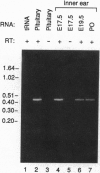
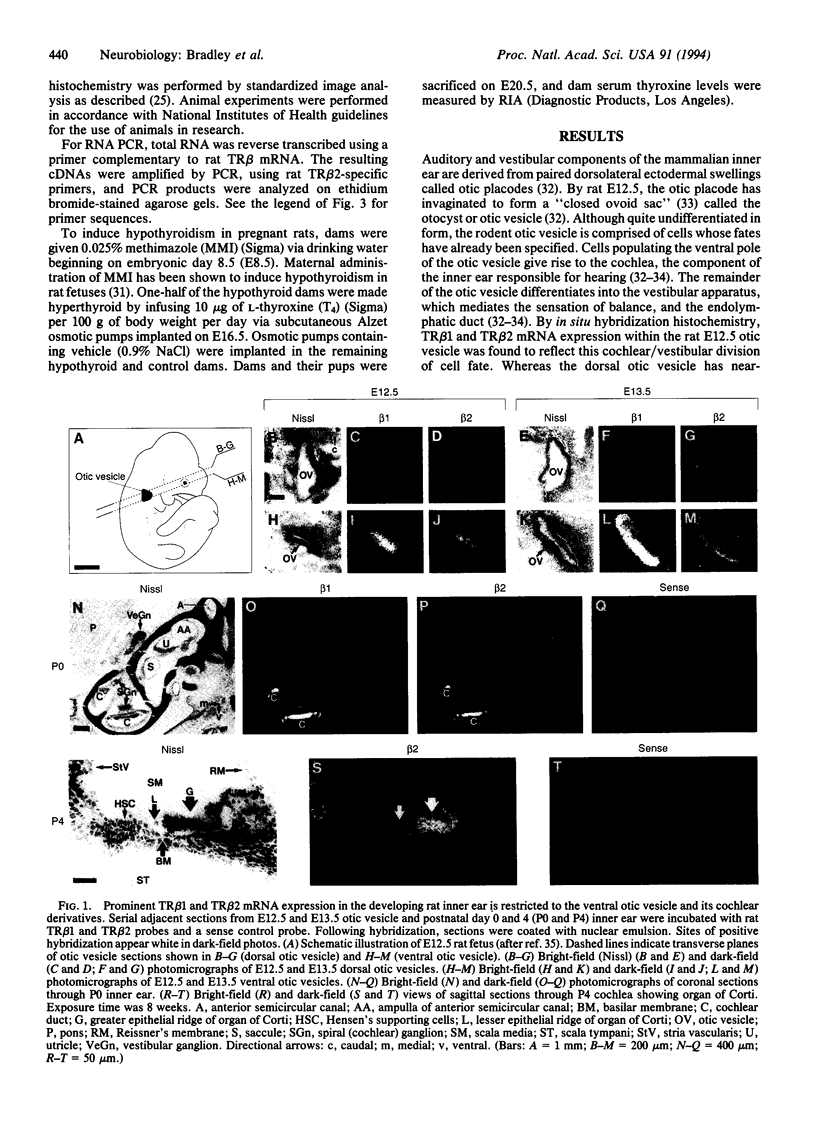
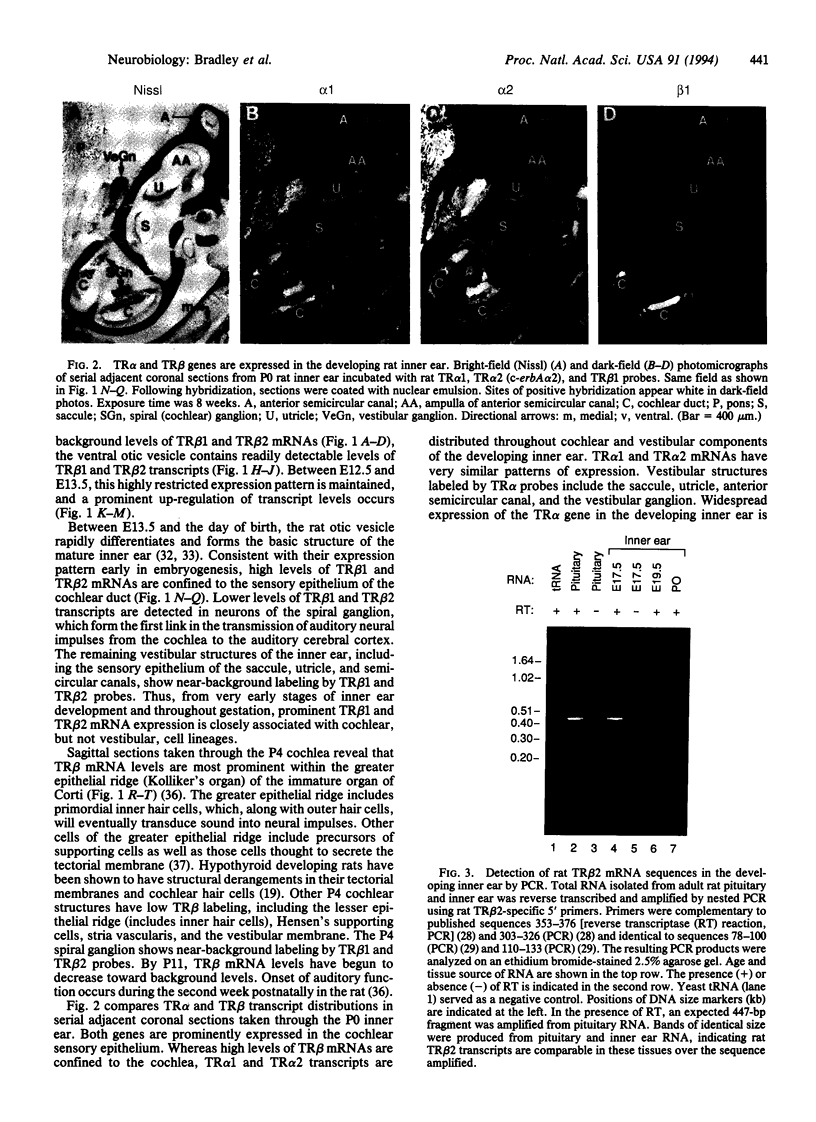
Abstract
Clinicians have long recognized that congenital deficiency of iodine (a component of thyroid hormone) somehow damages the human embryonic nervous system, causing sensori-neural deafness. Recently, a deletion encompassing most of the human beta thyroid hormone receptor (TR beta) gene has been found in children who are neurologically normal except for one striking defect: profound sensori-neural deafness. We now show that the TR beta gene is prominently expressed very early in rat inner ear development. This expression is remarkable because both TR beta 1 and TR beta 2 mRNAs are restricted, as early as embryonic day 12.5, to that portion of the embryonic inner ear that gives rise to the cochlea, the structure responsible for converting sound into neural impulses. The timing of this expression, when correlated with human inner ear development, raises the possibility that TRs may act in human ontogenesis earlier than previously suspected. These results provide a rare correlation between a specific human neurologic deficit (deafness) and transcription factor expression in a highly discrete embryonic cell population (ventral otocyst). TR alpha gene expression is also prominent in the developing cochlea, but, in contrast to the restricted pattern of TR beta gene expression, TR alpha 1 and TR alpha 2 transcripts are also found in inner ear structures responsible for balance. Deafness in children homozygous for a large deletion in the TR beta gene suggests that cochlear alpha 1 TRs cannot functionally compensate for the absence of TR beta 1 and TR beta 2. The developing inner ear may, therefore, represent an example of TR isoform-specific transcriptional regulation in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adan R. A., Cox J. J., van Kats J. P., Burbach J. P. Thyroid hormone regulates the oxytocin gene. J Biol Chem. 1992 Feb 25;267(6):3771–3777. [PubMed] [Google Scholar]
- Arriza J. L., Simerly R. B., Swanson L. W., Evans R. M. The neuronal mineralocorticoid receptor as a mediator of glucocorticoid response. Neuron. 1988 Nov;1(9):887–900. doi: 10.1016/0896-6273(88)90136-5. [DOI] [PubMed] [Google Scholar]
