Abstract
Background:
The purpose of this study was to compare the effectiveness of different ultrasonic scalers and a periodontal curette on the root surfaces for calculus removal and root surface roughness.
Materials and Methods:
40 single rooted teeth with subgingival calculus destined for extraction were assigned to one of three experimental groups (n = 10, in each group) and one control group (untreated, n = 10). Experimental groups were: Group 1: Piezoelectric ultrasonic group; Group 2: Magnetostrictive ultrasonic group; Group 3: Hand instrumentation group (Curette). After instrumentation, the teeth were extracted and the presence of residual deposits and root surface roughness were analyzed using Planimetric analyzing tool (Tool that measures the area of a plane figure as a mechanically coupled pointer traversing the perimeter of figure) and Surface Profilometer (Instrument used for profiling of an object). Root surface characteristics were evaluated qualitatively using SEM. Standardization of force, angulations and adaptation of instrument couldn’t be achieved in our study due to in vivo study design rather than in vitro design in previous studies where procedure was done on the extracted teeth samples.
Results:
The results of the study showed that residual deposits were similar in all experimental groups. With respect to roughness parameters, Rq (Root mean square roughness) and Rt (Total roughness) a significant difference was observed (P < 0.001) among hand instrumentation and ultrasonic devices. SEM analysis revealed a similar root surface pattern for the ultrasonic devices, but curette showed many instrument scratches, gouges, and removal of large amount of cementum.
Conclusions:
Curette produced the rougher root surfaces than two ultrasonic devices used in the study and caused more root surface removal. Piezoelectric devices produced minimum root surface roughness but caused more root substance removal and more cracks than Magnetostrictive ultrasonic devices.
Keywords: Dental calculus, hand curette, magnetostrictive ultrasonic, piezoelectric ultrasonic, profilometer, surface roughness, scanning electron microscope, scaling and root planing
INTRODUCTION
Bacterial plaque and calculus are recognized etiological agents in the initiation and progression of periodontal disease, and their accumulation and attachment are facilitated by a roughened root surface.[1] A primary goal in the treatment of periodontitis is the removal of bacterial deposits and the arrest of disease progression.[2] The creation of a plaque- and calculus-free surface may be achieved by routine nonsurgical treatment, or with direct vision after reflection of gingival flaps.[3,4] Thus, as a final goal of scaling and root planing procedures, the complete removal of adherent plaque, calculus and infected root cementum (Superficial layer of cementum) is desirable, although a complete removal is rarely attained.[5,6,7,8,9]
Hand and ultrasonic scalers are common instruments used for debridement of root surfaces as a part of periodontal therapy. For plaque and calculus removal, ultrasonic scalers are frequently used in the clinical practice and have become increasingly popular for subgingival debridement due to less operator strain, equal efficacy with hand instruments and better debridement due to newer design tips.[10,11,12] In several studies utilizing various methods, no consistent results have been reported regarding the effect of instrumentation on periodontally diseased tooth surfaces.[13,14,15]
Previous reports have differed regarding biologic significance of surface roughness.[10,11] Surface irregularities are known to increase bacterial colonization and plaque formation in vitro and in vivo, contribute to retention and attachment of dental calculus.[10,16] A number of studies have compared tooth surfaces after treatment with various ultrasonic and hand instruments demonstrating a smoother surface with curettes.[11,12,16]
Thus, the present study was carried out to compare the effectiveness of a piezoelectric ultrasonic instrument, a magnetostrictive ultrasonic instrument and a periodontal curette on the root surfaces in teeth exhibiting advanced periodontal disease regarding calculus removal and root surface roughness.
MATERIALS AND METHODS
Sample
The study was approved by the Joint Research and Ethics Committee of AECS Maaruti College of Dental Science and Research Centre, Bangalore. All participants who were included in the study were asked to sign a consent form, after being informed of the nature of the study. Each patient attended the Department of Periodontology at AECS Maaruti College of Dental Science and Research Centre and had one or more teeth extracted for periodontal and/or, prosthetic reasons.
Inclusion criteria
Human single rooted teeth (i.e., Incisors, Canines and Premolars) with evidence of chronic inflammatory periodontal disease and a 3-5 mm pocket on at least any one surface of the tooth
A clinical attachment loss of 8-12 mm or more
Bone loss ≥two-third of the root length (radiographic exam)
Presence of sub-gingival calculus detected with an explorer/probe, and
Mobility grade II/III.
