Abstract
A 63-year-old man presented with generalized fatigue, chills, malaise, dyspnea, intermittent fevers, and 50-pound weight loss of 4 months' duration. Blood cultures were positive for pan-sensitive Streptococcus anginosus. Transesophageal echocardiography showed an 11 mm × 3 mm mobile mass attached to the mitral valve, a 16 mm × 16 mm mobile mass attached to the pulmonary valve, and a small membranous ventricular septal defect. The patient received 12 weeks of intravenous (IV) antibiotics with eventual resolution of the masses. Multi-valve endocarditis involving both the left and right chambers is rarely reported without prior history of IV drug use or infective endocarditis. Our case emphasizes the importance of careful assessment for ventricular septal defects or extra-cardiac shunts in individuals who present with simultaneous right and left-sided endocarditis.
Keywords: Echocardiography, infective endocarditis, pulmonary valve endocarditis, ventricular septal defect
BRIEF REPORT
A 63-year-old man with history of hypertension, hyperlipidemia, diabetes mellitus, and hepatitis C secondary to blood transfusion more than 20 years prior presented with fatigue, chills, malaise, dyspnea, intermittent fevers, and 50-pound weight loss of 4 months' duration. He had no history of intravenous (IV) drug use or recent dental procedures. Physical examination was notable for a harsh 4/6 holosystolic murmur heard along the left upper sternal border, a separate 2/6 holosystolic murmur heard at the left lower sternal border and apex, and lower extremity petechiae.
Initial laboratory studies were significant for normocytic anemia and mild thrombocytopenia. Blood cultures were positive for pan-sensitive Streptococcus anginosus. Transesophageal echocardiography (TEE) showed an 11 mm × 3 mm mobile mass attached to the mitral valve, a 16 mm × 16 mm mobile mass attached to the pulmonary valve, and a small membranous ventricular septal defect (VSD) [Figure 1 and Videos 1–3]. Mild mitral valve regurgitation, but no pulmonary valve regurgitation, was noted. Chest computed tomography (CT) demonstrated evidence of septic emboli with associated pulmonary infarctions [Figure 2].
Figure 1.
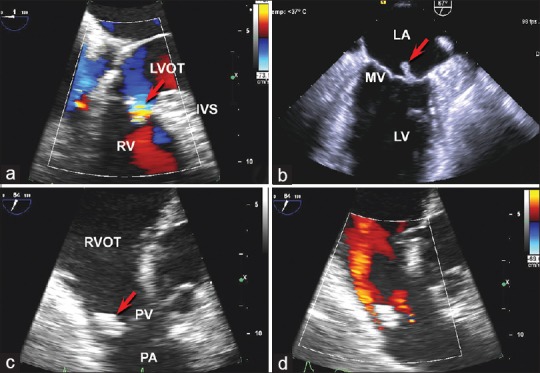
Transesophageal echocardiography was significant for a very small membranous interventricular septal defect shown in panel (a) with color Doppler demonstrating flow through the ventricular septal defect from the left ventricle to the right ventricle during systole (arrow). Panel (b) demonstrates the mobile vegetation of the posterior leaflet of the mitral valve (arrow). panel (c) shows the large pulmonary valve vegetation (arrow) while panel (d) is a color doppler evaluation of the pulmonary valve demonstrating the pulmonary regurgitation jet in red. IVS = Interventricular septum, LA = Left atrium, LV = Left ventricle, LVOT = Left ventricular outflow tract, PA = Pulmonary artery, PV = Pulmonary valve, RV = Right ventricle, RVOT = Right ventricular outflow tract
Figure 2.
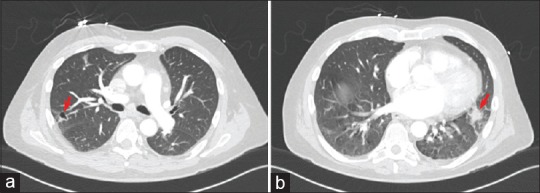
Chest computed tomography showed evidence of pulmonary infarcts secondary to septic emboli of the pulmonary valve infective endocarditis. Panel (a) shows a nodule in the right upper lobe posterolaterally which is partially cavitated (arrow). panel (b) demonstrates an irregular 17 mm nodule in the lingula posteriorly (arrow)
Penicillin, gentamicin, ceftriaxone, and vancomycin were initiated. Once bacterial sensitivities were available, antibiotics were reduced to daily ceftriaxone. Blood cultures were negative after 1 week of antibiotic therapy. Follow-up TEE completed after 6 weeks of antibiotic therapy revealed that the mitral valve mass had decreased in size (6 mm × 2 mm), but the pulmonic valve mass size was unchanged. The patient was asymptomatic and blood cultures remained negative. Following consultations with cardiology, infectious diseases, and cardiovascular surgery, the decision was made to proceed with two additional weeks of antibiotic therapy. One week later, however, the patient was hospitalized for acute pleuritic chest pain. Chest CT demonstrated findings suggestive for a new septic embolus. Repeat blood cultures were negative.
