Aggressive hepatic resection for patients with pyogenic liver abscess and APACHE II score>or=15. Am J Surg. 2008;196:346–50. doi: 10.1016/j.amjsurg.2007.09.051. [DOI] [PubMed] [Google Scholar]