Exclusion criteria
Acute periodontal or, endodontic infection
Periodontal treatment in the last 5 years
Root surface caries or, any sub-gingivally placed restorations, and
Aggressive periodontitis.
Teeth were assigned into three experimental and a control group consisting of 10 teeth each. Two root surfaces (buccal and lingual) of each tooth were then subjected to debridement in the experimental group.
The experimental and control groups of the study were:
Group I – Piezoelectric ultrasonic device group. EMS™* PS tips were used [Figure 1]. The vibrations are produced by oscillations using a quartz crystal hand piece, set at a frequency of 25,000 Hz. PS tips are fine diameter tips used for general subgingival deposit removal on all root surfaces and can also be used for supragingival maintenance therapy
Group II – Magnetostrictive ultrasonic device group. Cavitron™† TFI p-10 tips were used [Figure 1]. The vibrations are produced by a resonating stack of metal strips, at a frequency of 25,000 Hz. TFI p-10 tips are used for removal of light to moderate subgingival calculus removal in all quadrants of the mouth as well as for deplaquing pockets ≤4 mm. It is tip of choice for fine periodontal debridement and provide excellent access and adaptation to root anatomy ≥4 mm
Group III – Hand instrumentation. Gracey curette§ # 5/6 [Figure 1] and
Group IV – Untreated teeth (Control), included only in planimetric analysis.
Figure 1.
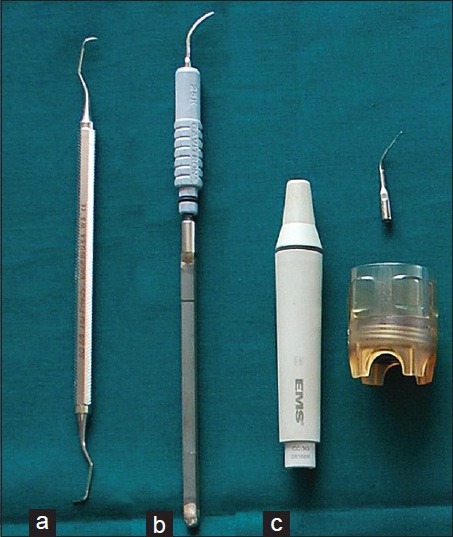
Instrument tips used in the study, (a) Curette # 5/6, (b) Cavitron™† TFI p-10 tip, (c) EMS™* PS tip
The water cooled ultrasonic devices were operated at medium power. The allocation process was randomized (tooth-based).
Clinical procedures
Supra- and sub-gingival instrumentation was performed by a single operator with local anesthesia in one session in vivo. Scaling and root planing were judged to be complete when the curved explorer indicated a smooth hard surface. No time limit was placed on the operator for mechanical debridement.
Following instrumentation, a small diamond round bur (0.2 mm) on a airotor hand piece was used to mark the level of free gingival margin, buccally and lingually, which acted as landmark for future evaluation and the tooth was extracted as atraumatically as possible, with the beak of extraction forceps above the gingival margin. The tooth was then washed in running water for 30 s to remove food, blood and debris and stored in a 10% buffered formalin solution till the analysis is carried out.
Staining of teeth and photographs
The teeth were transferred to 1% methylene blue for 2 min to stain attached connective tissues and then rinsed with running water for 2-3 min. They were then aligned parallel to horizontal plane and photographed by a digital camera** using a stereomicroscope.*** The image for each tooth surface had black background below the tooth.
Evaluation of stained deposits
The presence of plaque and calculus on root surfaces was measured using a planimetric analysis tool™.**** The surface area under investigation was determined coronally by gingival groove (bur mark) and apically by coronal borders of connective tissue attachment. Laterally the margins were set 0.5 mm apart for the line angle of tooth [Figure 2]. Within these boundaries, the root surface area covered by residual deposits was measured by a single trained examiner. The measurements for deposits were repeated three times on different days (within 24 days) and the average was taken. Final measurements were obtained in square millimeters (mm2) on total analyzed stained deposits.
Figure 2.