Transesophageal echocardiogram performed 1 week later demonstrated an 11 mm × 3 mm mass attached to the mitral valve and a 15 mm × 8 mm mass attached to the pulmonary valve. The case was again reviewed by cardiology, infectious diseases, and cardiovascular surgery. As the patient remained hemodynamically stable without evidence of significant valvular dysfunction and there was concern for a possible new cavitary pulmonary lesion, the decision was made to proceed with four additional weeks of IV antibiotics. Follow-up TEE obtained 4 weeks later demonstrated complete resolution of the masses, trivial mitral regurgitation, and moderate pulmonary regurgitation. Valve surgery was aborted. The patient subsequently has done well without symptom recurrence.
DISCUSSION
We present an unusual case of infective endocarditis (IE) affecting the mitral and pulmonary valves associated with a small congenital VSD in an adult patient without additional predisposing risk factors. The incidence of a first episode of IE in patients with isolated VSDs has been estimated to range from 0.3 to 3.8 per 1,000 person-years.[1] While IE typically involves left-sided cardiac structures, the presence of a VSD is a risk factor for the development of right-sided cardiac involvement due to left-to-right shunting across the ventricular septum. Similarly, extra-cardiac shunts (as in pulmonary arteriovenous malformations) can also predispose to biventricular IE.
While the pulmonary valve remains the least commonly affected valve in IE, several cases of isolated pulmonary valve IE associated with VSD have been reported. However, multi-valvular IE, as seen in our patient, is uncommon and simultaneous right and left-sided valve involvement, particularly involving the pulmonary valve, is rarely reported. Among multi-valvular IE cases reported in the literature, aortic and mitral valve involvement comprise the majority of cases, with the pulmonary valve rarely affected.[2] In a series of eight cases of pulmonary valve IE, only one patient had simultaneous pulmonary and mitral valve involvement.[3]
The precise mechanism underlying concurrent right and left-sided valve involvement in IE remains unclear. With a primary left-sided lesion, it is thought that infective organisms are shunted through a VSD onto tissue damaged by turbulent flow. The large size of the pulmonic valve vegetation in our case, however, suggests that this may have been the primary lesion. We speculate that the high pressure flow created by the left-to-right shunt across the diminutive VSD damaged the pulmonary valve, making the valve more susceptible to bacterial infection. While it has been proposed that “seeding” of the mitral valve might result from transient right-to-left shunting across a VSD,[4] this seems unlikely in our case, given the small size of the VSD. Alternatively, the presence of an extra-cardiac shunt, such as a pulmonary arteriovenous malformation, could be a plausible mechanism for seeding the mitral valve, but no extra-cardiac shunts were seen in our patient. It is also possible that septic emboli within the pulmonary vasculature allowed bacterial organisms to extend into the left heart and subsequently infect the mitral valve; however, the seeding of a previously undamaged valve with one of the less virulent organisms, such as S. anginosus in this case, is atypical.
There is ample evidence suggesting improved outcomes in IE treated with surgery, primarily in patients with concomitant heart failure, hemodynamic instability, large left-sided vegetations, and persistent sepsis.[5] However, it is noteworthy that these studies mainly involved patients with left-sided endocarditis. Given the hemodynamic stability in our patient, absence of sepsis, and resolution of the mitral vegetation after the initial course of antibiotic therapy, the decision was made to pursue prolonged antibiotic therapy rather than surgery.
Learning points
Ventricular septal defects are a well-described risk factor for the development IE involving right heart structures. Current evidence does not support routine VSD closure after an isolated case of IE.[1] Our case emphasizes the importance of careful assessment for VSD or an extra-cardiac shunt in individuals with no apparent risk factors of IE who present with simultaneous right- and left-sided endocarditis. Antibiotic prophylaxis is indicated in all patients with VSD or prior history of IE. Our case also demonstrates that prolonged antibiotic therapy can successfully eradicate simultaneous large vegetations on the mitral and pulmonary valves while preventing irreversible damage to these valves, thereby eliminating the need for surgical intervention.
Video Available on www.avicennajmed.com
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Guntheroth WG, Spiers PS. Is operative closure of a small ventricular septal defect required after an episode of infective endocarditis? Am J Cardiol. 2005;95:960–2. doi: 10.1016/j.amjcard.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 2.López J, Revilla A, Vilacosta I, Sevilla T, García H, Gómez I, et al. Multiple-valve infective endocarditis: Clinical, microbiologic, echocardiographic, and prognostic profile. Medicine (Baltimore) 2011;90:231–6. doi: 10.1097/MD.0b013e318225dcb0. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura K, Satomi G, Sakai T, Ando M, Hashimoto A, Koyanagi H, et al. Clinical and echocardiographic features of pulmonary valve endocarditis. Circulation. 1983;67:198–204. doi: 10.1161/01.cir.67.1.198. [DOI] [PubMed] [Google Scholar]
- 4.Itoh N, Shigematsu H, Itoh M, Yamada H. Right-sided infective endocarditis combined with mitral involvement in a patient with ventricular septal defect. Acta Pathol Jpn. 1985;35:459–71. doi: 10.1111/j.1440-1827.1985.tb00588.x. [DOI] [PubMed] [Google Scholar]
- 5.Kang DH, Kim YJ, Kim SH, Sun BJ, Kim DH, Yun SC, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med. 2012;366:2466–73. doi: 10.1056/NEJMoa1112843. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


