Abstract
BACKGROUND:
The effects of exercise, metformin, and orlistat on anthropometric parameters, lipid profile, endocrine parameters, and ovulation in polycystic ovarian syndrome (PCOS) women were compared.
AIM:
The aim was to study the efficacy of orlistat compared with metformin and exercise in PCOS.
DESIGN:
Randomized control trial.
METHODS:
A total of 90 eligible PCOS women were randomly assigned to receive either of the two drugs (orlistat or metformin) in combination with lifestyle interventions or as controls where they received lifestyle interventions alone. Anthropometric parameters were assessed at baseline and 4 weekly intervals for 3 months. Androgen levels, insulin resistance, ovulation and conception rates and lipid profile were also assessed at the end of study.
STATISTICAL ANALYSIS:
Statistical analysis was performed using the SPSS version 17.0.
RESULTS:
The levels of fasting blood sugar, fasting insulin and homeostatic model assessment insulin resistance were comparable in three treatment groups. Mean total testosterone, serum hormone binding globulin, free androgen index, dehydroepiandrosterone sulfate in all arms were comparable and statistically nonsignificant. However, orlistat and metformin were more effective in reducing weight, body mass index, waist circumference and waist-hip ratio. However, side-effects were less with orlistat. Ovulation rate was 33.3%, 23.35% with orlistat and metformin group respectively, but were not statistically significant. In orlistat group, significant improvement was observed in lipid profile at the end of 3 months. Conception rates were 40% and 16.7% and 3.3% in orlistat, metformin group and control group respectively (P - 0.003). Weight loss was found to be the best predictor of ovulation with sensitivity with good sensitivity.
CONCLUSION:
Orlistat is as effective as metformin in reducing weight and achieves similar ovulation rates in obese PCOS patients. However, orlistat has minimal side-effects and is better tolerated compared with metformin.
KEY WORDS: Control, metformin, orlistat, polycystic ovarian syndrome
INTRODUCTION
Polycystic ovarian syndrome (PCOS) is the most common endocrinopathy of women with a prevalence of 6-10% based on the National Institute of Health criteria and as high as 15% when the broader Rotterdam criteria are applied.[1] Obesity has reached it epidemic proportion. Its is present in 40-60% of women with PCOS mostly of central type.[2,3,4] Weight loss is the first-line treatment evidence-based approach in overweight PCOS women.[4]
Pharmacotherapy aiming to achieve weight loss for the group of obese PCOS patients comprises use of insulin-sensitizing drugs such as metformin and use of antiobesity drugs like orlistat.
There have been few randomized studies, which have compared the effects of orlistat and metformin in obese PCOS patients and have generally reported favorable results with equal efficacy of both drugs on weight reduction.[5,6,7] Orlistat being long acting and gastric lipase inhibitor which prevent the hydrolysis of dietary fat into absorbable free fatty acids and monoglyceride with increase of fecal fat excretion. Metformin insulin sensitizers various other mechanisms have been proposed. Therefore in our study, we attempted to evaluate the role of orlistat in PCOS.
Methods
The present study is a randomized controlled trial, which was carried out between October 2011 and June 2012 at Manipal Assisted Reproduction Center, Kasturba Medical College, Manipal University, Manipal (Karnataka).
Study population
The study population consisted of 90 overweight and obese women who fulfilled the criteria mentioned below were enrolled with 30 in each, orlistat, and metformin and control group.
Using a two-tailed alpha value (0.05) and a beta value (0.2) 30 observation per group would be sufficient to detect a significance difference.
Inclusion criteria
Age: <40 years, body mass index (BMI): ≥23 (taken as cut-off for overweight in the study as per the recommendations[8,9,10]), euthyroid, PCOS patients after a full work up done for infertility. Good antral follicles were taken into consideration for the study even though some women were nearing 40 years of age.
The diagnosis of PCOS was made according to the revised 2003 Rotterdam criteria.
Exclusion criteria
Male factor
Untreated hypothyroidism
Renal or hepatic impairment
Malabsorption syndrome
Diabetes (type 2 or type 1).
Study design
This was a randomized controlled clinical trial. All patients enrolled were randomly assigned to orlistat (Reshape, Meyer Organics), metformin (Obimet, Abbott), or control groups. Randomization was done by block randomization.
