Abstract
Intracranial space occupying lesion [SOL] during pregnancy presents several challenges to the neurosurgeons, obstetricians and anaesthesiologists in not only establishing the diagnosis, but also in the perioperative management as it requires a careful plan to balance both maternal and foetal well-being. It requires modification of neuroanaesthetic and obstetric practices which often have competing clinical goals to achieve the optimal safety of both mother and foetus. Intracranial tuberculoma should be considered in the differential diagnosis of intracranial SOL in pregnant women with signs and symptoms of raised intracranial pressure with or without neurological deficits, especially when they are from high incidence areas. We report a 28-week pregnant patient with intracranial SOL who underwent craniotomy and excision of the lesion, subsequently diagnosed as cranial tuberculoma.
Keywords: Neuro anaesthesia, pregnancy, tuberculoma
INTRODUCTION
Anaesthetic management of a pregnant patient with an intracranial space occupying lesion (SOL) requires modification of neuroanaesthetic and obstetric practices, which have competing clinical goals to achieve the optimal safety of both mother and foetus.[1] Maternal alterations during pregnancy may complicate the anaesthetic management and increase monitoring requirements for safety of both mother and foetus.[2,3] Neuroanaesthesia for the pregnant patient is required infrequently, as overall frequency for non-obstetric surgery during pregnancy is low and surgery for intracranial SOL during pregnancy is rare.[4] Intracranial tuberculomas are clinically and radio graphically indistinguishable from enhancing neoplastic lesions.[5]
Tuberculosis (TB) of central nervous system accounts for about 5% of extra pulmonary cases and manifests as meningitis or uncommonly as tuberculoma.[6] We present a 28-week pregnant patient with an intracranial SOL undergoing craniotomy.
CASE REPORT
A 22-year-old woman, gravida2 para1, 28 weeks pregnant, weighing 56 kg presented with headache, right hemi paresis and focal seizures in the right upper limb. The neurosurgical team made a clinical diagnosis of left frontal intracranial SOL. Magnetic resonance imaging (MRI) brain revealed a hyper intense mass in the left fronto-parietal region with peri-lesional oedema, mass effect and midline shift to the right. MRI brain [Figure 1] showed a hyper intense mass in the left fronto-parietal region with peri-lesional oedema. MR spectroscopy showed grossly elevated choline and reduced N-acetylaspartate and creatinine levels within the lesion. MRI brain and MR spectroscopic features were suggestive of neoplastic lesion-glioma. As the patient was 28 weeks pregnant with signs and symptoms of raised intracranial pressure (ICP) with progressive neurological deficits, not manageable with drugs, elective craniotomy was planned for decompression and excision of the SOL. Treatment was started with dexamethasone 8 mg BD, carbamazepine 200 mg BD, to reduce ICP and to prevent seizures. Patient was examined by the obstetric team and foetal well-being was determined. Risks of preterm labour and miscarriages were explained to the patient. Pre-anaesthetic evaluation was normal and haematological and biochemical parameters were within normal limits. Patient was pre-medicated with tab.ranitidine 150 mg and tab.metoclopramide 10 mg previous night and on morning of surgery as prophylaxis against aspiration. In the operating theatre, initial monitoring consisted of electrocardiogram, pulse oximeter, non-invasive blood pressure and capnography. Two large bore [16G] peripheral intravenous (IV) cannulae were secured. Patient was slightly tilted to the left and a wedge placed under the right buttock to avoid aorto-caval compression. After pre-oxygenation, modified rapid sequence induction and intubation was performed using thiopentone 5 mg/kg, fentanyl 2 μg/kg, lignocaine 1 mg/kg and rocuronium 1 mg/kg. Re-inforced cuffed endotracheal tube size 7 was used and an intra-arterial line was secured. Anaesthesia was maintained with isoflurane 0.7 MAC in oxygen and air, vecuronium and intermittent boluses of morphine. Additional monitoring included urinary catheter, oesophageal temperature probe and arterial blood pressure (BP). Foetal heart rate [FHR] was monitored.
Figure 1.
