Abstract
OBJECTIVE:
To characterize double and multiple aneuploidies in spontaneous abortions (SAB).
MATERIALS AND METHODS:
Retrospective analysis of cytogenetics data obtained by culturing/harvesting products of the conception material at our center from 2006 to 2009 was performed. The abnormal cytogenetic results, maternal age, gestational age, and previous pregnancy history were recorded and compared.
RESULTS:
Double and multiple aneuploidies are rare, however, a high percentage of double (4.6%) and multiple (0.4%) chromosomal aneuploidies were observed in our study of 1502 cases of SAB. Of 1502 cases of SAB evaluated, 70 cases (4.6%) showed double aneuploidy, whereas 6 cases (0.4%) had multiple aneuploidies. The chromosomes most frequently involved in double aneuploidy in the decreasing order were 21, 16, ± X, 22, 18, 13, and 15. The most frequent chromosome combinations observed were: Loss of X/21 (8.5%), 21/22 (4.4%), 16/21 (4.4%), and 7/16 (4.4%). The chromosome combinations in multiple aneuploidy included trisomy of chromosomes X/5/8, 8/20/22, 16/20/22, 14/21/22, and loss of X with 21/21 and 7/21. These abnormalities were significantly observed in women between the age group 40-44 years (59.2%). A high success rate (94%) of obtaining metaphase cells was observed in this study mainly due to the use of direct and long-term cultures.
CONCLUSIONS:
We observed a high percentage of double (4.6%) and multiple (0.4%) aneuploidies, frequently involving the acrocentic chromosomes 13, 15, 21, and 22 and nonacrocentric chromosomes X, 16, and 18.
KEY WORDS: Double/multiple chromosomal aneuploidies, products of conception, Cytogenetics
INTRODUCTION
Chromosomal analysis on products of conception (POC) is extremely useful to determine the cause for pregnancy loss and aid in estimating recurrence risks for future pregnancies. In previous studies, chromosomal abnormalities were observed in approximately 50-65% of spontaneous abortions (SAB) miscarriages[1,2,3] Among the cytogenetically abnormal cases, ~65% showed single trisomies (gain of one chromosome) followed by triploidy (~10%), and 45, X (~7%). The common trisomies observed in SAB in the decreasing frequencies are trisomy of chromosomes 16, 22, 15, and 21.[3]
Double trisomy (DT)/aneuploidy (gain of two chromosomes or gain/loss of at least two chromosomes) are observed in 0.21-2.8% of the aborted fetuses.[4,5,6] Since the first description of a case of double aneuploidy with 48, XXY, +21 karyotype,[7] approximately 385 cases with double aneuploidy are reported in the literature.[3,4,5,6] Autosomal double trisomies are observed in SAB but are rarely reported in live born infants. Most double aneuploidies are associated with an increased maternal age, abnormal sonogram, and pregnancy loss at a very early gestational age.[5] However, sex chromosome aneuploidy and trisomies involving chromosomes 16, 18, and 21 can survive for longer gestation.[8] Multiple aneuploidies (gain or loss of three or more chromosomes) are rarely reported in the literature.
The mechanism underlying the origin of double aneuploidy is unclear. It is hypothesized that double aneuploidy results either from two nondisjunctional events in gametogenesis or a single nondisjunctional event in a trisomic zygote.[5] The published literature shows that there is no specific chromosome association in double aneuploidy formation; however, the most frequently involved chromosomes are the sex chromosomes and acrocentric chromosomes. The chromosomes from the groups A and B are rarely observed in double aneuploidy formation.[6]
Cytogenetic analysis of POC samples is provided as a standard diagnostic tool in our laboratory for the clinical management of patients experiencing pregnancy loss in our hospital. The present study reviews the cytogenetic results of 1502 successfully karyotyped POC samples and report the high incidence of double and multiple aneuploidies encountered at our institution during a period of 4 years.
MATERIALS AND METHODS
A retrospective review of the cytogenetic data was performed on 1599 cases of POCs received from January 2006 to December 2009 at our institution. This study has been approved by the Institutional Review Board of our institution.
Cytogenetic analysis
Products of conception specimens from SABs received were processed on the same day. Cytogenetic studies were performed mostly on chorionic villi. In the absence of villi, a part of the skin, sac, membrane, or muscle was used. Direct (24 h) and long-term (LT) cultures were set up in AmnioMAX media (Gibco, Invitrogen Corp., Grand Island, NY) as per the protocol used in the laboratory.