All overweight and obese infertile PCOS women were recruited in the study and randomized to receive either of the two drugs (orlistat or metformin) in combination with lifestyle interventions or as controls where they received lifestyle interventions alone. BMI was based on an adapted version for Asians.
Drug formulation
Patients in the metformin arm received the drug with fertility fitness program. Metformin was incremented stepwise to maximum 500 mg 3 times a day.
In orlistat arm subjects received orlistat in a standard dose of 120 mg twice a day and underwent fertility fitness program.
The control arm patients underwent fertility fitness program only.
Fertility fitness program
All subjects in the three arms were given specific hypocaloric dietary advice according to their weight and fertility fitness program was part of the treatment in all the groups. The fertility fitness program included diet, exercise and lifestyle modification.
Dietetic treatment
Dietary management was targeted for gradual weight loss (0.5-1 kg/week) through energy intake reduction and increasing physical activity with daily calorie intake between 1200 and 1800 kcal/day depending on their activity and BMI. Diet was composed of low fat 25% of energy moderate protein of 15% of energy and high carbohydrate intake of 55% of energy with increased consumption of fiber whole grain bread, cereals, fruits and vegetables.
Physiotherapy
Exercise was advised by a physiotherapiest. Total duration of exercise was around 1 h/day. It included warm up session, aerobic exercise session and cooled down session.
Ethical committee
Ethical committee clearance was done.
Methodology
At the first visit following were performed
History
Menstrual cyclicity and the presence or absence of hirsutism and acne.
Physical examination
Body mass index (was taken as the adapted version for Asian people), waist circumference, waist-hip ratio. Waist circumference was measured as the smallest measurement between the iliac crest and the 12th rib.
Endocrine profile
Baseline serum luteinizing hormone (LH) and follicle-stimulating hormone (FSH) (Roche Co.): Performed on day 2 of the spontaneous cycle or progesterone-induced withdrawal bleed.
Androgen profile
Total testosterone, dehydroepiandrosterone sulfate (DHEAS), sex hormone-binding globulin (SHBG) and free androgen index (FAI). The FAI was calculated from the following formula: (Total testosterone/SHBG) × 100. Androgen estimation was done by immuno electric chermluminence method (ng/ml).
Parameters for measuring insulin resistance - Fasting insulin, fasting glucose and homeostatic model assessment insulin resistance (HOMA-IR).
Lipid profile - High-density lipoprotein, low-density lipoprotein (LDL), total cholesterol, triglycerides. Lipid profile was estimated by ELISA (mg/dl).
Transvaginal ultrasonography
Follow-up visits
The following were recorded at 4 weekly intervals for 3 months-history and physical examination, especially anthropometric evaluation.
At study conclusion
At 12 weeks follow-up, androgen profile, serum LH and FSH, lipid profile, fasting insulin, and fasting blood sugar were repeated.
Subjects who ovulated spontaneously (by ultrasound) were advised timed intercourse and were evaluated for pregnancy for a period of 3 months.
The subjects who did not have spontaneous ovulation as observed by ultrasound underwent one cycle of ovulation induction with clomiphene citrate 50 mg from day 2 to day 6 of the cycle.
Transvaginal sonography was done on day 2 for antral follicle count for ovarian reserve and following ovulation induction for follicular monitoring.
At least two follicles with a size of 18 mm were considered to be a good response and if less poor response. Those who responded to one cycle of ovulation induction underwent intrauterine insemination.
Outcome measures
Primary outcome measures
Ovulation
Weight loss
Secondary outcome measures
Change in the BMI and waist circumference, predictors of response to treatment (ovulation, detected by ultrasound) for the three arms.
Statistical analysis
Statistical analysis was performed using the SPSS program for Windows, version 17.0 (SPSS Chicago, Illinious, USA). For all statistical tests, a P < 0.05 was taken to indicate a significant difference. Continuous variables are presented as mean ± standard error, and categorical variables are presented as absolute numbers and percentage. Data were checked for normality before statistical analysis using Shapiro-Wilk test. Normally distributed continuous variables were compared using ANOVA. If the F value was significant and variance was homogeneous, Bonferroni multiple comparison test was used to assess the differences between the individual groups; otherwise, Tamhane's T2 test was used. The Kruskal-Wallis test was used for those variables that were not normally distributed and further complications were done using Mann-Whitney U-test. Categorical variables were analyzed using the Chi-square test. For all statistical tests, a P < 0.05 was taken to indicate a significant difference.
