Abstract
The neurotrophin receptor (p75) activates the c-Jun N-terminal kinase (JNK) pathway. Activation of JNK and its substrate c-Jun can cause apoptosis. Here we evaluate the role of p75 in spinal motoneurons by comparing immunoreactivity for p75 and phosphorylated c-Jun (p-c-Jun), the production of JNK activation in axotomized motoneurons in postnatal day (PN)1, PN7, PN14 and adult rats. Intensive p-c-Jun was induced in axotomized motoneurons in PN1 and PN7. In PN14, p-c-Jun expression was sharply reduced after the same injury. The decreased expression of p-c-Jun at this age coincided with a developmental switch of re-expression of p75 in axotomized cells. In adult animals, no p-c-Jun but intensive p75 was detected in axotomized motoneurons. These results indicate differential expression or turnover of phosphorylation of c-Jun and p75 in immature versus mature spinal motoneurons in response to axonal injury. The non-co-occurrence of p75 and p-c-Jun in injured motoneurons indicated that p75 may not activate JNK pathway, suggesting that the p75 may not be involved in cell death in axotomized motoneurons.
Keywords: apoptosis, transcription factor, c-Jun N-terminal kinase, nerve growth factor receptor, motoneuron, spinal cord, axotomy, neonatal, adult, axonal regeneration
Research Highlights
-
(1)
Intensive phosphorylated c-Jun (p-c-Jun) was induced in axotomized motoneurons in postnatal day (PN)1 and PN7 but not in adults.
-
(2)
Intensive p75 was induced in axotomized motoneurons in adult but not in PN1 and PN7.
-
(3)
Both weak p-c-Jun and p75 were induced in PN14.
-
(4)
Intensive p-c-Jun was induced in axotomized motoneurons in postnatal day 1 and PN7, which coincided with massive motoneuron death in the animals.
-
(5)
Intensive p75 was induced in axotomized motoneurons in adults. This coincided with non-neuronal death in adults.
-
(6)
These results indicate differential expression or turnover of phosphorylation of c-Jun and p75 in immature versus mature spinal motoneurons in response to axonal injury.
-
(7)
The non-co-occurrence of p75 and p-c-Jun in injured motoneurons suggest that p75 may not activate JNK pathway.
INTRODUCTION
P75 is the first discovered neurotrophin receptor and can essentially bind to all neurotrophins with equal affinity in most cells[1]. The main function of p75, however, remains elusive. It has been shown to promote cell survival either in association with Trk receptors or by itself[1,2,3,4,5,6,7,8,9,10,11,12]. However, p75 has also shown to induce apoptotic cell death[13,14,15,16]. Distinctly different roles of p75 have also been suggested in injured motoneurons. On one hand, p75 has been demonstrated to promote cell death following axotomy[14]. Application of antisense p75NTR oligodeoxynucleotides to the proximal nerve stumps of neonatal rats significantly reduces the loss of axotomized motoneurons[17,18,19]. On the other hand, p75 is associated with motoneuron survival and axonal regeneration[20,21,22,23,24].
Previous studies have shown that in p75-induced cell death, c-Jun N-terminal kinase (JNK) activation is required[25]. Prevention of JNK activation with CEP-1347/KT7515 blocked p75-mediated death of hippocampal neurons, suggesting that the JNK pathway is essential for p75-mediated death of hippocampal neurons[10,23,26]. It is shown that in p75-mediated neuronal death pathway, p75 firstly activates JNK pathway and then neuronal death is carried out by JNK activation[27,28].
Here we re-evaluate the role of p75 in motoneurons following axotomy by investigating whether p75 induces the activation of JNK in spinal motoneurons following distal axotomy. Activation of JNK was studied by analyzing the induction of the phosphorylated c-Jun 63 and phosphorylated c-Jun 73, activating members of the JNK cascade[29] in axotomized spinal motoneurons in immature and mature animals. Our present results support the idea that one gene may function differently in different developmental stages[29].
RESULTS
Age-dependent motoneuron loss after distal axotomy
In postnatal day (PN1) (Figures 1A and B) and PN7 (Figures 1C and D) age groups, distal axotomy induced massive motoneuron death at 1 week post-lesion (Figures 1B, D and I) compared with their contralateral normal control (Figures 1A and C). In PN14 (Figures 1E and F) or adult rats (Figures 1G and H), distal axotomy did not lead to significant motoneuron death (Figures 1F, H and I) compared with their contralateral normal control (Figures 1E and G).