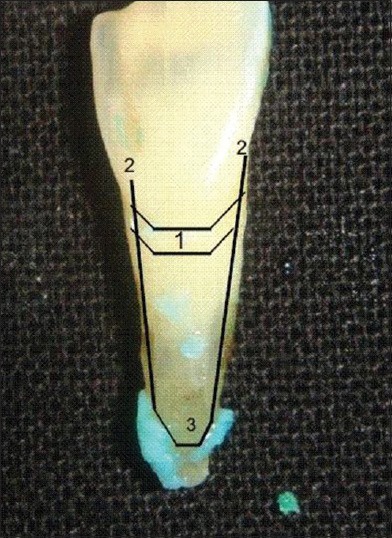
Measurement of root surface roughness within landmarks, 1. Gingival groove, 2. Lateral borders of connective tissue attachment, 3. Coronal borders of connective tissue attachment
Roughness parameters evaluation
After deposit analysis, all teeth were longitudinally sectioned using straight micro motor hand piece and diamond disk so as to obtain two surfaces (buccal and lingual) for each tooth and total of 20 surfaces per group were obtained. After sectioning teeth were dried in order to get their surface profile. All 20 surfaces (10 buccal and 10 lingual) per group were used for roughness evaluation. The roughness of the root surface was measured in micrometer (μm) using a non-contact optical surface profilometer.***** For this purpose, center of instrumented root surface 1-2 mm below the gingival groove was considered the instrumented area. Before starting the measurement instrument was calibrated against a standard object. Profilometer light was focused on area of interest which covered the entire instrumented area and measurements were obtained as interference pattern (fringes) given by profilometer Figure 3. Measurements were obtained at 41× magnification (Maximum magnification of profilometer). Surface profile was determined as average roughness (Ra), defined as the mean between peaks and valleys of the surface profile, total roughness (Rt) which means that the distance between maximum peak-to-valley height, Rq is the largest roughness considering all the cutoffs (highest peak and highest valley) and Rz is the mean of 10 peaks and 10 valley heights in each cutoff. Ra and Rz are average roughness parameters and they are not enough to distinguish surfaces that differ in spacing or shape. Therefore, it was necessary to calculate other parameters for a surface that measure peaks and valleys such as Rq and Rt. Data for the root surface roughness values were then plotted separately for Ra, Rt, Rq and Rz.
Figure 3.
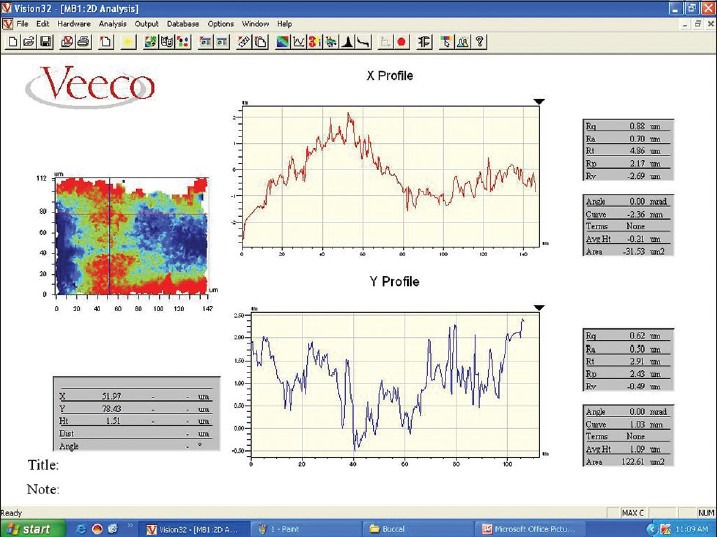
Roughness profile of surface obtained using profilometer
Scanning electron microscopy
After profilometric analysis, teeth were subjected to SEM examination. A total of 6 surfaces/group (3 buccal and 3 lingual) were evaluated by SEM.****** The samples were fixed in 1% glutaraldehyde phosphate-buffered saline solution for 1 h and rinsed in phosphate-buffered saline. The specimens were then post-fixed in 1% osmium tetroxide solution in phosphate-buffered saline for 1 h. and dehydrated in an ethanol ascending series, substituted with isoamyl acetate. Samples were processed with a critical point dryer. Each specimen was mounted on a metal stub before examination and coated with standard amount of gold needed for the observation with an Ion Coater.******* Finally, they were observed using a Scanning electron microscope. Two micrographs were obtained per surface at 100× magnification below the gingival groove for each experimental tooth surface [Figure 4]. The SEM examination was done to evaluate structure loss, amount of cementum present, damage, corrugations, scratches, and cracks.