Direct culture
About 10-12 mg of chorionic villi from POCs was isolated and placed in 3 ml of AmnioMax medium and incubated overnight in a 37°C CO2 incubator, and the cells were harvested on the next day after Velban/Colcemid treatment (Gibco, Invitrogen Corp., Grand Island, NY).
Long term culture
Following Collagenase (0.3 mg/ml) treatment of the chorionic villi (~15 mg), two cultures were set up and incubated at 37°C with 5% CO2. When sufficient growth of cells was observed, Velban/Colcemid was added and incubated for 1-2 h. The cells were trypsinized using 0.1% trypsin solution, harvested, and Giemsa-trypsin-Giemsa banded as per standard protocols. Twenty metaphase cells from LT cultures were analyzed in cases with an abnormal/normal male karyotype. Metaphase cells from direct cultures were used when mosaicism, tissue culture artifact, a female chromosome complement was observed in the LT or LT cultures failed to yield metaphase cells for analysis. The final karyotypes were described according to ISCN, 2009.[9]
RESULTS
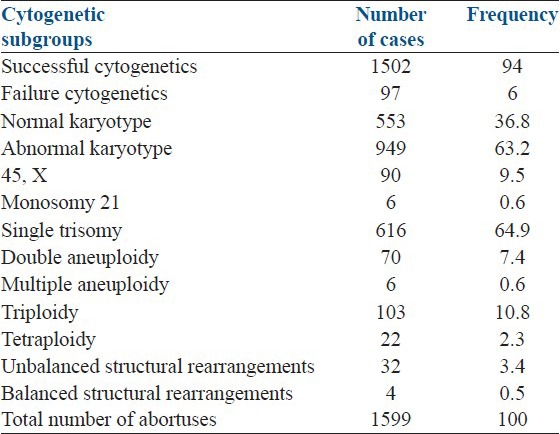
Of the 1599 POCs received for cytogenetic analysis, successful karyotypes were obtained in 1502 (94%) cases. Of these, 949 (63.2%) had an abnormal karyotype, and the rest 553 (36.8%) showed a normal karyotype. The female to male sex ratio among the normal karyotype cases was 1.72. The following abnormalities were observed among the cytogenetically abnormal cases (n = 949): Single trisomies including mosaic trisomy (64.9%), triploidy (10.8%), 45, X (9.5%), double aneuploidy (7.4%), tetraploidy (2.3%), multiple aneuploidy (0.6%), monosomy 21 (0.6%), and unbalanced (3.4%) and balanced structural rearrangements (0.5%) [Table 1].
Table 1.
The frequency of different chromosomal abnormalities observed in this study
Single chromosomal aneuploidy
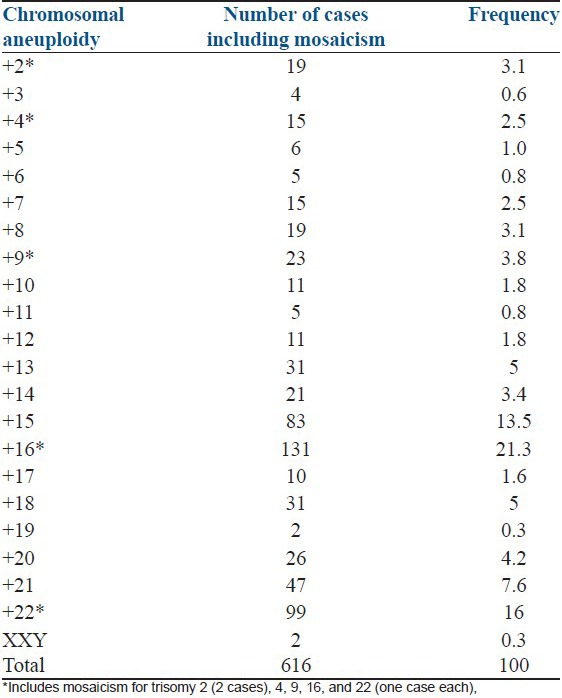
Single chromosome aneuploidy included 45, X (90 cases), XXY (2 cases), monosomy 21 (6 cases), and trisomies of chromosomes 16 (131 cases), 22 (99 cases), 15 (83 cases), 21 (47 cases), 13 (31 cases), 18 (31 cases), 20 (26 cases), 9 (23 cases), 14 (21 cases), 2 (19 cases), 8 (19 cases), 4 (15 cases), 7 (15 cases), 10 (11 cases), 12 (11 cases), 17 (10 cases), 5 (6 cases), 6 (5 cases), 11 (5 cases), 3 (4 cases), and 19 (2 cases) [Table 2]. The different types of mosaic aneuploidies observed were mosaic monosomy 21 (1 case), mosaic trisomy 2 (2 cases), mosaic trisomy of chromosomes 4, 9, 16, and 22 (one case each).