Figure 1.
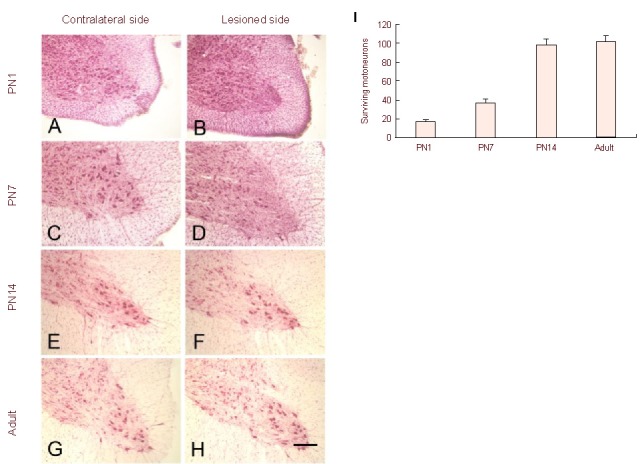
Photomicrographs of neutral red stained transverse sections of nonlesioned (A, C, E, G) and lesioned side (B, D, F, H) of the C7 spinal cord of postnatal day (PN)1 (A, B), PN7 (C, D), PN14 (E, F) and adult (G, H) rats at 1 week following distal axotomy.
Few motoneurons are found in the ventral horn of the lesion side spinal cord in PN1 (B) and PN7 (D) compared with their normal control (A, C, respectively). Many motoneurons are found in the lesioned side of PN14 (F) and adult (H) rats, which is comparable to their normal control (E, G, respectively). Scale bar: 200 μm.
Quantitative analysis of axotomized motoneuron survival showed that distal axotomy induced massive motoneuron loss in PN1 and PN7 rats. In contrast, distal axotomy did not induce significant motoneuron loss in PN14 and adult rats at 1 week post-lesion (I).
Data are expressed as a percentage (mean ± SEM) of the contralateral (normal) cell numbers.
c-Jun 63 and 73 but not p75 was induced following distal axotomy during early development of PN1 and PN7
In normal control rats, no p75 (Figure 2A), c-Jun 63 (Figure 2B) or c-Jun 73 (Figure 2C) immunostaining was present in ventral horn of spinal cord at every developmental age (PN 1 as a representative). Following distal axotomy, intensive staining for c-Jun 63 (Figures 2E and H, Table 1) or 73 (Figures 2F and I, Table 1) was seen in the lesion side of ventral horn of spinal cord in PN1 (Figures 2E and F, Table 1) and PN7 (Figures 2H and I, Table 1). No or few p75 positive motorneurons could be detected during the same time points following axotomy (Figures 2D and G, respectively, Table 1).
Figure 2.
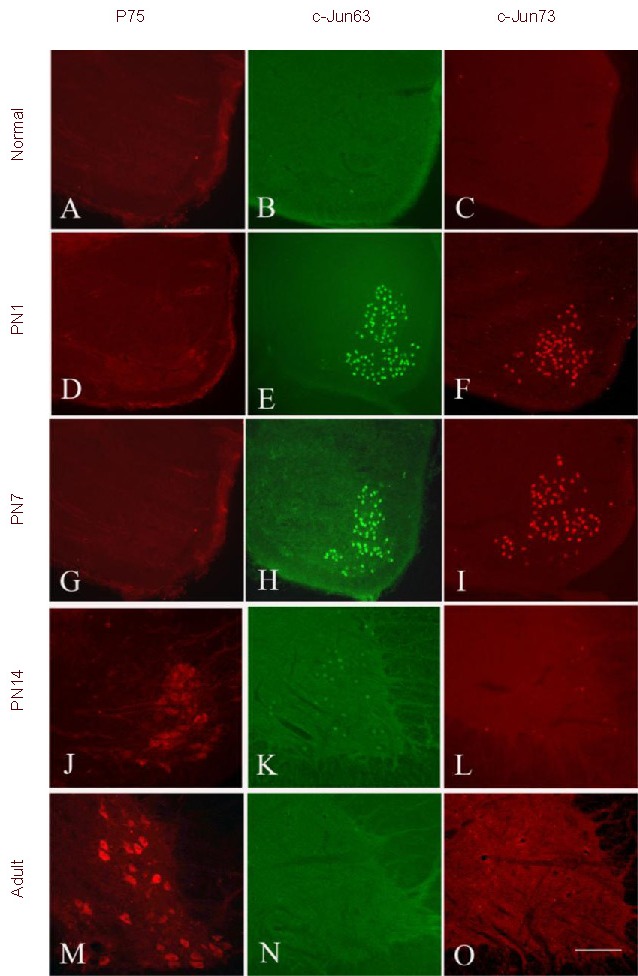
Photomicrographs of transverse sections of nonlesioned (A, B, C) and lesioned sides of the C7 spinal cord of postnatal (PN)1 (D, E, F), PN7 (G, H, I), PN14 (J, K, L) and adult (M, N, O) rats stained with p75 (A, D, G, J, M), c-Jun 63 (B, E, H, K, N) and c-Jun 73 (C, F, I, L, O) at 3 days following distal axotomy. Scale bar: 200 μm.