Figure 4.
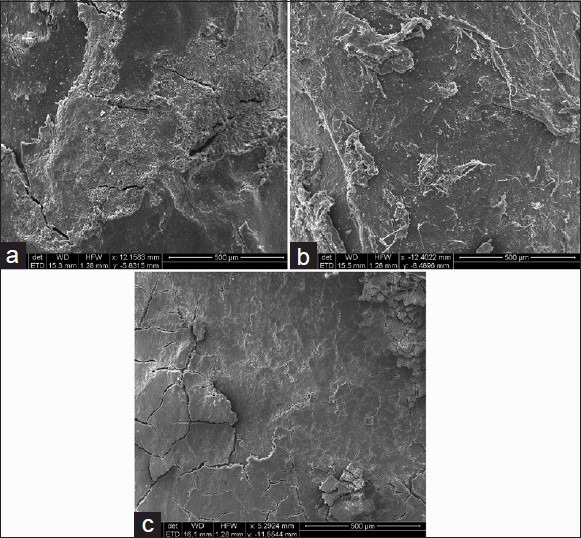
SEM measurement of samples below gingival groove at ×100, (a) ultrasonic instrumentation on root surface, (b) Magnetostrictive ultrasonic instrumentation on root surface, (c) Hand instrumentation (Gracey Curette) on root surface
Statistical analysis
Before the planimetric analysis, the examiner was trained and calibrated in two phases:
Measurements in five teeth to standardized area measured
Repeat measurements in 20 randomly selected surfaces at two different time points (within a 24 h interval). The intra-examiner repeatability was made by the Bland and Altman procedure [Graph 1].
Graph 1.
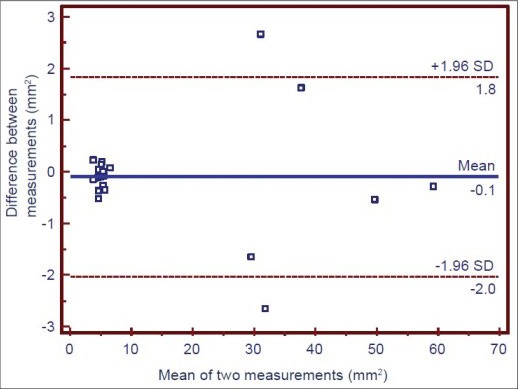
Intra-examiner repeatability. Bland-Altman plot of the data obtained by repeat measurements in two different time points. One point is superposed
The results of planimetric and roughness parameters were computed as means and standard error (SE). Statistically significant differences among groups (planimetric and roughness parameters) were evaluated by one way ANOVA with Tukey's post hoc test. 95% confidence interval was taken and for planimetric and roughness parameters, statistical significance was considered when P ≤ 0.01 and moderate statistically significance was considered for 0.01 <P ≤ 0.05.
The normal distribution of data was tested by the Shapiro–Wilks test with P > 0.05 and the homogeneity of variances was tested using Levene's statistic. The relationship between radicular stained deposits and surface roughness parameters was obtained using the Pearson correlation test [Graph 2]. The SEM evaluation was done qualitatively. The analyses were performed using statistical programs SAS 9.2, SPSS 15.0, Stata 10.1, MedCalc 9.0.1 etc.
Graph 2.
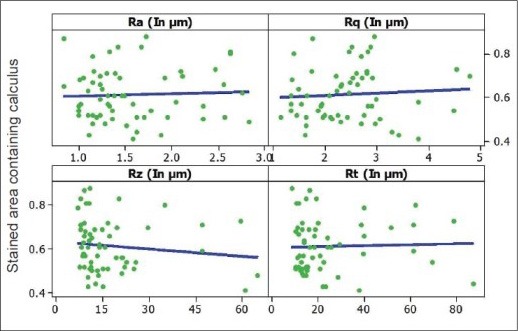
Pearson correlation test among surface roughness parameters and radicular stained deposits (independent of the treatment)
RESULTS
This study included a total of 14 participants (8 males and 6 females) with generalized chronic periodontitis aged between 34 and 70 years.