RESULTS
The baseline demographic and clinical characteristics were similar in three groups with no significant difference. The mean age, clinical characteristics such as menstrual history, androgenic symptoms, weight, BMI, waist circumference, waist-hip ratio were comparable in three groups.
Primary outcome
Ovulation rates
Subjects in orlistat and metformin arms had similar ovulation rates which were significantly better than that of the control group with P - 0.008 as shown Table 1.
Table 1.
Comparison of ovulation rate in three groups
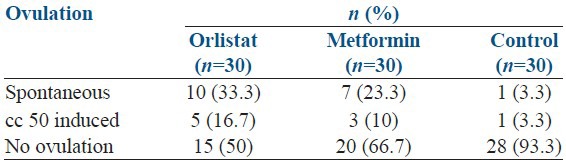
Conception rates
As shown in Table 2, 12 participants (40%) conceived in orlistat group, 8 (16.7%) participants conceived in the metformin arm and 1 (3.3%) participant conceived in the control arm (P - 0.003).
Table 2.
Comparison of conception rates in three groups
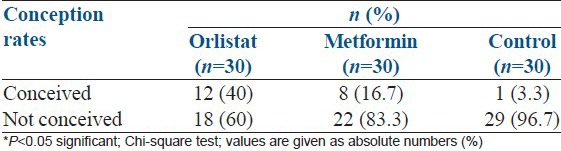
Weight loss
Comparisons within group
Over the course of the study, there was overall statistically significant fall in all anthropometric measurements in the three groups with P < 0.001 as highlighted in Figures 1–3.
Figure 1.
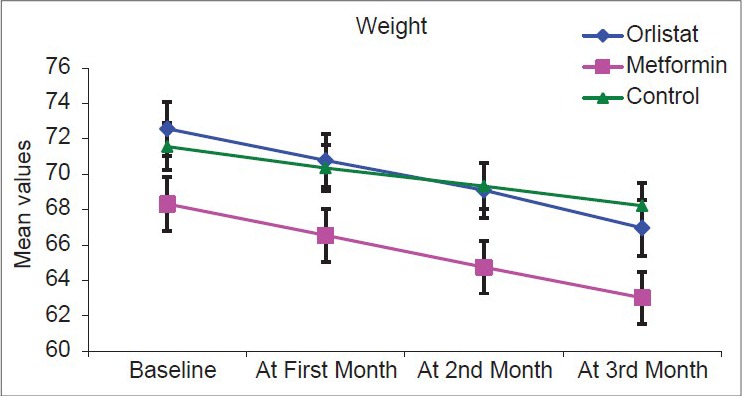
Graph showing weight in the three arms at follow-up visits
Figure 3.
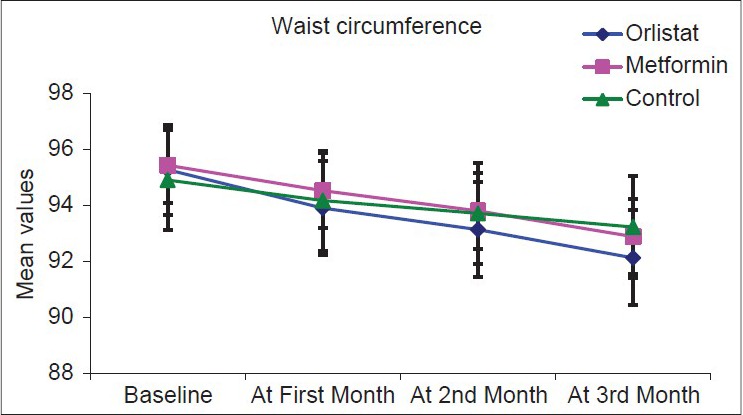
Graph showing waist circumference in the three arms at follow-up visits
Figure 1 shows a significant fall in weight over 3 months in all three groups, but fall is significantly more in two drug arms.
Figure 2 shows a significant decrease in BMI over 3 months in all three groups, but fall is more in two drug arms compared with control arm.
Figure 2.