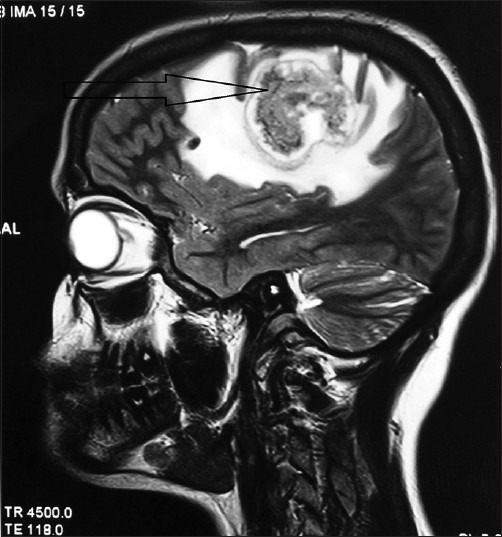
Magnetic resonance imaging brain showing a hyper intense mass in the left fronto-parietal region with peri-lesional oedema
Antibiotic prophylaxis with ceftriaxone 1 g, and dexamethasone 8 mg IV, and mannitol 0.25 g/kg (IV infusion after skin incision) were administered. Warming blanket was used to keep the patient warm. Craniotomy and excision of the intracranial SOL was done. Patient was haemodynamically stable throughout the procedure, with systolic BP between 100 and 120 mm Hg, heart rate 60–80 beats/min. SpO2 (98–100%), EtCO2 (28–34 mm Hg) and body temperature (36–37°C) were maintained. FHR remained stable throughout. Total duration of procedure was 4 h, during which 2000 ml of crystalloids was infused. Estimated blood loss was 400 ml and urine output was maintained at >1 ml/kg/h. Intra-operative arterial blood gas analysis showed values as below: pH 7.42, pCO2 32.6 mm Hg, pO2 239.9 mm Hg, HCO3 23.0 mmol/l, O2 Sat 99.6%. At the end of the procedure, neuromuscular blockade was reversed with neostigmine and glycopyrrolate. Patient was extubated fully awake, with no new neurological deficits, in the operating theatre and shifted to intensive care unit for recovery and observation. Foetal status assessed by foetal heart rate was normal. After a steady recovery, she was shifted to the ward 48 h after surgery. The histopathology examination report [HPE] revealed features of granulomatous encephalitis, consistent with tuberculoma. Ziehl-Nielssen stain showed positive result. Patient was started on antituberculous therapy [ATT] in consultation with obstetric team and was discharged from hospital. At 38th week of gestation, patient delivered a healthy male baby weighing 2.8 kg by normal vaginal delivery.
DISCUSSION
Evidence based recommendations for neuroanaesthetic management in pregnancy are sparse and so planning and decision making must be made largely on general principles of neurosurgical and obstetric anaesthesia. The foetus may be compromised indirectly by maternal hypotension, uterine artery vasoconstriction, maternal hypoxaemia and acid-base changes. Indeed any change in maternal physiology that reduces utero-placental perfusion compromises foetal gas exchange.[7]
The adverse foetal effects of drugs administered in the perioperative period need to be carefully considered.[1] All the drugs administered in our case belonged to either Food and Drug Administration (FDA) pregnancy category B (Animal studies fail to demonstrate risk to foetus) or category C (Potential benefits may warrant use of drugs in pregnant women despite potential risks).[8] Ranitidine, metoclopramide, lignocaine, ceftriaxone and glycopyrrolate are included in FDA category B. Mannitol, dexamethasone, thiopentone, fentanyl, morphine, rocuronium, vecuronium, isoflurane and neostigmine are included in FDA category C.
Foetal heart rate monitoring may help in detecting foetal hypoxia and metabolic acidosis which may result in tissue damage or foetal death.[2] After 16th week of gestation, continuous FHR monitoring may help in early detection of foetal hypoxia in the peri-operative period.[4,9] In our case, we used continuous FHR monitoring intraoperatively with external cardiotocography. The anaesthesiologist must understand the physiological changes of pregnancy, their implications, and the specific risks of anaesthesia during pregnancy.[10] Following surgery during pregnancy, the risk of preterm labour and abortion are increased.[3] Prophylactic tocolytics aim to stop uterine contractions and to temporarily delay delivery with the objective of improving neonatal outcome by preventing preterm labour.[11] However, they are controversial as they have considerable maternal side effects and their efficacy in non-obstetric surgery is not established.[3]
Tuberculosis is primarily a lung disease, but extra pulmonary involvement is common. Our patient was afebrile without pulmonary manifestations of TB, a normal chest X-ray and was HIV negative. Intracranial tuberculomas are thought to arise when tubercles in the brain parenchyma enlarge without rupturing into the subarachnoid space.[12] A high index of suspicion of tuberculoma is warranted in situations like focal or generalized seizures, focal neurological deficits and in patients with symptoms and signs of raised intra-cranial tension such as headache, vomiting and papilloedema.[13] Our patient presented with headache, focal seizures and right hemi paresis. Pre-operatively, based on the MRI and MR spectroscopic features and clinical presentation, the diagnosis was a tumour, possibly glioma. However, there are limitations of MR Spectroscopy for the diagnosis of intracranial SOL as it may not be able to distinguish brain tumours such as glioma from inflammatory lesions such as tuberculomas and may be inconclusive in tuberculomas.[5] During surgery, initial impression was that of a tumour, rather than an inflammatory process. However, the HPE report clearly suggested it was an intracranial tuberculoma and the patient responded well to ATT.