No positive signals for p75 (A), c-Jun 63 (B) and c-Jun 73 (C) could be found in normal spinal cord.
Intensive c-Jun 63 (E, H) and c-Jun 73 (F, I) was observed in PN1 (E, F) and PN7 (H, I) rats at 3 days following the injury.
Weak c-Jun 63 (K) and c-Jun 73 (L) was seen in PN14 rats.
No c-Jun 63 (N) and c-Jun 73 (O) was seen in adult rats.
In contrast, no p75 positive signals could be found in PN1 (D) and PN7 (G) rats. In contrast, intensive p75 positive signals were found in PN14 rats (J) and adult rats (M).
Table 1.
Percentages of phosphorylated c-Jun (p-c-Jun) or p75 in surviving motoneurons in postnatal (PN) and adult rats

P75 but not c-Jun 63 or 73 was induced following distal axotomy in PN14 and adult rats
Following distal axotomy, many weak p75 positive motoneurons were detected in the lesion side of ventral horn of spinal cord in PN14 (Figure 2J, Table 1) but intensive in adult rats (Figure 2M, Table 1). In PN14, a few weak c-Jun 63 (Figure 2K, Table 1) or 73 (Figure 2L, Table 1) could be detected in axotomized motoneurons, whereas in adult rats, no c-Jun 63 (Figure 2N, Table 1) or 73 (Figure 2O, Table 1) could be found in axotomized motoneurons.
DISCUSSION
P75 may not be involved in motoneuron death following axonal injury
P75 and c-Jun have many common properties. First, p75 and c-Jun are well known for their induction after injurious stimuli[1,10,30,31]. Second, both have been linked to either survival or death[18,32,33]. Third, their expression can be regulated by neurotrophic factors after axonal injury[34,35]. Fourth, both are intensively investigated in motoneuron system[15,17,36,37]. Their roles for motoneurons are controversial and have not been clarified[15,17,36,37]. The findings for p75-mediated cell death pathway in hippocampus bring these two molecules together[10,23,25,38]. For example, Friedman et al[25] find that blockade of JNK activation prevents p75-mediated cell death in hippocampal neurons, suggesting that JNK activation is a prerequisite for p75-mediated cell death. Thus, co-occurrence of p75 and JNK activation is necessary when p75-mediated cell death pathway is activated. This concept triggered us to reevaluate the role of p75 in motoneurons by comparing expression of p75 and p-c-Jun (ser63 and ser73), production of JNK activation. We found that both p-c-Jun and p75 induction in axotomized motoneurons are age-dependent. P-c-Jun expression in axotomized motoneurons declined with age. In contrast, p75 expression in axotomized motoneuron increased with age, which is consistent with our previous studies[10,25]. Further, we found that there was a developmental shift from p-c-Jun to p75 expression in axotomized motoneurons from childhood to adulthood. PN14 may be the shift age point characterized by a weak p-c-Jun and p75 expression. Intensive p-c-Jun expression never co-occurred with p75 expression in axotomized motoneurons, vice verse. These results suggest that p75 may not regulate the activity of the two signaling proteins of JNK pathway in axotomized motoneurons as observed in hippocampal neurons[40,41]; and p75-mediated cell death pathway may not occur in rat spinal motoneurons. Therefore, p75 expression may not be related with motoneuron degeneration following axonal injury.