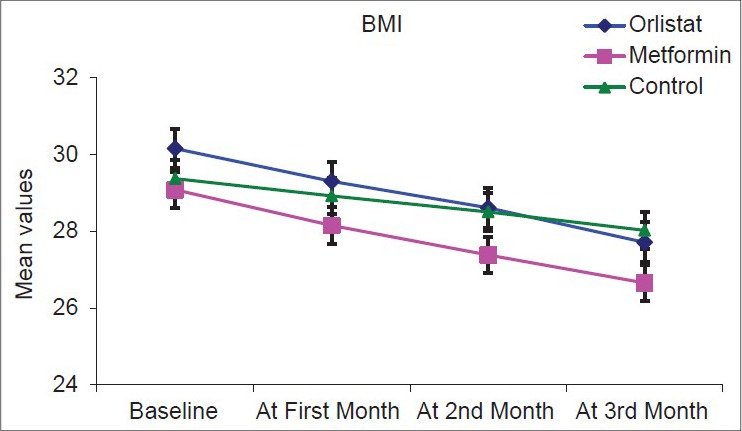
Graph showing body mass index in the three arms at follow-up visits
Between groups comparisons
Both drug arms have similar efficacy in reducing weight, BMI, waist circumference and waist-hip ratio as highlighted in Table 3.
Table 3.
Percentage change in parameters for measuring weight loss at follow-up between the groups
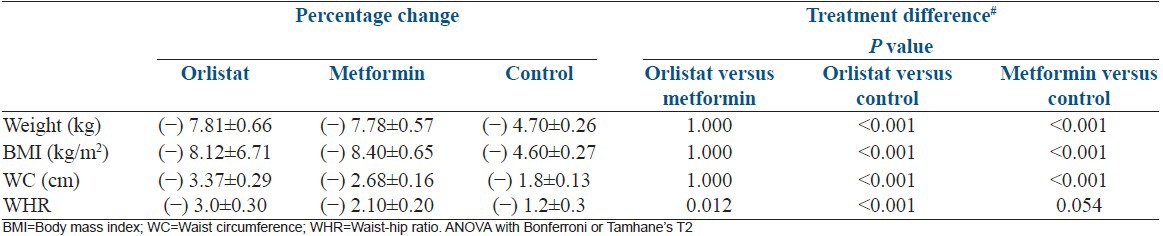
Secondary outcome
Androgen profile
Comparisons within groups
As shown in Table 4 in all the arms, there was a significant reduction in testosterone, FAI by the end of 12 weeks whereas there was no significant change in SHBG or DHEAS levels.
Table 4.
Percentage change in androgen profile at follow-up between three groups
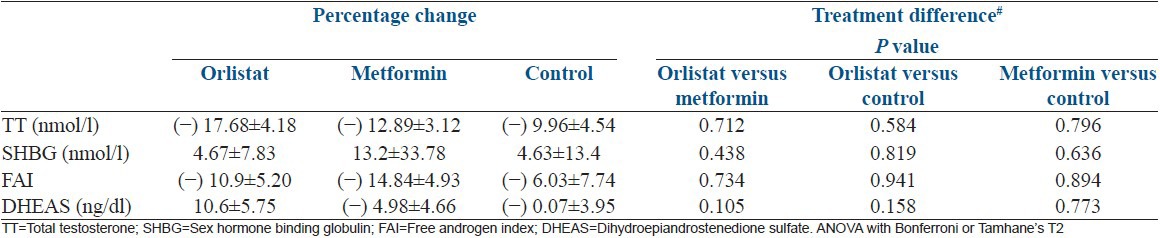
Comparisons between groups
There was no significant difference in the mean serum testosterone, DHEAS, SHBG concentrations or FAI between the three arms at the start and end of the study as shown in Table 4.
Lipid profile
Comparisons within group
As summarized in Table 5, the levels of total cholesterol, LDL, triglycerides decreased significantly in orlistat arm.
Table 5.
Percentage change in lipid profile at follow-up between the three groups
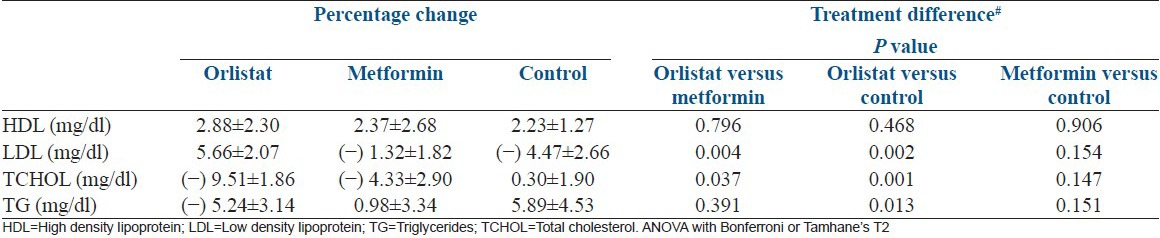
Comparisons between groups
Metformin and control arm orlistat showed significant improvement in LDL, triglycerides and total cholesterol at the start and end of the study as depicted in Table 5.