There were 40 teeth included in the study for evaluation, providing 80 root surfaces for the analysis.
Intra-examiner repeatability (stained deposits measurements) was in the limits of agreement as shown by Bland and Altman plot. The graph indicates that stained area measured at 2 different time points (Within a 24 hour interval) in 20 randomly selected surfaces gave nearly similar value and are in agreement with each other.
Evaluation of stained deposits
The total area analyzed in the study was not significantly different among the groups (P = 0.399, ANOVA, power test = 80%).
The remaining identifiable stained deposits were similar in all experimental groups. Stained area containing calculus in mm2 showed statistically significant difference among groups (P < 0.01, ANOVA) [Table 1].
Table 1.
Comparison of stained area containing calculus (mm2) to the total stained area measured (mm2) between various groups

Percentage stained area to the total area showed statistically significant difference among groups (P < 0.01, ANOVA).
Thus, there were statistically significant differences (P < 0.01, Tukey's post hoc test) between the control group and all the experimental groups [Table 1] with respect to percentage of stained area deposits.
Roughness parameters evaluation
A mean roughness value was calculated for each experimental tooth for different roughness parameters.
The roughness values, Ra and Rz of the roots treated showed a similar pattern among the groups (P > 0.05, ANOVA, power test = 80%, not statistically significant difference) [Table 2].
Table 2.
Comparison of surface roughness values of root surfaces in test groups
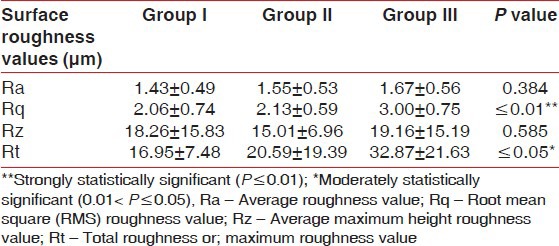
However, significant differences for Rq and Rt were observed (P < 0.01 for Rq and P ≤ 0.05 for Rt, ANOVA, power test = 80%) among experimental groups [Table 2, Graph 3].
Graph 3.
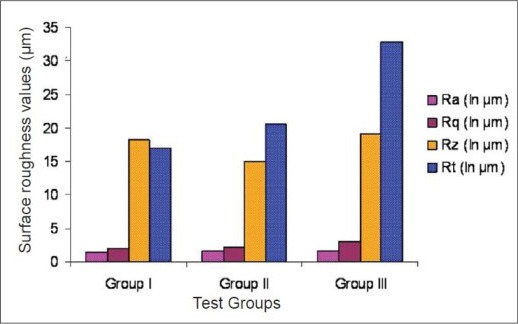
Surface roughness values in test groups
These findings showed that curette produced deepest gouges.
However, Ra values among various experimental groups were not statistically significant while Rt values were statistically significant.
In the Group IV (untreated or control group teeth) roughness parameters were not evaluated because surface profilometer sensor was out of the reading range and unable to focus light on a specific area of root surface (Irregular radicular surface due to the presence of heavy calculus deposits).
Scanning electron microscope evaluation
The SEM examination was done qualitatively (at 100× magnification) to evaluate the amount of structure loss, amount of cementum present, damage, corrugations, scratches, and cracks on the root surfaces after instru-mentation.
In Group I (piezoelectric), in all subjects, cementum was present and the radicular surface appeared irregular with few corrugations. A reduced number of instrumental scratches and gouges were observed. Few surface cracks were presented in this group.
In Group II (magnetostrictive), the majority of the samples had all the cementum present and the radicular surfaces appeared irregular with few corrugations. Decreased number of instrumental scratches and gouges were observed. Dentine substance was lost to a lesser extent than that observed in Groups I and III. Few surface cracks were observed in this group.
In Group III (hand instrumentation, Curette), cementum was observed in few points and the radicular surface appeared regular with few corrugations. Many instrument scratches and deep gouges were observed. A significant amount of the dentine layer was removed. Surface cracks were maximum in this group.