Different mechanisms involved in induction of p75 and p-c-Jun
Axonal injury leads to response of neuronal cell bodies including anatomical changes and modification of gene expression. The cell body of a lesioned neuron must receive accurate and timely information on the site and extent of axonal damage, in order to exert an appropriate response. Specific mechanisms must therefore exist to transmit such information along the length of the axon from the lesion site to the cell body. Two main types of signals have been postulated to underlie this process including the loss of retrograde signals initiated by disconnection of the injured neuron from its target, thereby interrupting the normal supply of retrogradely transported trophic factors from the target and introduction of novel retrograde signals at the site of axonal injury or intrinsic signals within axons activated by nerve injury[35,39,42].
In the present study, we observed that massive motoneuron death occurred in PN1 and PN7 but not in PN14 and adult rats following distal axotomy. One of assumed reasons is that immature motoneurons are more dependent on the target-derived neurotrophic factors[34,37]. Immature but not mature injured motoneurons expressed p-c-Jun, suggesting that loss of retrograde signals of target-derived neurortrophic factors by axonal injury may be responsible to p-c-Jun induction. Indeed, it is demonstrated that application of exogenous neurotrophic factor can reverse p-c-Jun induction after axonal injury[43]. This is consistent with a previous study revealing that increases in c-Jun expression can be elicited by blockade of axonal transport in the absence of a lesion[44].
In contrast, p75 was induced in more mature axotomized motoneurons of PN14 and adult rats, but not in PN1 and PN7 rats, when they do not depend on target-derived neurotrophic factors for survival. Lack of p75 induction after distal axotomy in immature motoneurons indicates that loss of retrograde signals of target-derived neurotrophic factors is not a cause for p75 induction. Whereas, induction of novel retrograde signals at the site of axonal injury could be the candidate to regulate p75 expression in adult axotomized motoneurons. In support of this, a comparison study on p75 induction in adult hypoglossal motoneurons after axonal crush and axonal injury plus blockade of axonal transport, showed that p75 is not induced in crushed motoneurons with axonal transport blockade, while p75 is induced in crushed motoneurons with normal axonal transport[45]. These results demonstrate that induction of a novel retrograde signal at the site of axonal injury rather than a loss of retrograde signals from target by axonal injury is a prerequisite for p75 induction in injured motoneurons.
In conclusion, while the identification of genes induced in motoneurons after injury has been well described, those genes implicated in the survival or death of cells have never brought to an end. Motivated by the findings that p75 mediated hippocampal neuronal death requires activation of JNK pathway, the present study was undertaken to look for evidence of co-expression of p75 and p-c-Jun in the injured spinal motoneurons. We found that intensive p-c-Jun expression nerve co-occurred with p75 expression in axotomized motoneurons. This is the first demonstration that there was a developmental shift from p-c-Jun to p75 expression in axotomized motoneurons from childhood to adulthood. Our results suggest that p75 re-expression in motoneurons may not be related with motoneuron death, although p75 functions as a pro-apoptotic receptor for embryonic motoneuron apoptosis during embryogenesis[45]. Our present results support the idea that one gene may function differently in different developmental stages[29]. For example, overexpression of Akt is an essential component in the neurotrophin survival pathway. It can prevent motoneuron cell death in the neonate after axotomy[29]. However, Akt does not play an essential role in adult motoneuron survival since they found that adult motoneurons can survive very well following axotomy in the animals with dysfunction of Akt. Further studies on signaling mechanisms of p75 will be required to understand the precise role of p75 in injured motoneurons.
MATERIALS AND METHODS
Design
A prospective experimental study.
Time and setting
The experiment was performed at the University of Hong Kong (Hong Kong) from July 2010 to December 2011.
Materials
Female Sprague-Dawley postnatal day (PN) 1, 7, 14 and adult rats were used. Animals were anesthetized under deep hypothermia (for PN1 and PN7 rats) or with ketamine (80 mg/kg) and xylazine (8 mg/kg) (for PN14 and adult rats). All surgical interventions and subsequent care and treatment were approved by the Committee on the Use of Live Animals for Teaching and Research of the University of Hong Kong.