Table 2.
The frequency of single chromosome trisomies identified in SABs
Double and multiple aneuploidies
Of the total 1502 successfully karyotyped POCs, 70 cases (4.6%) with double aneuploidy and 6 cases (0.4%) with multiple aneuploidies were identified. The clinical and cytogenetic findings of double aneuploidy are given in Table 3 (Unique patient number (UPN) 1-70). Among the 70 cases with double aneuploidy, DT was observed in 59 (84%) cases, and the remaining 11 (16%) cases had pseudodiploidy. The pseudodiploid cases consisted of the loss of one sex chromosome with a gain of either chromosomes 21 (6 cases), 18 (2 cases), 16 (2 cases), and 22 (1 case). Most of the chromosomes were represented in double and multiple aneuploidies except chromosomes 1, 3, 4, 17, and 19. The most frequently involved chromosomes in the decreasing order were chromosomes 21 (24 cases), 16 (19 cases), ±X (17 cases), 22 (11 cases), 18 (10 cases), 13 (8 cases), and 15 (8 cases). The most frequent chromosome combinations involved in double aneuploidy were loss of X/21 (6 cases, 8.5%), 7/16 (3 cases, 4%), 21/22 (3 cases, 4%), and 16/21 (3 cases, 4%). Seven new chromosome combinations of double aneuploidy identified in this study were chromosomes 7/16 (3 cases), 12/18 (2 cases), and one each of chromosomes 2/10, 6/11, 10/11, 13/14, and 22/Y [Figure 1 modified from Reddy et al.].[5]