After instrumentation, all groups were covered by smear layer, which made difficult to distinguish cementum from dentine but root surface preparation prior to SEM evaluation using Glutaraldehyde and ethanol ascending series remove Smear layer completely. Gouges, probably corresponding to the instruments tips, were found on all surfaces.
DISCUSSION
The goal of periodontal procedures (SRP mainly) is to produce a smooth surface with minimal alteration of the root surfaces, which can be obtained with ultrasonic and hand instruments.[12] The cumulative effect of minor substance removal per instrumentation may lead to severe root damage over time. Some studies suggested that ultrasonic devices lead to less damage to the root surface than hand instruments,[17] and in ultrasonic devices, piezoelectric devices are less aggressive in substance removal than magnetostrictive devices[18] but leave a more rougher surface after instrumentation.[17]
With respect to stained deposits, significant variation in the appearance of stained areas was observed on all teeth surfaces. The stained deposits present in the three groups were not significantly different from each other (ANOVA). All instruments significantly reduced the amount of stained deposits on the root surfaces. For all the analyzed teeth, the mean percentages of stained deposits obtained in the present study were: Piezoelectric (4.5%), Magnetostrictive (5.05%), and Hand Instrumentation (5.72%). The results of the present study are in agreement with results of Hurzerler et al.,[19] Eberhard et al.[18] and Yukna et al.[20] but showed higher values than those obtained by Busslinger et al.[17] and Kocher et al.[21] The results of the present study were lower than those obtained by Santos et al.[16]
The reasons for these differences are probably due to differences in methodology used in present study such as: Different tips used in the study, instrumentation without reflection of mucoperiosteal flap, tooth selection criteria with shallow probing depths and use of highly sophisticated ProgRes image analysis software with high degree of precision. Several potential factors were responsible for the less favorable results reported in this study with curette instrumentation. These include teeth conditions, increased grade of mobility (grade 2 and 3) and decreased precision with curette movement.
With regard to the roughness parameters after instrumentation, Ra and Rq values showed a similar pattern. However, Rz and Rt values were higher for hand instrumentation than for ultrasonic devices. The present study values for average roughness values (Ra) were lower for hand instrumentation than investigations of Santos et al.,[16] Cross-Poline et al.[14] and Bye et al.[22] with mean Ra values being 5.2, 4.70 and 4.90 microns but are in agreement with studies of Kishida et al.,[1] Busslinger et al.,[17] Schlagater et al.,[23] Huerzeler et al.[19] and Vastardis et al.[24] with mean Ra values being 0.54, 1.42, 1.90, 1.96 and 0.78 microns respectively. Similarly, average roughness values (Ra) were lower for Piezoelectric ultrasonic instrumentation than investigations of Santos et al.,[16] Cross-Poline et al.[11] and Bye et al.[22] with mean Ra values being 4.1, 7.35 and 7.39 μm, respectively, but are in agreement with studies of Vastardis et al.[24] with mean Ra value being 0.68μm. Average roughness values were lower for Magnetostrictive ultrasonic instrumentation than investigations of Cross-Poline et al.,[11] Bye et al.[22] and Santos et al.[16] with mean Ra values being 7.19, 9.920 and 4.40 μm but are in agreement with studies of Busslinger et al.[17] with mean Ra value of 1.36μm. The main reasons for the difference in Ra values with other studies for all experimental groups are the methodology used such as in vivo study design, different instrument tips, precision of profilometer, type of profilometer used, time period for instrumentation, medium power setting of the ultrasonic instruments.
The present study was unique in the manner that instrumentation was performed in vivo under Local Anesthesia where as previous studies of Schmidlin et al.,[25] Volkinbdrg et al.,[26] Flemmig et al.[27] etc., were performed on extracted teeth in vitro. Thus, standardization of force, adaptation and angulation of instrumentation was not possible in our study. For ultrasonic scalers, medium power setting was used in our study. It has been demonstrated that defect depth and volume for piezoelectric and magnetostrictive ultrasonic instruments depend on instrumentation time, lateral forces, power setting and tip angulation but for curettes defect depth and volume depend on manual dexterity, proper adaptation and experience of the operator.[27]
The goal of periodontal debridement is the creation of a biologically acceptable root surface while protecting the healthy dental tissues. When creating, maintaining and following-up healthy periodontal tissues, severe root damage can occur during recall interventions, when aggressive instruments are thoughtlessly used. This can be clarified by the contrasting nature of two opinions: In 1st opinion,[28] more aggressive concept of root surface debridement was suggested. They investigated the distribution of bacteria within the radicular dentin and pulp of periodontally diseased caries free teeth and finally suggested that the roots of periodontally diseased teeth could act as bacterial reservoirs for recolonization of mechanically treated root surfaces and re-infection of the dental pulp. In contrast, another opinion[29] suggested that excessive removal of root substance including removal of “soft” cementum during periodontal therapy is not necessary. Therefore for clinical application meticulous scaling and root planing procedure should be performed during initial cause-related therapy, whereas more conservative approach is recommended during maintenance care.
