Abstract
Introduction:
Paroxysmal sympathetic hyperactivity (PSH) is a clinical disorder mainly caused by traumatic brain injury, stroke, encephalitis and other types of brain injury. The clinical features are episodes of hypertension, tachycardia, tachypnea, fever and dystonic postures. In this study, we described clinical profile and outcome of six patients of PSH admitted in neurocritical care unit.
Materials and Methods:
This was a prospective observational study conducted at neurology critical care unit of a tertiary care center. All patients admitted at neurology critical unit during 6-month period from August 2013 to January 2014 were screened for the occurrence of PSH. The clinical details and outcome was documented.
Results:
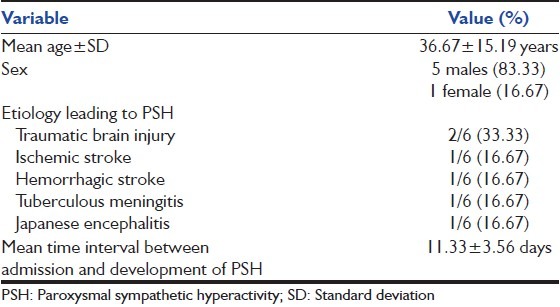
PSH was observed in 6 patients. Male to female ratio was 5:1. Mean age ± SD was 36.67 ± 15.19 years. The leading causes were traumatic brain injury (two patients), stroke (two patients) and Japanese encephalitis (JE) (one patient) and tuberculous meningitis (one patient).
Conclusion:
PSH is an unusual complication in neurocritical care. It prolonged the hospitalization and hampers recovery. The other life-threatening conditions that mimic PSH should be excluded. The association with JE and tuberculous meningitis was not previously described in literature.
Keywords: Autonomic seizures, encephalitis, paroxysmal sympathetic hyperactivity, traumatic brain injury
Introduction
Paroxysmal sympathetic hyperactivity (PSH) is a complication of severe brain injury which is characterized by episodes of hypertension, tachycardia, tachypnea, diaphoresis, fever and dystonic posturing.[1] The first case of PSH was reported by Penfield in 1929, he gave the term diencephalic autonomic epilepsy to describe this condition.[2] Since then various terms like paroxysmal sympathetic storms, diencephalic seizures, or midbrain dysregulatory syndrome and paroxysmal autonomic instability with dystonia (PAID) have been used to describe this condition.[3] PSH is an important clinical entity because it leads to poor outcome, and it represents a significant additional burden on health care system.[4] PSH can mimic other common and life-threatening conditions, and early diagnosis can lead to the rational approach to management.[3] Traumatic brain injury, cerebral hypoxia and cerebrovascular accidents are amongst the most common conditions leading to PSH.[4] Despite the important impact of this condition the literature on this syndrome is still debatable, with no consensus regarding nomenclature, diagnosis and evidence-based management of PSH.[4] Therefore, we conducted this study on patients admitted in neurology critical care unit with the aim to determine the frequency, etiology, clinical features, and outcome of PSH patients admitted in our referral center.
Materials and Methods
This was a prospective observational study conducted at neurology critical care unit of a tertiary care center. All patients admitted at neurology critical unit during 6 months period from August 2013 to January 2014 were screened for the occurrence of PSH.
Paroxysmal sympathetic hyperactivity was diagnosed on the basis of diagnostic criteria proposed by Blackman et al.[3] This proposed criteria defines PSH or PAID in the clinical setting of traumatic brain injury. The signs of PAID syndrome include: (1) Severe brain injury (Rancho Los Amigos level IV), (2) temperature of at least 38.5°C, (3) pulse of at least 130 beats/min, (4) respiratory rate of at least 140 breaths/min, (5) agitation, (6) diaphoresis, and (7) dystonia (i.e. rigidity or decerebrate posturing). The duration is at least 1 cycle/day for at least 3 days. Finally, other related conditions must be ruled out.[3]
Other clinical disorders mimicking PSH like neuroleptic malignant syndrome, malignant hyperthermia, pheochromocytoma, hyperthyroidism, sepsis and other infections were ruled out clinically and by specific investigations like creatine phosphokinase (CPK), urine for myoglobin, septic screen, thyroid profile etc.