Methods
Lesion model and surgical procedures
Distal axotomy was performed following the procedure as described previously[30,31,46]. Briefly, anesthetized animals were placed on the surgical table; the brachial plexus was exposed from the outlet of the vertebrae down to the axilla, by a ventral middle line incision extending to the axilla. After the skin and subcutaneous tissues had been cut, the pectoralis major and sternohyoid muscles were separated from the midline. The muscles were retracted laterally so that the cervical vertebrae could be exposed. The seventh cervical (C7) spinal nerve was cut about 10 mm away from the spinal cord. A 3 mm segment of nerve distal to the lesion site was removed to avoid reinnervation. The wounds were sutured following the lesions and animals were allowed to survive for 3, 7 days with five rats in each postoperative time period.
Perfusion and tissue processing
At the end of the postoperative survival period, the rats were deeply anesthetized with a lethal dose of ketamine and xylazine and were perfused intracardially with normal saline, followed by 4% paraformaldehyde in 0.1 M PBS (pH 7.4). The C7 spinal segment was removed and immersion-fixed in the same fixative for 6 hours, and then placed into 30% sucrose in 0.1 M PBS overnight. Transverse serial sections were cut at 40 μm, collected in wells containing PBS.
Immunocytochemistry
After rinsing with PBS, sections were incubated overnight with a rabbit IgG polyclonal antibody against p75 (1:1 000; Promega, Madison, WI, USA) and a rabbit IgG polyclonal antibody against p-c-Jun (ser63 or 73) (1:1 000; cell signaling, Danvers, MA, USA) at room temperature. The primary antibody was diluted in PBS containing 0.2% Triton X-100, and 3% normal goat serum. The sections were incubated overnight at room temperature with the rabbit polycononal antibody against p75 and the rabbit polycononal antibodies against p-c-Jun. After rinsing with PBS, they were incubated for 2 hours at room temperature with their corresponding secondary antibodies conjugated with Alexa-488 or 568 (1:400, Molecular Probes, Eugene, OR, USA).
Then, the sections were mounted on gelatin-coated glass slides and coverslipped in mounting medium (Dako, Denmark). Fluorescent images were captured with Zeiss microscope (Zeiss, Gottingen, Germany) equipped with Spot digital camera (Diagnostic Instruments, Sterling Heights, MI, USA).
Quantification of motoneuron survival following distal axotomy
In order to evaluate the neuronal survival after distal axotomy, the serial sections were stained with neutral red. The total number of surviving motoneurons was counted on both the lesioned and the contralateral intact sides. Surviving motoneurons in the lesion side were expressed as a percentage of cells on the contralateral intact side.
Quantitative analysis of percentage of p-c-Jun or p75 in surviving motoneurons
Quantitative analysis of percentage of p-c-Jun and p75 in surviving motoneurons was performed as described previously. Briefly, sections immunostained with antibody against p-c-Jun or p75 were counterstained with neutral red, the ratio of number of p-c-Jun or p75 staining to number of neutral red staining would be the percentage of p-c-Jun or p75.
Statistical analysis
The motoneuron survival following distal axotomy was presented as mean ± SEM and analyzed with one-way analysis of variance followed by Turkey Kramer multiple comparison test using GraphPad Prism 4 software (CA, USA). Statistical significance was set at P < 0.05.
Footnotes
Funding: This study was supported by Direct Grant (Project No. 2030392) of the Chinese University of Hong Kong and HK Spinal Cord Injury Foundation and National Key Basic Research Support Foundation (973 Project: 2011CB504402).
Conflicts of interest: None declared.