Table 3.
Clinical and cytogenetic findings in double aneuploidy (n=70 cases, UPN 1-70) and multiple aneuploidies (n=6 cases, UPN 71-76)
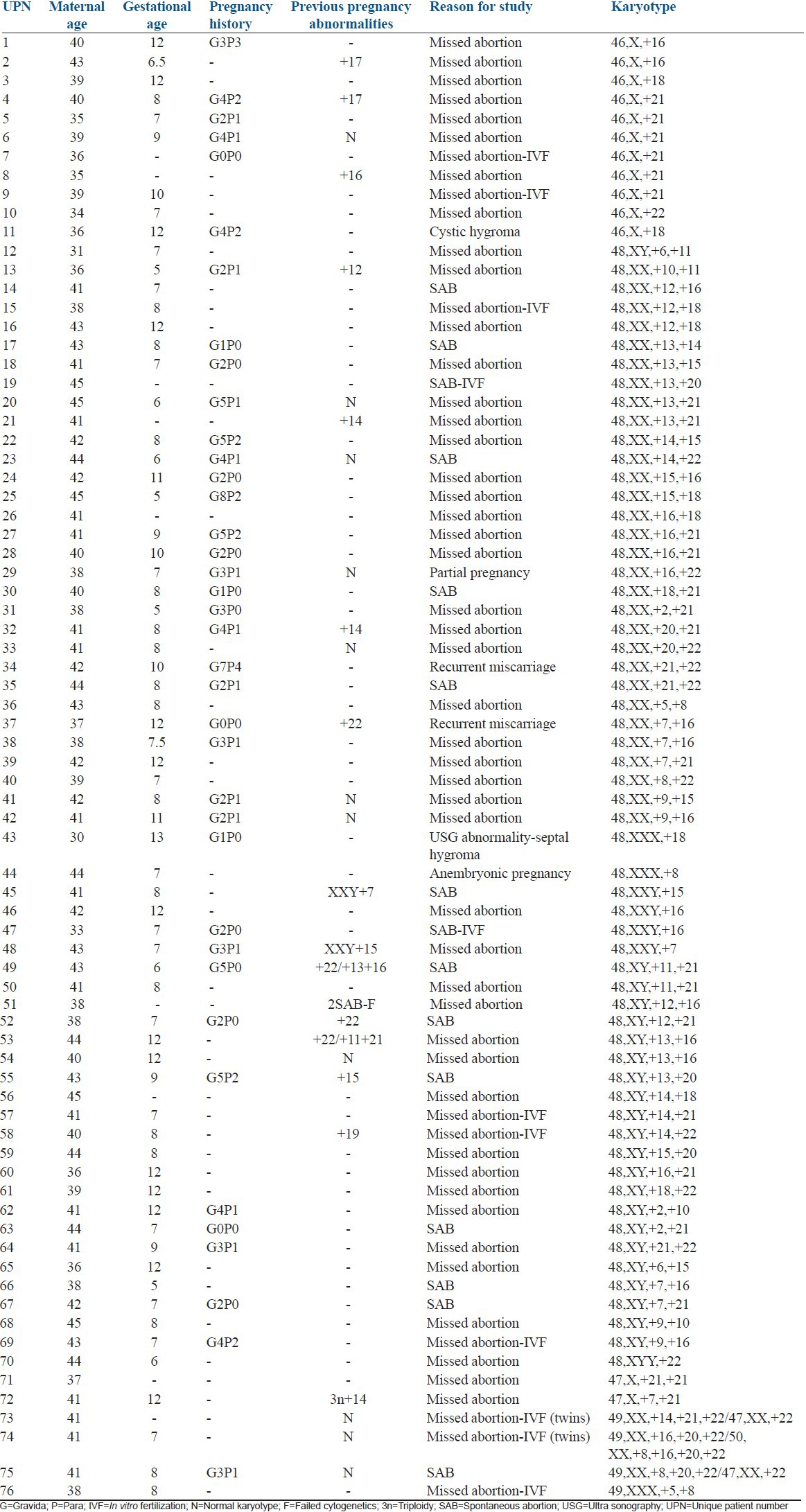
Figure 1.
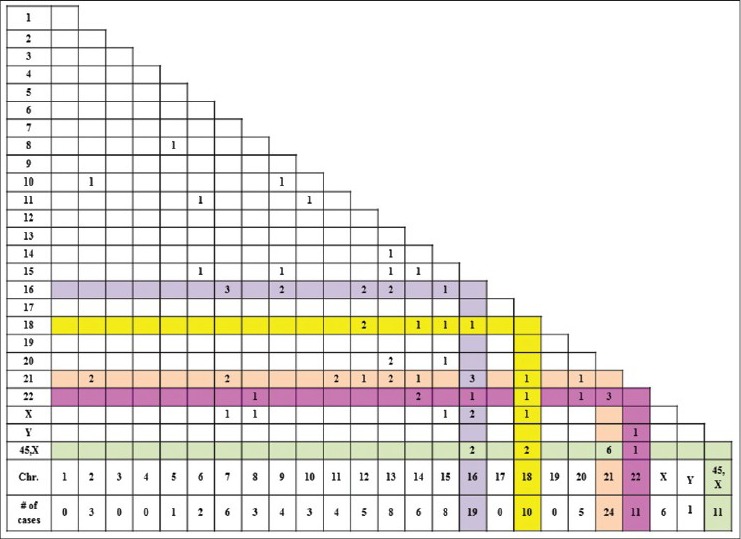
Chromosomal combinations observed in double aneuploidy. X and Y axis represent the chromosomes involved in double aneuploidy. Numbers in the grid correspond to the number of cases with the combinations[5]
In the present study, multiple aneuploidies were observed in 6 cases. The clinical and cytogenetic findings of multiple aneuploidies are given in Table 3 (UPN 71-76). Three of these six cases had in vitro fertilization treatments. Two cases (73 and 74) had twin pregnancies. Case 73 had one of the twins with triple trisomy, whereas the other twin had only trisomy 22. Case 74 had triple trisomy in one twin and trisomy of four chromosomes 8, 16, 20 and 22 in the other twin. The chromosome combinations in this group included trisomy of chromosomes X/5/8, 8/20/22, 16/20/22, 14/21/22, and loss of sex chromosome with 21/21 and 7/21.