Endocrine profile
Comparisons within group
As shown in Table 6, the groups showed no significant improvement in the biochemical parameters for assessing insulin resistance except metformin group but it did not reach the level of significance.
Table 6.
Percentage change in endocrine profile of insulin resistance
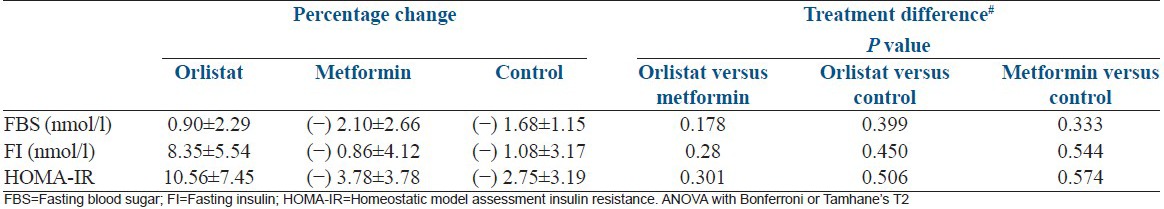
Comparsions between groups
As shown in Table 6, there was no significant difference in the fasting blood sugar, fasting insulin and HOMA-IR between the three arms.
Predictors of response to ovulation
Figure 4 depicts receiver operating characteristic (ROC) curve with low baseline LH as predictor for ovulation with area under curve (AUC) =0.619.
Figure 4.
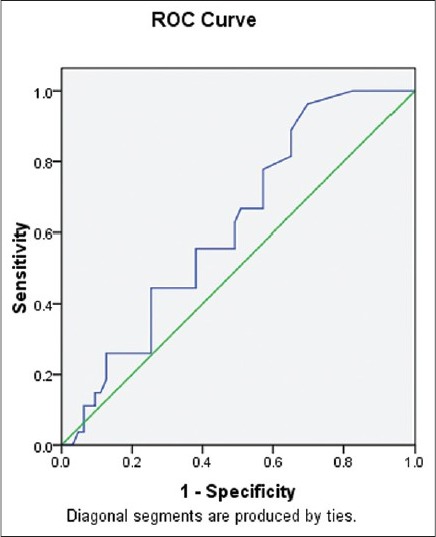
Receiver operating characteristic curve demonstrating low baseline luteinizing hormone as predictor of ovulation
Figure 5 depicts ROC curve with percentage weight loss as the best marker for ovulation with AUC = 0.839.
Figure 5.
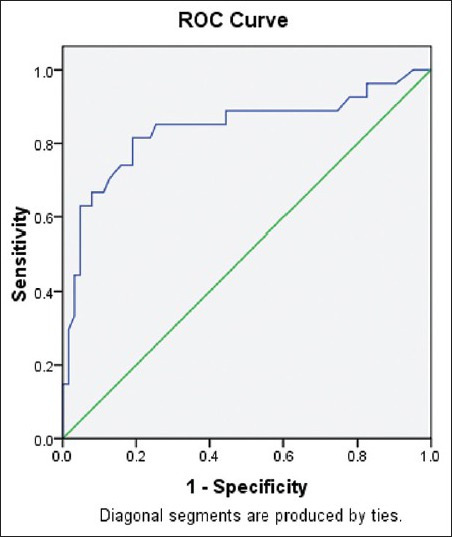
Receiver operating characteristic curve demonstrating percentage weight loss as best marker for ovulation
Subjects in orlistat and metformin group showed statistically significant response as compared to the control group (P - 0.001) in terms of folliculogenesis. However, the two drug arms namely orlistat and metformin were similar as far as their effect on folliculogenesis is concerned (P - 0.190).
Side-effects
Patients in the metformin group reported side-effects such as nausea, epigastric pain. However, those in orlistat group tolerated the drug well. Gastrointestinal symptoms were present in 6.7% of patients who received metformin and none of the orlistat group had any symptoms.