CONCLUSION
Cerebral tuberculoma should be considered in the differential diagnosis of SOL in a pregnant woman presenting with signs and symptoms of raised ICP, especially in women coming from high incidence areas, even in the absence of pulmonary involvement. Neurosurgery is uncommonly required during pregnancy, but it requires a multidisciplinary approach with a specific anaesthetic plan, keeping in mind the safety requirements of both the mother and the foetus.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Wang LP, Paech MJ. Neuroanesthesia for the pregnant woman. Anesth Analg. 2008;107:193–200. doi: 10.1213/ane.0b013e31816c8888. [DOI] [PubMed] [Google Scholar]
- 2.Cok OY, Akin S, Aribogan A, Acil M, Erdogan B, Bagis T. Anesthetic management of 29 week pregnant patient undergoing craniotomy for pituitary macroadenoma – A case report. Middle East J Anaesthesiol. 2010;20:593–6. [PubMed] [Google Scholar]
- 3.Van De Velde M, De Buck F. Anesthesia for non-obstetric surgery in the pregnant patient. Minerva Anestesiol. 2007;73:235–40. [PubMed] [Google Scholar]
- 4.Balki M, Manninen PH. Craniotomy for suprasellar meningioma in a 28-week pregnant woman without fetal heart rate monitoring. Can J Anaesth. 2004;51:573–6. doi: 10.1007/BF03018400. [DOI] [PubMed] [Google Scholar]
- 5.Omuro AM, Leite CC, Mokhtari K, Delattre JY. Pitfalls in the diagnosis of brain tumours. Lancet Neurol. 2006;5:937–48. doi: 10.1016/S1474-4422(06)70597-X. [DOI] [PubMed] [Google Scholar]
- 6.Gandhi R, Prabhakar H. Emergency caesarean section in a patient with intracerebral tuberculoma. Indian J Anaesth. 2007;51:244–6. [Google Scholar]
- 7.Bassel GM, Marx GF. Optimization of fetal oxygenation. Int J Obstet Anesth. 1995;4:238–43. doi: 10.1016/0959-289x(95)82917-y. [DOI] [PubMed] [Google Scholar]
- 8.Boothby LA, Doering PL. FDA labeling system for drugs in pregnancy. Ann Pharmacother. 2001;35:1485–9. doi: 10.1345/aph.1A034. [DOI] [PubMed] [Google Scholar]
- 9.Giannini A, Bricchi M. Posterior fossa surgery in the sitting position in a pregnant patient with cerebellopontine angle meningioma. Br J Anaesth. 1999;82:941–4. doi: 10.1093/bja/82.6.941. [DOI] [PubMed] [Google Scholar]
- 10.Heidemann BH, McLure JH. Changes in maternal physiology during pregnancy. CEPD Reviews. Br J Anaesth. 2003;3:65–8. [Google Scholar]
- 11.Katz VL, Farmer RM. Controversies in tocolytic therapy. Clin Obstet Gynecol. 1999;42:802–19. doi: 10.1097/00003081-199912000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Rock RB, Olin M, Baker CA, Molitor TW, Peterson PK. Central nervous system tuberculosis: Pathogenesis and clinical aspects. Clin Microbiol Rev. 2008;21:243–61. doi: 10.1128/CMR.00042-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar A, Kannan R. Tuberculoma of brain. Pulmon. 2011;13:9–11. [Google Scholar]