- Bradley D. J., Towle H. C., Young W. S., 3rd Spatial and temporal expression of alpha- and beta-thyroid hormone receptor mRNAs, including the beta 2-subtype, in the developing mammalian nervous system. J Neurosci. 1992 Jun;12(6):2288–2302. doi: 10.1523/JNEUROSCI.12-06-02288.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. J., Young W. S., 3rd, Weinberger C. Differential expression of alpha and beta thyroid hormone receptor genes in rat brain and pituitary. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7250–7254. doi: 10.1073/pnas.86.18.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent G. A., Moore D. D., Larsen P. R. Thyroid hormone regulation of gene expression. Annu Rev Physiol. 1991;53:17–35. doi: 10.1146/annurev.ph.53.030191.000313. [DOI] [PubMed] [Google Scholar]
- DeLong G. R., Stanbury J. B., Fierro-Benitez R. Neurological signs in congenital iodine-deficiency disorder (endemic cretinism). Dev Med Child Neurol. 1985 Jun;27(3):317–324. doi: 10.1111/j.1469-8749.1985.tb04542.x. [DOI] [PubMed] [Google Scholar]
- Deol M. S. An experimental approach to the understanding and treatment of hereditary syndromes with congenital deafness and hypothyroidism. J Med Genet. 1973 Sep;10(3):235–242. doi: 10.1136/jmg.10.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussault J. H., Ruel J. Thyroid hormones and brain development. Annu Rev Physiol. 1987;49:321–334. doi: 10.1146/annurev.ph.49.030187.001541. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsetti A., Mitsuhashi T., Desvergne B., Robbins J., Nikodem V. M. Molecular basis of thyroid hormone regulation of myelin basic protein gene expression in rodent brain. J Biol Chem. 1991 Dec 5;266(34):23226–23232. [PubMed] [Google Scholar]
- Forrest D., Hallbök F., Persson H., Vennström B. Distinct functions for thyroid hormone receptors alpha and beta in brain development indicated by differential expression of receptor genes. EMBO J. 1991 Feb;10(2):269–275. doi: 10.1002/j.1460-2075.1991.tb07947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffner M. E., Su F., Ross N. S., Hershman J. M., Van Dop C., Menke J. B., Hao E., Stanzak R. K., Eaton T., Samuels H. H. An arginine to histidine mutation in codon 311 of the C-erbA beta gene results in a mutant thyroid hormone receptor that does not mediate a dominant negative phenotype. J Clin Invest. 1993 Feb;91(2):538–546. doi: 10.1172/JCI116233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Rosenfeld M. G. Mechanisms of complex transcriptional regulation: implications for brain development. Neuron. 1991 Aug;7(2):183–196. doi: 10.1016/0896-6273(91)90257-z. [DOI] [PubMed] [Google Scholar]
- Hodin R. A., Lazar M. A., Chin W. W. Differential and tissue-specific regulation of the multiple rat c-erbA messenger RNA species by thyroid hormone. J Clin Invest. 1990 Jan;85(1):101–105. doi: 10.1172/JCI114398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodin R. A., Lazar M. A., Wintman B. I., Darling D. S., Koenig R. J., Larsen P. R., Moore D. D., Chin W. W. Identification of a thyroid hormone receptor that is pituitary-specific. Science. 1989 Apr 7;244(4900):76–79. doi: 10.1126/science.2539642. [DOI] [PubMed] [Google Scholar]