(Edited by Gioltzoglou T, Cesca F/Zhao LJ/Song LP)
REFERENCES
- [1].Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol. 2002;67:203–233. doi: 10.1016/s0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- [2].Bui NT, Konig HG, Culmsee C, et al. p75 neurotrophin receptor is required for constitutive and NGF-induced survival signalling in PC12 cells and rat hippocampal neurones. J Neurochem. 2002;81:594–605. doi: 10.1046/j.1471-4159.2002.00841.x. [DOI] [PubMed] [Google Scholar]
- [3].Barker PA. p75NTR: A study in contrasts. Cell Death Differ. 1998;5:346–356. doi: 10.1038/sj.cdd.4400375. [DOI] [PubMed] [Google Scholar]
- [4].Fobian K, Owczarek S, Budtz C, et al. Peptides derived from the solvent-exposed loops 3 and 4 of BDNF bind TrkB and p75(NTR) receptors and stimulate neurite outgrowth and survival. J Neurosci Res. 2010;88:1170–1181. doi: 10.1002/jnr.22285. [DOI] [PubMed] [Google Scholar]
- [5].Bachis A, Avdoshina V, Zecca L, et al. Human immunodeficiency virus type 1 alters brain-derived neurotrophic factor processing in neurons. J Neurosci. 2012;32:9477–9484. doi: 10.1523/JNEUROSCI.0865-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bhakar AL, Howell JL, Paul CE, et al. Apoptosis induced by p75NTR overexpression requires Jun kinase- dependent phosphorylation of Bad. J Neurosci. 2003;23:11373–11381. doi: 10.1523/JNEUROSCI.23-36-11373.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Head BP, Patel HH, Niesman IR, et al. Inhibition of p75 neurotrophin receptor attenuates isoflurane-mediated neuronal apoptosis in the neonatal central nervous system. Anesthesiology. 2009;110:813–825. doi: 10.1097/ALN.0b013e31819b602b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Panni JK, Panni MK. Role of p75 neurotrophin receptor in isoflurane-mediated neuronal changes. Anesthesiology. 2009;111:1162–1163. doi: 10.1097/ALN.0b013e3181bbc177. [DOI] [PubMed] [Google Scholar]
- [9].Troy CM, Friedman JE, Friedman WJ. Mechanisms of p75-mediated death of hippocampal neurons Role of caspases. J Biol Chem. 2002;277:34295–34302. doi: 10.1074/jbc.M205167200. [DOI] [PubMed] [Google Scholar]
- [10].Volosin M, Trotter C, Cragnolini A, et al. Induction of proneurotrophins and activation of p75NTR-mediated apoptosis via neurotrophin receptor-interacting factor in hippocampal neurons after seizures. J Neurosci. 2008;28:9870–9879. doi: 10.1523/JNEUROSCI.2841-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ferraiuolo L, Kirby J, Grierson AJ, et al. Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;7:616–630. doi: 10.1038/nrneurol.2011.152. [DOI] [PubMed] [Google Scholar]
- [12].Ferraiuolo L, Higginbottom A, Heath PR, et al. Dysregulation of astrocyte-motoneuron cross-talk in mutant superoxide dismutase 1-related amyotrophic lateral sclerosis. Brain. 2011;134:2627–2641. doi: 10.1093/brain/awr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Barbeito LH, Pehar M, Cassina P, et al. A role for astrocytes in motor neuron loss in amyotrophic lateral sclerosis. Brain Res Brain Res Rev. 2004;47:263–274. doi: 10.1016/j.brainresrev.2004.05.003. [DOI] [PubMed] [Google Scholar]
- [14].Lowry KS, Murray SS, Coulson EJ, et al. Systemic administration of antisense p75(NTR) oligodeoxynucleotides rescues axotomised spinal motor neurons. J Neurosci Res. 2001;64:11–17. doi: 10.1002/jnr.1048. [DOI] [PubMed] [Google Scholar]
- [15].Perlson E, Jeong GB, Ross JL, et al. A switch in retrograde signaling from survival to stress in rapid-onset neurodegeneration. J Neurosci. 2009;29:9903–9917. doi: 10.1523/JNEUROSCI.0813-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sorensen B, Tandrup T, Koltzenburg M, et al. No further loss of dorsal root ganglion cells after axotomy in p75 neurotrophin receptor knockout mice. J Comp Neurol. 2003;459:242–250. doi: 10.1002/cne.10625. [DOI] [PubMed] [Google Scholar]