Gestational age in double and multiple aneuploidies
The median gestational age for the 76 cases with double and multiple aneuploidies was 8 weeks (range = 5-13 weeks), and the median maternal age was 41 years (range = 31-44 years). Double and multiple aneuploidies were significantly observed in women >35 years when compared with women <35 years (94.7% vs. 5.3%, P = 0.03). Thirteen women had an abnormal karyotype in the previous pregnancy, of which two cases had double aneuploidy, with no recurrence of the same chromosomes [Table 3]. Comparison of double and multiple aneuploidies with single trisomies among the different maternal age groups, showed that double and multiple aneuploidies were frequently present in the maternal age group 40-44 years of age (59.2% vs. 32.6%), whereas single trisomies were common in the maternal age group 35-39 years (38.5% vs. 28.9%) [Figure 2].
Figure 2.
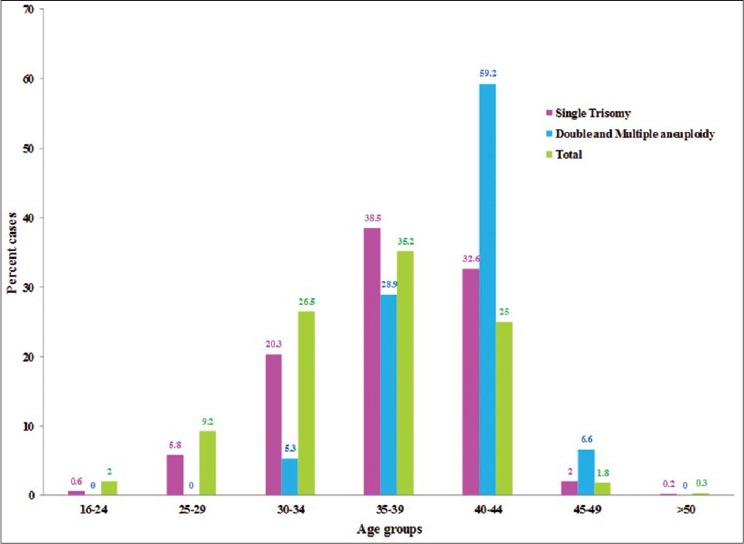
Frequency of single trisomy and double and multiple aneuploidies among the different maternal age groups
Direct and long-term cultures
Of the 269 cases studied by direct and LT cultures, chromosomal analysis on direct cultures helped us to determine the cytogenetic findings in 67 cases, of which 40 cases (15%) had an abnormal karyotype. These cases would have been missed if only LT cultures were evaluated.
DISCUSSION
Over the past few decades, cytogenetic analysis has been used as a valid tool in determining the cause for pregnancy loss. The frequency of different chromosomal abnormalities is difficult to determine as most of the SABs occur early in pregnancy and sometimes even before the recognition of a pregnancy. A very high culture failure rate (~20%) and maternal cell contamination (MCC) are often encountered in SABs.[10] However, the problem of MCC can be circumvented either by fluorescence in situ hybridization/molecular methods on direct chorionic villi, or by chromosomal studies of direct cultures as MCC is very minimal in direct cultures. In this study, by the use of both direct and LT cultures, we were able to obtain cytogenetic results in 94% of the cases studied. In the present study, analysis of direct cultures was helpful in getting cytogenetic results in 67 (25%) of 269 cases studied by both direct and LT cultures. Forty (15%) of these cases showed an abnormal karyotype that was missed by LT culture analysis. These results clearly demonstrate the importance of using direct cultures instead using more expensive molecular techniques.[11,12] Our high abnormal rate may in part be due to fresh specimens we receive and as well as the use of direct culture.
In the present study, a high percentage (63.2%) of SABs showed an abnormal karyotype. The most common abnormality observed in SABs is single chromosome trisomies (~65%),[1,2,3,13] trisomy 16 being the most frequent chromosomal abnormality followed by trisomy of chromosomes 21 and 22. As in previous studies, our study also identified trisomy 16 as the most common trisomy followed by trisomy 22, trisomy 15, and trisomy 21. The frequency of other chromosomal aneuploidies was similar to the data published in the literature. Trisomy 1 and 19 are very rarely reported in the literature. Interestingly, we observed two cases with trisomy 19 (1 case at 8th week and the other at 12th week gestational age), whereas trisomy 1 was not observed in this study. Studies from few case presentations suggest that embryos with trisomy 1 and 19 are spontaneously terminated at an early gestational age suggesting these trisomies as highly lethal.[14] Study of chromosomal analysis of oocytes by Pellestor et al.,[15] showed a lower rate of nondisjunction in the larger group chromosomes (groups A and C), which could be one of the reasons explaining the rarity of trisomies in larger group chromosomes. On the contrary, even though chromosome 19 is small when compared to the A, B, and C group chromosomes, it is an infrequent trisomy in SAB. We hypothesize that chromosome 19 may carry genes which are developmentally regulated and lethal if the gene dosage is affected.