- Lazar M. A., Hodin R. A., Darling D. S., Chin W. W. Identification of a rat c-erbA alpha-related protein which binds deoxyribonucleic acid but does not bind thyroid hormone. Mol Endocrinol. 1988 Oct;2(10):893–901. doi: 10.1210/mend-2-10-893. [DOI] [PubMed] [Google Scholar]
- Lazar M. A. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev. 1993 Apr;14(2):184–193. doi: 10.1210/edrv-14-2-184. [DOI] [PubMed] [Google Scholar]
- Lezoualc'h F., Hassan A. H., Giraud P., Loeffler J. P., Lee S. L., Demeneix B. A. Assignment of the beta-thyroid hormone receptor to 3,5,3'-triiodothyronine-dependent inhibition of transcription from the thyrotropin-releasing hormone promoter in chick hypothalamic neurons. Mol Endocrinol. 1992 Nov;6(11):1797–1804. doi: 10.1210/mend.6.11.1480171. [DOI] [PubMed] [Google Scholar]
- Li C. W., Van De Water T. R., Ruben R. J. The fate mapping of the eleventh and twelfth day mouse otocyst: an in vitro study of the sites of origin of the embryonic inner ear sensory structures. J Morphol. 1978 Sep;157(3):249–267. doi: 10.1002/jmor.1051570302. [DOI] [PubMed] [Google Scholar]
- Mandel G., McKinnon D. Molecular basis of neural-specific gene expression. Annu Rev Neurosci. 1993;16:323–345. doi: 10.1146/annurev.ne.16.030193.001543. [DOI] [PubMed] [Google Scholar]
- Mellström B., Naranjo J. R., Santos A., Gonzalez A. M., Bernal J. Independent expression of the alpha and beta c-erbA genes in developing rat brain. Mol Endocrinol. 1991 Sep;5(9):1339–1350. doi: 10.1210/mend-5-9-1339. [DOI] [PubMed] [Google Scholar]
- Mixson A. J., Renault J. C., Ransom S., Bodenner D. L., Weintraub B. D. Identification of a novel mutation in the gene encoding the beta-triiodothyronine receptor in a patient with apparent selective pituitary resistance to thyroid hormone. Clin Endocrinol (Oxf) 1993 Mar;38(3):227–234. doi: 10.1111/j.1365-2265.1993.tb00999.x. [DOI] [PubMed] [Google Scholar]
- Morreale de Escobar G., Obregon M. J., Escobar del Rey F. Fetal and maternal thyroid hormones. Horm Res. 1987;26(1-4):12–27. doi: 10.1159/000180681. [DOI] [PubMed] [Google Scholar]
- Morreale de Escobar G., Obregon M. J., Ruiz de Oña C., Escobar del Rey F. Transfer of thyroxine from the mother to the rat fetus near term: effects on brain 3,5,3'-triiodothyronine deficiency. Endocrinology. 1988 Apr;122(4):1521–1531. doi: 10.1210/endo-122-4-1521. [DOI] [PubMed] [Google Scholar]