All patients suspected of having PSH underwent detailed clinical history and examination, including continuous heart rate and electrocardiogram monitoring, noninvasive blood pressure (BP) monitoring and temperature monitoring frequently. These patients were also subjected to routine hematological and biochemical investigations along with septic screen (blood, urine, tracheal aspirate and sputum culture) chest X-ray, CPK, urine for myoglobin and thyroid profile.
Patients developing PSH were managed using adequate hydration, morphine sulfate, propranolol and clonidine.
Statistical analysis
Categorical variables will be expressed as percentages; continuous variables will be expressed as mean ± standard deviation.
Observation
During the study period of 6 months, a total of 57 patients were admitted to neurology critical care unit, PSH developed in only 6 of these patients, therefore incidence of PSH in these 6 months in our setup was 10.53%.
The baseline characteristics of these 6 patients are given in Table 1.
Table 1.
Baseline characteristics of PSH patients
Case 1
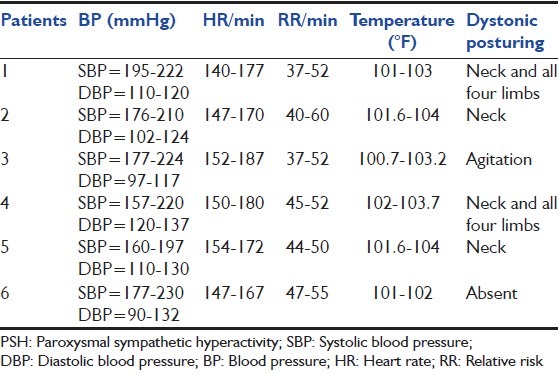
A 35-year-old male was admitted to our center after sustaining a head injury during a road traffic accident (RTA). At presentation, his vitals were stable, Glasgow Coma Scale (GCS) was E2M4V2. Computed tomography (CT) scan (Cranium) was suggestive of multiple hemorrhagic contusions. Patient was managed conservatively, he was doing well and recovering but on 8th day of admission he developed episodes of fever, tachycardia and hypertension along with dystonic posturing, each episode lasted for a few minutes and there were several such episodes per day, details of such episodes are summarized in Table 2. Patient was diagnosed to be suffering from PSH after ruling out other conditions mimicking PSH as described above. He was treated using propranolol 40 mg thrice a day, clonidine 0.1 mg thrice a day. The frequency of these episodes reduced markedly on this treatment after several days, and these episodes stopped after 1 week.
Table 2.
Details of PSH episodes
Case 2
A 62-year-old female who was admitted with complaints of sudden onset unconsciousness and weakness of the right side of the body. At presentation, her BP was 220/130, GCS was E3M5V1, right hemiparesis was present and right plantar was extensor. Noncontrast CT scan of the head was suggestive of left thalamic bleed. Patient was given injection labetalol 20 mg intravenous to control the BP; she was managed conservatively using cerebral decongestants and physiotherapy. Her condition showed improvement and on 9th day of admission she was fully conscious, although weakness of her right upper and lower limb persisted. She was about to discharge, when she developed episodes of fever, tachycardia, diaphoresis and hypertension along with dystonic posturing. These episodes were diagnosed as PSH after exclusion of other closely related conditions. A repeat CT scan of the brain was repeated, which was not suggestive of any other abnormality apart from resolving thalamic hematoma. Her episodes also responded well to propranolol and clonidine. Details of her PSH episodes are described in Table 2.
Case 3
A 17-year-old male who was admitted with fever, altered sensorium and seizures. He was diagnosed to be having Japanese encephalitis (JE) as his cerebrospinal fluid (CSF) was positive for JE antibodies and magnetic resonance imaging (MRI) brain was showing bilateral thalamic hyperintensities. He was managed conservatively and was showing gradual improvement when on 18th day of admission he started to develop episodes of PSH. He was treated using propranolol and clonidine, but his episodes did not stopped completely so gabapentin 600 mg/day was added, after few days of adding gabapentin his episodes stopped.