- [17].Li F, Li L, Song XY, et al. Preconditioning selective ventral root injury promotes plasticity of ascending sensory neurons in the injured spinal cord of adult rats--possible roles of brain-derived neurotrophic factor, TrkB and p75 neurotrophin receptor. Eur J Neurosci. 2009;30:1280–1296. doi: 10.1111/j.1460-9568.2009.06920.x. [DOI] [PubMed] [Google Scholar]
- [18].Wu W, Li L, Yick LW, et al. GDNF and BDNF alter the expression of neuronal NOS, c-Jun, and p75 and prevent motoneuron death following spinal root avulsion in adult rats. J Neurotrauma. 2003;20:603–612. doi: 10.1089/089771503767168528. [DOI] [PubMed] [Google Scholar]
- [19].Wu W, Chai H, Zhang J, et al. Delayed implantation of a peripheral nerve graft reduces motoneuron survival but does not affect regeneration following spinal root avulsion in adult rats. J Neurotrauma. 2004;21:1050–1058. doi: 10.1089/0897715041651006. [DOI] [PubMed] [Google Scholar]
- [20].Bamji SX, Majdan M, Pozniak CD, et al. The p75 neurotrophin receptor mediates neuronal apoptosis and is essential for naturally occurring sympathetic neuron death. J Cell Biol. 1998;140:911–923. doi: 10.1083/jcb.140.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Casaccia-Bonnefil P, Carter BD, Dobrowsky RT, et al. Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Natur. 1996;383:716–719. doi: 10.1038/383716a0. [DOI] [PubMed] [Google Scholar]
- [22].Hammarberg H, Piehl F, Cullheim S, et al. GDNF mRNA in Schwann cells and DRG satellite cells after chronic sciatic nerve injury. Neuroreport. 1996;7:857–860. doi: 10.1097/00001756-199603220-00004. [DOI] [PubMed] [Google Scholar]
- [23].Yano H, Torkin R, Martin LA, et al. Proneurotrophin-3 is a neuronal apoptotic ligand: evidence for retrograde-directed cell killing. J Neurosci. 2009;29:14790–14802. doi: 10.1523/JNEUROSCI.2059-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yoon SO, Casaccia-Bonnefil P, Carter B, et al. Competitive signaling between TrkA and p75 nerve growth factor receptors determines cell survival. J Neurosci. 1998;18:3273–3281. doi: 10.1523/JNEUROSCI.18-09-03273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Friedman WJ. Neurotrophins induce death of hippocampal neurons via the p75 receptor. J Neurosci. 2000;20:6340–6346. doi: 10.1523/JNEUROSCI.20-17-06340.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Linggi MS, Burke TL, Williams BB, et al. Neurotrophin receptor interacting factor (NRIF) is an essential mediator of apoptotic signaling by the p75 neurotrophin receptor. J Biol Chem. 2005;280:13801–13808. doi: 10.1074/jbc.M410435200. [DOI] [PubMed] [Google Scholar]
- [27].Karin M, Hunter T. Transcriptional control by protein phosphorylation: signal transmission from the cell surface to the nucleus. Curr Biol. 1995;5:747–757. doi: 10.1016/s0960-9822(95)00151-5. [DOI] [PubMed] [Google Scholar]
- [28].Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- [29].Namikawa K, Honma M, Abe K, et al. Akt/protein kinase B prevents injury-induced motoneuron death and accelerates axonal regeneration. J Neurosci. 2000;20:2875–2886. doi: 10.1523/JNEUROSCI.20-08-02875.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Herdegen T, Skene P, Bahr M. The c-Jun transcription factor--bipotential mediator of neuronal death, survival and regeneration. Trends Neurosci. 1997;20:227–231. doi: 10.1016/s0166-2236(96)01000-4. [DOI] [PubMed] [Google Scholar]
- [31].Herdegen T, Waetzig V. AP-1 proteins in the adult brain: facts and fiction about effectors of neuroprotection and neurodegeneration. Oncogene. 2001;20:2424–2437. doi: 10.1038/sj.onc.1204387. [DOI] [PubMed] [Google Scholar]