DISCUSSION
Weight loss
In the present study, statistically significant weight loss in terms of weight, BMI, waist circumference and waist-hip ratio was achieved with the degree of decline being same in both metformin and orlistat arms (P - 0.418) compared to the control arm. It is similar to results in previous studies by Metwally et al.[5] and Ghandi et al.[6] as shown in Table 7.
Table 7.
Comparison of the outcome of the current study with that of Metwally et al. and Ghandi et al. studies
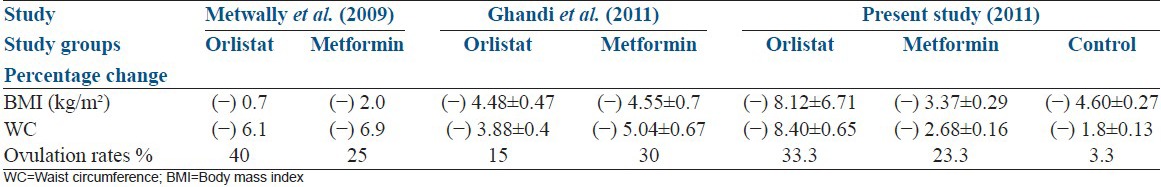
The pilot study in 2005 by Jayagopal et al.[7] demonstrated that those on orlistat showed more significant reduction in weight compared to metformin with P - 0.002 for orlistat versus 0.006 for metformin.
Ovulation rates
The result of the current study suggests that both orlistat and metformin can induce ovulation in overweight and obese PCOS women at similar rates (33.3% vs. 23.3%, P - 0.418). These results are consistent with previous studies by Metwally et al.[5] and Ghandi et al.[6]
The best predictor of ovulation according to the present and the previous study is percentage weight loss and a low concentration of baseline LH.
Androgen profile
Our study demonstrated a statistically significant fall in total serum testosterone levels and improvements in the FAI in both drug arms.
Similar to the results of present study Metwally et al.[5] and Jayagopal et al.[7] found a significant decrease in the values of total testosterone and FAI in both the orlistat and metformin group.
Lipid profile
In the current study treatment with orlistat produced significant change in the lipid parameters including significant fall in total cholesterol, LDL cholesterol and triglycerides (P < 0.05), whereas subjects in metformin and control arm showed no change in lipid profile.
Insulin resistance
In the current study, none of the groups showed improvement in any of the biochemical parameters for assessing insulin resistance. Nevertheless, there was fall in levels of fasting blood sugar, fasting insulin and HOMA-IR in the metformin group but it did not reach the level of significance.
When plasminogen activator-1 (PAI-1) levels were analyzed in a study, orlistat did not show any reduction in PAI-1, compared with metformin. The reduction in circulating androgens during Metformin treatment might be implicated in this decline.[11]
A study on 101 PCOS woman showed orlistat combined with lifestyle changes induces substantial weight loss in PCOS.[12] However, emphasis on lifestyle changes when combined with anti-obesity agents, exert beneficial effects on the endocrine abnormalities of obese patients with PCOS and improve metabolic parameters.[13] A study with, 24 weeks of orlistat with diet and physical exercise resulted in significant weight loss, improvement of hyperandrogenism and insulin sensitivity and increased anti-Müllerian hormone levels.[14] The importance of lifestyle modification should be stressed before initiation of pharmacological intervention.[15]
CONCLUSION
However, all these studies including our study have given substantial evidence that both orlistat and metformin are more efficacious in reducing weight, and ovulation in obese PCOS patients compared to control thereby demonstrating the therapeutic potential of orlistat in PCOS. In addition, subjects in orlistat group showed significant improvement in lipid profile. However, orlistat has minimal side-effects and is better tolerated compared to metformin. Though there was no statistically significant difference in term of reduction of BMI, waist circumference and waist-hip ratio between orlistat and metformin groups, ovulation rates in both groups were comparable. The significant improvements observed in lipid profile including LDL, Triglycerides and total cholesterol at the end of 3 months, is an observation for reducing cardiovascular problems on long-term intake of orlistat.
ACKNOWLEDGMENTS
The authors would like to acknowledge Dr. Lavanya Rai who participated in literature search. Mrs. Preetha from Department of Physiotherapy, for training subjects physiotherapy exercises.