Case 4
A 42-year-old male was diagnosed as a case of acute ischemic stroke with infarct in right middle cerebral artery territory, he presented to us with left hemiparesis. He was being managed using aspirin 150 mg/day and atorvastatin 20 mg/day and other conservative measures. His hospital course was uneventful till day 10 when he started to develop episodes of PSH. He also responded well to propranolol and clonidine.
Case 5
A 37-year-old male, who sustained head injury following RTA; he was admitted with GCS of E1M4V1. He developed episodes of PSH on 12th day of admission. He responded poorly to propranolol and clonidine, but on adding gabapentin 600 mg/day his episodes subsided within a few days.
Case 6
A 27-year-old male presented to us with fever, headache and altered sensorium. He was diagnosed as a case of tuberculous meningitis on the basis of CSF findings, and MRI brain revealed meningeal enhancement, basal exudates and communicating hydrocephalous. He was being treated with antitubercular drugs and steroids; he developed PSH episodes on 11th day of admission, his episodes responded to morphine 15 mg thrice daily, clonidine 0.1 mg thrice daily and propranolol 80 mg twice daily.
Discussion
Penfield was the first to describe PSH in the year 1929, he hypothesized that these episodes are caused by epileptiform discharges in thalamic nuclei irritated by increased intracranial pressure; therefore he called the condition as “diencephalic autonomic seizures.”[5] Since then this condition have been given various terms by various authors; 31different terms were found in the literature to describe this condition.[4] PSH was the term introduced by Rabinstein in 2007,[6] since then this term is being considered most clinically accurate.[4] We have also used the term PSH through this article to describe this condition.
The pathophysiology of PSH is still unclear and a matter of debate. Most authors have invoked a disconnection theory; according to this theory brainstem excitatory centers are released from higher control leading to a hyper sympathetic state.[7]
The exact incidence of occurrence of PSH is also variable, various studies have reported estimated incidence of PSH following traumatic brain injury from 7.7% to 33%.[4,6,8,9] Dolce et al. found that 1/3 of their severe traumatic brain injury patients developed PSH.[10] We found the incidence of PSH in our set up as 10.53% that lies within the range of previously reported incidence.
Traumatic brain injury is the most commonly reported etiology of PSH.[4,6] Second most commonly reported etiology is hypoxia.[4] Third most etiology usually reported in PSH patients is stroke.[4] We also found that most common etiology leading to PSH was traumatic brain injury (33.33%), we found PSH in one case of hemorrhagic stroke and in one case of ischemic stroke. We also found occurrence of PSH in one case of tuberculous meningitis and in one case of JE; this was an interesting finding as PSH had been very rarely reported in association with tuberculous meningitis previously.[11] We could not find any previous report of the occurrence of PSH following JE.
As per the review of Perkes et al. in 2010 PSH was found in males more commonly than females.[4] We also found a higher percentage of males (83.33%) developing PSH.
Mean age of our patients developing PSH was 36.67 ± 15.19 years. Previous studies have also reported occurrence of PSH commonly in younger individuals.[4] Perkes et al. in their review reported mean age of PSH cases as 24.2 ± 11.8 years.[4]
Paroxysmal sympathetic hyperactivity is an important clinical problem as case-control studies have shown that the occurrence of PSH leads to poor outcome and prolongation of the length of hospital stay.[12] Although our study was not a case-control study, but we noted that time of hospitalization was prolonged in cases developing PSH. None of our patients developing PSH died.
Unfortunately, limited data are available regarding the management of PSH. The best available evidence supports the use of intravenous morphine, midazolam, and alpha agonists like clonidine, beta-blockers, bromocriptine and intrathecal baclofen.[13] Baguley et al. have also shown the efficacy of gabapentin in the management of PSH.[14] We also managed our patients using clonidine, propranolol and morphine; gabapentin was tried in one of our cases and showed good response.
The major limitation of our study was a short study period of 6 months, therefore, leading to a small number of PSH cases.