- Murray M. B., Zilz N. D., McCreary N. L., MacDonald M. J., Towle H. C. Isolation and characterization of rat cDNA clones for two distinct thyroid hormone receptors. J Biol Chem. 1988 Sep 5;263(25):12770–12777. [PubMed] [Google Scholar]
- Parrilla R., Mixson A. J., McPherson J. A., McClaskey J. H., Weintraub B. D. Characterization of seven novel mutations of the c-erbA beta gene in unrelated kindreds with generalized thyroid hormone resistance. Evidence for two "hot spot" regions of the ligand binding domain. J Clin Invest. 1991 Dec;88(6):2123–2130. doi: 10.1172/JCI115542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refetoff S., DeGroot L. J., Benard B., DeWind L. T. Studies of a sibship with apparent hereditary resistance to the intracellular action of thyroid hormone. Metabolism. 1972 Aug;21(8):723–756. doi: 10.1016/0026-0495(72)90121-7. [DOI] [PubMed] [Google Scholar]
- Refetoff S., DeWind L. T., DeGroot L. J. Familial syndrome combining deaf-mutism, stuppled epiphyses, goiter and abnormally high PBI: possible target organ refractoriness to thyroid hormone. J Clin Endocrinol Metab. 1967 Feb;27(2):279–294. doi: 10.1210/jcem-27-2-279. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Peña A., Ibarrola N., Iñiguez M. A., Muñoz A., Bernal J. Neonatal hypothyroidism affects the timely expression of myelin-associated glycoprotein in the rat brain. J Clin Invest. 1993 Mar;91(3):812–818. doi: 10.1172/JCI116301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai A., Miyamoto T., DeGroot L. J. Cloning and characterization of the human thyroid hormone receptor beta 1 gene promoter. Biochem Biophys Res Commun. 1992 May 29;185(1):78–84. doi: 10.1016/s0006-291x(05)80957-x. [DOI] [PubMed] [Google Scholar]
- Sakurai A., Miyamoto T., Hughes I. A., DeGroot L. J. Characterization of a novel mutant human thyroid hormone receptor beta in a family with hereditary thyroid hormone resistance. Clin Endocrinol (Oxf) 1993 Jan;38(1):29–38. doi: 10.1111/j.1365-2265.1993.tb00969.x. [DOI] [PubMed] [Google Scholar]
- Sakurai A., Miyamoto T., Refetoff S., DeGroot L. J. Dominant negative transcriptional regulation by a mutant thyroid hormone receptor-beta in a family with generalized resistance to thyroid hormone. Mol Endocrinol. 1990 Dec;4(12):1988–1994. doi: 10.1210/mend-4-12-1988. [DOI] [PubMed] [Google Scholar]
- Sakurai A., Nakai A., DeGroot L. J. Structural analysis of human thyroid hormone receptor beta gene. Mol Cell Endocrinol. 1990 Jun 18;71(2):83–91. doi: 10.1016/0303-7207(90)90245-4. [DOI] [PubMed] [Google Scholar]