- [32].Lindwall C, Dahlin L, Lundborg G, et al. Inhibition of c-Jun phosphorylation reduces axonal outgrowth of adult rat nodose ganglia and dorsal root ganglia sensory neurons. Mol Cell Neurosci. 2004;27:267–279. doi: 10.1016/j.mcn.2004.07.001. [DOI] [PubMed] [Google Scholar]
- [33].Lindwall C, Kanje M. The role of p-c-Jun in survival and outgrowth of developing sensory neurons. Neuroreport. 2005;16:1655–1659. doi: 10.1097/01.wnr.0000183324.75499.fc. [DOI] [PubMed] [Google Scholar]
- [34].Sun W, Gould TW, Newbern J, et al. Phosphorylation of c-Jun in avian and mammalian motoneurons in vivo during programmed cell death: an early reversible event in the apoptotic cascade. J Neurosci. 2005;25:5595–5603. doi: 10.1523/JNEUROSCI.4970-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yuan Q, Hu B, Wu Y, et al. Induction of c-Jun phosphorylation in spinal motoneurons in neonatal and adult rats following axonal injury. Brain Res. 2010;1320:7–15. doi: 10.1016/j.brainres.2010.01.038. [DOI] [PubMed] [Google Scholar]
- [36].Raivich G, Bohatschek M, Da CC, et al. The AP-1 transcription factor c-Jun is required for efficient axonal regeneration. Neuron. 2004;43:57–67. doi: 10.1016/j.neuron.2004.06.005. [DOI] [PubMed] [Google Scholar]
- [37].Vaudano E, Rosenblad C, Bjorklund A. Injury induced c-Jun expression and phosphorylation in the dopaminergic nigral neurons of the rat: correlation with neuronal death and modulation by glial-cell-line-derived neurotrophic factor. Eur J Neurosci. 2001;13:1–14. [PubMed] [Google Scholar]
- [38].Harrington AW, Kim JY, Yoon SO. Activation of Rac GTPase by p75 is necessary for c-jun N-terminal kinase-mediated apoptosis. J Neurosci. 2002;22:156–166. doi: 10.1523/JNEUROSCI.22-01-00156.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yuan Q, Hu B, So KF, et al. Age-related reexpression of p75 in axotomized motoneurons. Neuroreport. 2006;17:711–715. doi: 10.1097/01.wnr.0000214390.35480.1a. [DOI] [PubMed] [Google Scholar]
- [40].Hanz S, Perlson E, Willis D, et al. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron. 2003;40:1095–1104. doi: 10.1016/s0896-6273(03)00770-0. [DOI] [PubMed] [Google Scholar]
- [41].Michaelevski I, Medzihradszky KF, Lynn A, et al. Axonal transport proteomics reveals mobilization of translation machinery to the lesion site in injured sciatic nerve. Mol Cell Proteomics. 2010;9:976–987. doi: 10.1074/mcp.M900369-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rossiter JP, Riopelle RJ, Bisby MA. Axotomy-induced apoptotic cell death of neonatal rat facial motoneurons: time course analysis and relation to NADPH-diaphorase activity. Exp Neurol. 1996;138:33–44. doi: 10.1006/exnr.1996.0044. [DOI] [PubMed] [Google Scholar]
- [43].Leah JD, Herdegen T, Bravo R. Selective expression of Jun proteins following axotomy and axonal transport block in peripheral nerves in the rat: evidence for a role in the regeneration process. Brain Res. 1991;566:198–207. doi: 10.1016/0006-8993(91)91699-2. [DOI] [PubMed] [Google Scholar]
- [44].Bussmann KA, Sofroniew MV. Re-expression of p75NTR by adult motor neurons after axotomy is triggered by retrograde transport of a positive signal from axons regrowing through damaged or denervated peripheral nerve tissue. Neuroscience. 1999;91:273–281. doi: 10.1016/s0306-4522(98)00562-4. [DOI] [PubMed] [Google Scholar]
- [45].Sedel F, Bechade C, Triller A. Nerve growth factor (NGF) induces motoneuron apoptosis in rat embryonic spinal cord in vitro. Eur J Neurosci. 1999;11:3904–3912. doi: 10.1046/j.1460-9568.1999.00814.x. [DOI] [PubMed] [Google Scholar]
- [46].Wu W. Potential roles of gene expression change in adult rat spinal motoneurons following axonal injury: a comparison among c-jun, off-affinity nerve growth factor receptor (LNGFR), and nitric oxide synthase (NOS) Exp Neurol. 1996;141:190–200. doi: 10.1006/exnr.1996.0153. [DOI] [PubMed] [Google Scholar]