To conclude that PSH is an important clinical problem in critically ill neurological patients, it can lead to poor outcome and prolonged hospital stay. Therefore treating neurologist, neurosurgeon and critical care physicians should have an open eye to diagnose this condition, as early recognition can lead to improved outcome. Furthermore, large multicenter trials are required to form proper guidelines for diagnosing and managing this important condition.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Diamond AL, Callison RC, Shokri J, Cruz-Flores S, Kinsella LJ. Paroxysmal sympathetic storm. Neurocrit Care. 2005;2:288–91. doi: 10.1385/NCC:2:3:288. [DOI] [PubMed] [Google Scholar]
- 2.Penfield W. Diencephalic autonomic epilepsy. Arch Neurol Psychiatry. 1929;22:358–74. [Google Scholar]
- 3.Blackman JA, Patrick PD, Buck ML, Rust RS., Jr Paroxysmal autonomic instability with dystonia after brain injury. Arch Neurol. 2004;61:321–8. doi: 10.1001/archneur.61.3.321. [DOI] [PubMed] [Google Scholar]
- 4.Perkes I, Baguley IJ, Nott MT, Menon DK. A review of paroxysmal sympathetic hyperactivity after acquired brain injury. Ann Neurol. 2010;68:126–35. doi: 10.1002/ana.22066. [DOI] [PubMed] [Google Scholar]
- 5.Do D, Sheen VL, Bromfield E. Treatment of paroxysmal sympathetic storm with labetalol. J Neurol Neurosurg Psychiatry. 2000;69:832–3. doi: 10.1136/jnnp.69.6.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabinstein AA. Paroxysmal sympathetic hyperactivity in the neurological intensive care unit. Neurol Res. 2007;29:680–2. doi: 10.1179/016164107X240071. [DOI] [PubMed] [Google Scholar]
- 7.Baguley IJ, Heriseanu RE, Cameron ID, Nott MT, Slewa-Younan S. A critical review of the pathophysiology of dysautonomia following traumatic brain injury. Neurocrit Care. 2008;8:293–300. doi: 10.1007/s12028-007-9021-3. [DOI] [PubMed] [Google Scholar]
- 8.Baguley IJ, Slewa-Younan S, Heriseanu RE, Nott MT, Mudaliar Y, Nayyar V. The incidence of dysautonomia and its relationship with autonomic arousal following traumatic brain injury. Brain Inj. 2007;21:1175–81. doi: 10.1080/02699050701687375. [DOI] [PubMed] [Google Scholar]
- 9.Fernández-Ortega JF, Prieto-Palomino MA, Muñoz-López A, Lebron-Gallardo M, Cabrera-Ortiz H, Quesada-García G. Prognostic influence and computed tomography findings in dysautonomic crises after traumatic brain injury. J Trauma. 2006;61:1129–33. doi: 10.1097/01.ta.0000197634.83217.80. [DOI] [PubMed] [Google Scholar]
- 10.Dolce G, Quintieri M, Leto E, Milano M, Pileggi A, Lagani V, et al. Dysautonomia and clinical outcome in vegetative state. J Neurotrauma. 2008 doi: 10.1089/neu.2008.0536. [DOI] [PubMed] [Google Scholar]
- 11.Gil Antón J, López Bayón J, López Fernández Y, Pilar Orive J. Autonomic dysfunction syndrome secondary to tuberculous meningitis. An Pediatr (Barc) 2004;61:449–50. doi: 10.1016/s1695-4033(04)78427-1. [DOI] [PubMed] [Google Scholar]
- 12.Baguley IJ, Nicholls JL, Felmingham KL, Crooks J, Gurka JA, Wade LD. Dysautonomia after traumatic brain injury: A forgotten syndrome? J Neurol Neurosurg Psychiatry. 1999;67:39–43. doi: 10.1136/jnnp.67.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baguley IJ, Cameron ID, Green AM, Slewa-Younan S, Marosszeky JE, Gurka JA. Pharmacological management of dysautonomia following traumatic brain injury. Brain Inj. 2004;18:409–17. doi: 10.1080/02699050310001645775. [DOI] [PubMed] [Google Scholar]
- 14.Baguley IJ, Heriseanu RE, Gurka JA, Nordenbo A, Cameron ID. Gabapentin in the management of dysautonomia following severe traumatic brain injury: A case series. J Neurol Neurosurg Psychiatry. 2007;78:539–41. doi: 10.1136/jnnp.2006.096388. [DOI] [PMC free article] [PubMed] [Google Scholar]