- Sakurai A., Takeda K., Ain K., Ceccarelli P., Nakai A., Seino S., Bell G. I., Refetoff S., DeGroot L. J. Generalized resistance to thyroid hormone associated with a mutation in the ligand-binding domain of the human thyroid hormone receptor beta. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8977–8981. doi: 10.1073/pnas.86.22.8977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Damm K., Goldberg Y., Ghysdael J., Leutz A., Beug H., Vennström B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986 Dec 18;324(6098):635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Sher A. E. The embryonic and postnatal development of the inner ear of the mouse. Acta Otolaryngol Suppl. 1971;285:1–77. [PubMed] [Google Scholar]
- Sjöberg M., Vennström B., Forrest D. Thyroid hormone receptors in chick retinal development: differential expression of mRNAs for alpha and N-terminal variant beta receptors. Development. 1992 Jan;114(1):39–47. doi: 10.1242/dev.114.1.39. [DOI] [PubMed] [Google Scholar]
- Strait K. A., Schwartz H. L., Perez-Castillo A., Oppenheimer J. H. Relationship of c-erbA mRNA content to tissue triiodothyronine nuclear binding capacity and function in developing and adult rats. J Biol Chem. 1990 Jun 25;265(18):10514–10521. [PubMed] [Google Scholar]
- Strait K. A., Zou L., Oppenheimer J. H. Beta 1 isoform-specific regulation of a triiodothyronine-induced gene during cerebellar development. Mol Endocrinol. 1992 Nov;6(11):1874–1880. doi: 10.1210/mend.6.11.1282672. [DOI] [PubMed] [Google Scholar]
- Struhl K. Mechanisms for diversity in gene expression patterns. Neuron. 1991 Aug;7(2):177–181. doi: 10.1016/0896-6273(91)90256-y. [DOI] [PubMed] [Google Scholar]
- Takeda K., Balzano S., Sakurai A., DeGroot L. J., Refetoff S. Screening of nineteen unrelated families with generalized resistance to thyroid hormone for known point mutations in the thyroid hormone receptor beta gene and the detection of a new mutation. J Clin Invest. 1991 Feb;87(2):496–502. doi: 10.1172/JCI115023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K., Sakurai A., DeGroot L. J., Refetoff S. Recessive inheritance of thyroid hormone resistance caused by complete deletion of the protein-coding region of the thyroid hormone receptor-beta gene. J Clin Endocrinol Metab. 1992 Jan;74(1):49–55. doi: 10.1210/jcem.74.1.1727829. [DOI] [PubMed] [Google Scholar]
- Thompson C. C., Weinberger C., Lebo R., Evans R. M. Identification of a novel thyroid hormone receptor expressed in the mammalian central nervous system. Science. 1987 Sep 25;237(4822):1610–1614. doi: 10.1126/science.3629259. [DOI] [PubMed] [Google Scholar]
- Usala S. J., Bercu B. B., Refetoff S. Diverse abnormalities of the c-erbA beta thyroid hormone receptor gene in generalized thyroid hormone resistance. Adv Exp Med Biol. 1991;299:251–258. doi: 10.1007/978-1-4684-5973-9_15. [DOI] [PubMed] [Google Scholar]