Dr. Asha Kamath, Assistant Professor, Department of Community Medicine, Kasturba Medical College, Manipal, Karnataka, India who helped in statistics.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Consensus on women's health aspects of polycystic ovary syndrome (PCOS) Hum Reprod. 2012;27:14–24. [Google Scholar]
- 2.Yildirim B, Sabir N, Kaleli B. Relation of intra-abdominal fat distribution to metabolic disorders in nonobese patients with polycystic ovary syndrome. Fertil Steril. 2003;79:1358–64. doi: 10.1016/s0015-0282(03)00265-6. [DOI] [PubMed] [Google Scholar]
- 3.Kiddy DS, Sharp PS, White DM, Scanlon MF, Mason HD, Bray CS, et al. Differences in clinical and endocrine features between obese and non-obese subjects with polycystic ovary syndrome: An analysis of 263 consecutive cases. Clin Endocrinol (Oxf) 1990;32:213–20. doi: 10.1111/j.1365-2265.1990.tb00857.x. [DOI] [PubMed] [Google Scholar]
- 4.Moran LJ, Pasquali R, Teede HJ, Hoeger KM, Norman RJ. Treatment of obesity in polycystic ovary syndrome: A position statement of the Androgen Excess and Polycystic Ovary Syndrome Society. Fertil Steril. 2009;92:1966–82. doi: 10.1016/j.fertnstert.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Metwally M, Amer S, Li TC, Ledger WL. An RCT of metformin versus orlistat for the management of obese anovulatory women. Hum Reprod. 2009;24:966–75. doi: 10.1093/humrep/den454. [DOI] [PubMed] [Google Scholar]
- 6.Ghandi S, Aflatoonian A, Tabibnejad N, Moghaddam MH. The effects of metformin or orlistat on obese women with polycystic ovary syndrome: A prospective randomized open-label study. J Assist Reprod Genet. 2011;28:591–6. doi: 10.1007/s10815-011-9564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jayagopal V, Kilpatrick ES, Holding S, Jennings PE, Atkin SL. Orlistat is as beneficial as metformin in the treatment of polycystic ovarian syndrome. J Clin Endocrinol Metab. 2005;90:729–33. doi: 10.1210/jc.2004-0176. [DOI] [PubMed] [Google Scholar]
- 8.Misra A. Revisions of cutoffs of body mass index to define overweight and obesity are needed for the Asian-ethnic groups. Int J Obes Relat Metab Disord. 2003;27:1294–6. doi: 10.1038/sj.ijo.0802412. [DOI] [PubMed] [Google Scholar]
- 9.Choo V. WHO reassesses appropriate body-mass index for Asian populations. Lancet. 2002;360:235. doi: 10.1016/S0140-6736(02)09512-0. [DOI] [PubMed] [Google Scholar]
- 10.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 11.Koiou E, Tziomalos K, Katsikis I, Delkos D, Tsourdi EA, Panidis D. Disparate effects of pharmacotherapy on plasma plasminogen activator inhibitor-1 levels in women with the polycystic ovary syndrome. Hormones (Athens) 2013;12:559–66. doi: 10.14310/horm.2002.1444. [DOI] [PubMed] [Google Scholar]
- 12.Panidis D, Tziomalos K, Papadakis E, Chatzis P, Kandaraki EA, Tsourdi EA, et al. The role of orlistat combined with lifestyle changes in the management of overweight and obese patients with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2014;80:432–8. doi: 10.1111/cen.12305. [DOI] [PubMed] [Google Scholar]
- 13.Panidis D, Tziomalos K, Papadakis E, Vosnakis C, Chatzis P, Katsikis I. Lifestyle intervention and anti-obesity therapies in the polycystic ovary syndrome: Impact on metabolism and fertility. Endocrine. 2013;44:583–90. doi: 10.1007/s12020-013-9971-5. [DOI] [PubMed] [Google Scholar]
- 14.Vosnakis C, Georgopoulos NA, Rousso D, Mavromatidis G, Katsikis I, Roupas ND, et al. Diet, physical exercise and Orlistat administration increase serum anti-Müllerian hormone (AMH) levels in women with polycystic ovary syndrome (PCOS) Gynecol Endocrinol. 2013;29:242–5. doi: 10.3109/09513590.2012.736557. [DOI] [PubMed] [Google Scholar]
- 15.Panidis D, Tziomalos K, Papadakis E, Katsikis I. Infertility treatment in polycystic ovary syndrome: Lifestyle interventions, medications and surgery. Front Horm Res. 2013;40:128–41. doi: 10.1159/000341824. [DOI] [PubMed] [Google Scholar]