In the present study, a high frequency of double (4.6%) and multiple (0.4%) aneuploidies was observed. DT was observed in 59 of 1502 (3.9%) cases, thus bringing up to a total of 444 cases of DT in the literature. The remaining 11 cases showed loss of sex chromosome and gain of an autosome. The relative increase in DT in this study when compared to previously published data (3.9% vs. 0.21-2.8%)[4,5,6,7,16] may be due to an increased maternal age in this group (>35 years = 95%). However, direct harvest employed in the chorionic villi harvesting method in our laboratory and prompt initiation of culture upon receipt of the specimen may also attribute to the detection of double and multiple aneuploidies in our study. Reddy,[5] Diego-Alvarez et al.,[4] and Micale et al.,[6] have extensively reviewed the cases with DT and reported that sex chromosomes and all autosomes except the chromosomes 1, 3, and 19 were involved in DT. In the present study, all chromosomes except chromosomes 1, 3, 4, 17, and 19 were involved in double and multiple aneuploidies. The absence of these chromosomes in double and multiple aneuploidies might suggest survival in capacitance of the fetus due to the genes that reside on these chromosomes, which may be involved with survival. Similar to previous studies, our data also showed that the most frequently involved chromosomes in DT in the order of decreasing frequency were: Chromosomes 21, 16, X, 22, 18, 13, and 15. The most frequent chromosome combinations observed were: X/21, 21/22, 16/21, and 7/21. These observations indicate that the pregnancies with double and multiple aneuploidies get terminated earlier when involves a nonviable trisomic chromosome and pregnancies continue longer when a viable trisomic chromosome is involved. Seven new chromosome combinations of double aneuploidy were identified in this study. The chromosome combinations included: 7/16, 12/18, 2/10, 6/11, 10/11, 13/14, and 22/Y. These combinations were not reported in the literature, and hence, its significance is not clearly established. Even though the chromosomes which are frequently involved in single trisomies are also commonly associated with double and multiple aneuploidies, a specific chromosome combination of double and multiple aneuploidies was not identified in this study suggesting that the chromosomes involved in double and multiple aneuploidies are random and their survival depends on the type of chromosome (viable vs. nonviable trisomy) involved. The exact cause of double and multiple aneuploidies is not clearly established, chromosomal nondisjunction at maternal meiosis I seems to be one of the causes. It is possible to have a zygote with double aneuploidy, when a sperm with an extra chromosome fuses with an egg with a loss or gain of a chromosome. However, Li et al.,[16] showed that the chromosomes involved in double aneuploidy are mostly maternal and not paternal in origin. They suggested the possibility of simultaneous abnormal separation of two or more chromosomes in oogonia which is most likely due to advanced maternal age.
Unlike double aneuploidy, there is a paucity of data on multiple aneuploidies in SABs; two cases with multiple trisomies were reported in the literature.[17,18] The first case was an SAB at 12 weeks with trisomy of chromosomes 5, 16, and 20,[17] and the second case was a 17-week-old fetus with trisomy of chromosomes X, 5, and 13.[18] Six cases of multiple aneuploidies were observed in this study. A significant difference in the clinical and cytogenetic findings when compared to the double aneuploidy was not observed in this group. The precise mechanism of multiple aneuploidy formation is not clearly elucidated in the literature. Multiple nondisjunctional events in a single gamete or both gametes (sperm and ovum) leading to trisomy of multiple chromosomes could be one of the mechanisms in multiple aneuploidy formation. However, further investigation is warranted in this area.