- Usala S. J., Menke J. B., Watson T. L., Bérard W. E., Bradley C., Bale A. E., Lash R. W., Weintraub B. D. A new point mutation in the 3,5,3'-triiodothyronine-binding domain of the c-erbA beta thyroid hormone receptor is tightly linked to generalized thyroid hormone resistance. J Clin Endocrinol Metab. 1991 Jan;72(1):32–38. doi: 10.1210/jcem-72-1-32. [DOI] [PubMed] [Google Scholar]
- Usala S. J., Menke J. B., Watson T. L., Wondisford F. E., Weintraub B. D., Bérard J., Bradley W. E., Ono S., Mueller O. T., Bercu B. B. A homozygous deletion in the c-erbA beta thyroid hormone receptor gene in a patient with generalized thyroid hormone resistance: isolation and characterization of the mutant receptor. Mol Endocrinol. 1991 Mar;5(3):327–335. doi: 10.1210/mend-5-3-327. [DOI] [PubMed] [Google Scholar]
- Usala S. J., Tennyson G. E., Bale A. E., Lash R. W., Gesundheit N., Wondisford F. E., Accili D., Hauser P., Weintraub B. D. A base mutation of the C-erbA beta thyroid hormone receptor in a kindred with generalized thyroid hormone resistance. Molecular heterogeneity in two other kindreds. J Clin Invest. 1990 Jan;85(1):93–100. doi: 10.1172/JCI114438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uziel A., Gabrion J., Ohresser M., Legrand C. Effects of hypothyroidism on the structural development of the organ of Corti in the rat. Acta Otolaryngol. 1981 Nov-Dec;92(5-6):469–480. doi: 10.3109/00016488109133286. [DOI] [PubMed] [Google Scholar]
- Van Middlesworth L., Norris C. H. Audiogenic seizures and cochlear damage in rats after perinatal antithyroid treatment. Endocrinology. 1980 Jun;106(6):1686–1690. doi: 10.1210/endo-106-6-1686. [DOI] [PubMed] [Google Scholar]
- Weinberger C., Bradley D. J. Gene regulation by receptors binding lipid-soluble substances. Annu Rev Physiol. 1990;52:823–840. doi: 10.1146/annurev.ph.52.030190.004135. [DOI] [PubMed] [Google Scholar]
- Weinberger C., Thompson C. C., Ong E. S., Lebo R., Gruol D. J., Evans R. M. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986 Dec 18;324(6098):641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- Yaoita Y., Brown D. D. A correlation of thyroid hormone receptor gene expression with amphibian metamorphosis. Genes Dev. 1990 Nov;4(11):1917–1924. doi: 10.1101/gad.4.11.1917. [DOI] [PubMed] [Google Scholar]
- Yen P. M., Sugawara A., Refetoff S., Chin W. W. New insights on the mechanism(s) of the dominant negative effect of mutant thyroid hormone receptor in generalized resistance to thyroid hormone. J Clin Invest. 1992 Nov;90(5):1825–1831. doi: 10.1172/JCI116058. [DOI] [PMC free article] [PubMed] [Google Scholar]