As described in the previous studies, a higher maternal age and a lower gestational age for double and multiple aneuploidies were observed in our study. The double and multiple aneuploidies were more frequent in the 40-44 years maternal age group. A higher maternal age was observed in double and multiple aneuploidies when compared to single trisomies, thus emphasizing the importance of maternal age in nondisjunctional events. In the present study, 13 women with double and multiple aneuploidies had chromosomal abnormalities in the previous pregnancies. Aberrant recombination near the centromeres of chromosomes as well as of a previous trisomic pregnancy has been associated with nondisjunction.[19,20] However, there are other biological factors proposed correlating maternal age and nondisjunction. Age-related defect in folliculogenesis during late antral stage[21] or the decline of the ovarian oocyte pool (oocyte atresia) in elderly women[22] may also be considered as risk factors for meiotic error. However, these mechanisms have not been studied in double or multiple aneuploidies. The high percentage of double and multiple aneuploidies observed in the present study may be attributed to the patient population with increased maternal age and high success rate of obtaining metaphase cells for cytogenetic analysis.
CONCLUSIONS
Of the 1502 cases with repeated abortuses, we identified a high percentage (63.2%) of cases with an abnormal karyotype. Direct cultures helped to determine a correct cytogenetic diagnosis in 67 cases, of which 15% of them had an abnormal karyotype. These cases would have been missed if only LT cultures were setup. Hence, we emphasize the use of direct cultures for chorionic villi which can effectively rule out MCC as well as increase the sensitivity of obtaining abnormal karyotypes. When compared to the previous studies, a high percentage of double (4.6%) and multiple (0.4%) aneuploidies were observed in this series. DT constituted 6.2% of abnormal cases and 3.9% of the total karyotyped cases. Seven new chromosome combinations were identified with double aneuploidies in this study. The high percentage of double and multiple aneuploidies observed in the present study may be attributed to the patient population, increased maternal age observed in this group, and immediate handling of the specimen upon arrival to the laboratory.
ACKNOWLEDGMENTS
We thank Dr. Lita M Alonso for reviewing the cases, Ms. Serpouhi Popescu, Ms. Lynda R Royer-Fuller, Mr. Joseph F Ragusa, Ms. Lana Ioffe, and Mr. David Brooks for their technical help. We also thank all the clinicians in the Obstetrics and Gynecology department for referring the samples to the Cytogenetics Laboratory.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Hassold T. Chromosome abnormalities in human reproductive wastage. Trends Genet. 1986;2:105–10. [Google Scholar]
- 2.Kalousek DK, Pantzar T, Tsai M, Paradice B. Early spontaneous abortion: Morphologic and karyotypic findings in 3,912 cases. Birth Defects Orig Artic Ser. 1993;29:53–61. [PubMed] [Google Scholar]
- 3.Menasha J, Levy B, Hirschhorn K, Kardon NB. Incidence and spectrum of chromosome abnormalities in spontaneous abortions: New insights from a 12-year study. Genet Med. 2005;7:251–63. doi: 10.1097/01.gim.0000160075.96707.04. [DOI] [PubMed] [Google Scholar]
- 4.Diego-Alvarez D, Ramos-Corrales C, Garcia-Hoyos M, Bustamante-Aragones A, Cantalapiedra D, Diaz-Recasens J, et al. Double trisomy in spontaneous miscarriages: Cytogenetic and molecular approach. Hum Reprod. 2006;21:958–66. doi: 10.1093/humrep/dei406. [DOI] [PubMed] [Google Scholar]
- 5.Reddy KS. Double trisomy in spontaneous abortions. Hum Genet. 1997;101:339–45. doi: 10.1007/s004390050638. [DOI] [PubMed] [Google Scholar]
- 6.Micale M, Insko J, Ebrahim SA, Adeyinka A, Runke C, Van Dyke DL. Double trisomy revisited – a multicenter experience. Prenat Diagn. 2010;30:173–6. doi: 10.1002/pd.2429. [DOI] [PubMed] [Google Scholar]
- 7.Ford CE, Jones KW, Miller OJ, Mittwoch U, Penrose LS, Ridler M, et al. The chromosomes in a patient showing both mongolism and the Klinefelter syndrome. Lancet. 1959;1:709–10. doi: 10.1016/s0140-6736(59)91891-4. [DOI] [PubMed] [Google Scholar]
- 8.Begam M, Bekdache GN, Murthy SK, Mirghani HM. Double aneuploidy of trisomy 18 and Klinefelter syndrome: Prenatal diagnosis and perinatal outcome. J Perinat Med. 2010;38:565–6. doi: 10.1515/jpm.2010.075. [DOI] [PubMed] [Google Scholar]
- 9.Shaffer LG, Slovak ML, Campbell LJ. Basel: S. Karger; 2009. ISCN: An International System for Human Cytogenetic Nomenclature. [Google Scholar]
- 10.Shearer BM, Thorland EC, Carlson AW, Jalal SM, Ketterling RP. Reflex fluorescent in situ hybridization testing for unsuccessful product of conception cultures: A retrospective analysis of 5555 samples attempted by conventional cytogenetics and fluorescent in situ hybridization. Genet Med. 2011;13:545–52. doi: 10.1097/GIM.0b013e31820c685b. [DOI] [PubMed] [Google Scholar]
- 11.Christopoulou S, Christopoulou G, Hatzaki A, Hatzipouliou A, Donoghue J, Karkaletsi M, et al. The replacement of cytogenetic analysis by direct chorionic villi sampling preparation with quantitative fluorescence PCR. Gynecol Obstet Invest. 2009;68:255–61. doi: 10.1159/000240671. [DOI] [PubMed] [Google Scholar]
- 12.Jobanputra V, Esteves C, Sobrino A, Brown S, Kline J, Warburton D. Using FISH to increase the yield and accuracy of karyotypes from spontaneous abortion specimens. Prenat Diagn. 2011;31:755–9. doi: 10.1002/pd.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morales C, Sánchez A, Bruguera J, Margarit E, Borrell A, Borobio V, et al. Cytogenetic study of spontaneous abortions using semi-direct analysis of chorionic villi samples detects the broadest spectrum of chromosome abnormalities. Am J Med Genet A. 2008;146A:66–70. doi: 10.1002/ajmg.a.32058. [DOI] [PubMed] [Google Scholar]
- 14.Vicic A, Roje D, Strinic T, Stipoljev F. Trisomy 1 in an early pregnancy failure. Am J Med Genet A. 2008;146A:2439–41. doi: 10.1002/ajmg.a.32481. [DOI] [PubMed] [Google Scholar]
- 15.Pellestor F, Anahory T, Hamamah S. The chromosomal analysis of human oocytes. An overview of established procedures. Hum Reprod Update. 2005;11:15–32. doi: 10.1093/humupd/dmh051. [DOI] [PubMed] [Google Scholar]
- 16.Li QY, Tsukishiro S, Nakagawa C, Tanemura M, Sugiura-Ogasawara M, Suzumori K, et al. Parental origin and cell stage of non-disjunction of double trisomy in spontaneous abortion. Congenit Anom (Kyoto) 2005;45:21–5. doi: 10.1111/j.1741-4520.2005.00056.x. [DOI] [PubMed] [Google Scholar]
- 17.Soukup SW. Triple trisomy in a spontaneous abortion. Hum Genet. 1992;88:363. doi: 10.1007/BF00197280. [DOI] [PubMed] [Google Scholar]
- 18.Pettenati MJ, Rao N. Triple trisomy in a 17-week-old fetus. Hum Genet. 1991;87:239–40. doi: 10.1007/BF00204194. [DOI] [PubMed] [Google Scholar]
- 19.Hassold T, Hunt P. To err (meiotically) is human: The genesis of human aneuploidy. Nat Rev Genet. 2001;2:280–91. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- 20.Warburton D, Dallaire L, Thangavelu M, Ross L, Levin B, Kline J. Trisomy recurrence: A reconsideration based on North American data. Am J Hum Genet. 2004;75:376–85. doi: 10.1086/423331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volarcik K, Sheean L, Goldfarb J, Woods L, Abdul-Karim FW, Hunt P. The meiotic competence of in-vitro matured human oocytes is influenced by donor age: Evidence that folliculogenesis is compromised in the reproductively aged ovary. Hum Reprod. 1998;13:154–60. doi: 10.1093/humrep/13.1.154. [DOI] [PubMed] [Google Scholar]
- 22.Kline J, Kinney A, Reuss ML, Kelly A, Levin B, Ferin M, et al. Trisomic pregnancy and the oocyte pool. Hum Reprod. 2004;19:1633–43. doi: 10.1093/humrep/deh310. [DOI] [PubMed] [Google Scholar]