Generally, instrumentation during periodontal debridement causes damage to the integrity of the root surface[30] while a rough root surface may influence plaque formation,[31,32] the indicator for a clean and biologically acceptable root surface remains the healing and continuous reaction of periodontal soft tissues,[33] even though different surface roughness may show no difference concerning these periodontal parameters.[29,34] However, the clinically smooth root surface still remains the goal and indicator for a clean tooth surface.[35] Therefore, it can be assumed that parameters like “smooth” and “rough” will remain controversial related with scaling instruments discussed in the literature, and that further studies are needed to clarify the influence of root surface roughness on clinical results.
Our study results showed that Piezoelectric instrument produced smoothest surface followed by Magnetostrictive instrument and the Curette produced least smooth surface. The roughness of root surface after instrumentation does not seem to impair post-operative healing, and a micro irregular surface is conducive to the formation of new cementum.[36,37]
Our results with SEM in piezoelectric and magnetostrictive groups were similar. The root surfaces appeared irregular with occasional gouges or depressions, but greater losses of dentine substance were observed with piezoelectric than with magnetostrictive devices. After hand instrumentation, we observed instrumental scratches, deep gouges and large dentine layers were removed. The root surface treated with a Gracey curette was covered by a smear layer.[1] The results were similar to those obtained by Busslinger et al.[17] in that the magnetostrictive instrument produced a better surface finish than piezoelectric manipulation, with the curette revealing gouges that likely correspond to the curette tip.
Scaling and Root planing is helpful in regeneration which is achieved through application of Curettes but Ultrasonic scalers does not achieve this goal as studies done by Kishida et al.,[38] Wirthin et al.[39,40] and Kenji et al.[41] As number of fibroblasts and their function is unfavorable for growth in Ultrasonic group due to incomplete removal of root surfaces containing endotoxin While in Curette group, root substance loss is more and thus contaminant layer of root containing endotoxin removal is completely achieved. Thus, a large number of fibroblasts are attached on treated root surfaces with Curettes. Even excessive removal of cementum is not necessary for achieving regeneration as this can cause severe post-operative sensitivity and root substance loss.[42]
Thus, scaling and root planing using ultrasonic scalers are effective in removing large masses of calculus quickly but are unable to remove complete endotoxins from root surfaces to achieve regeneration. Hence, Ultrasonic scalers should be supplemented with curettes to achieve this goal.
Future investigations should consider the issues of clinical access to all areas of the root surface and the clinical significance of differences between the instruments.
CONCLUSION
The study results showed that amount of residual calculus present was minimum in all experimental groups as compared to control group and rough root surfaces were produced after using all the experimental devices but maximum root surface roughness were produced by Hand instruments followed by Magnetostrictive ultrasonic devices and Piezoelectric ultrasonic devices, respectively. Maximum amount of root substance removal, root surface cracks and corrugations were seen after using hand instruments followed by Piezoelectric ultrasonic devices and Magnetostrictive ultrasonic devices, respectively.
Key finding
Ultrasonic instruments produces less root surface roughness, less damage to the root surface than hand instruments as demonstrated by decreased roughness value, less scratches, corrugations and gouges on root surfaces by ultrasonic instrumentation. Piezoelectric instruments give smoother root surface than Magnetostrictive instruments.
ACKNOWLEDGEMENT
This study was supported by authors themselves. The authors report no conflicts of interest related to this study.
* Electro medical system, EMS, Switzerland.
† Dentsply, Dentsply international, USA.
§ Hu Friedy™, Chicago, USA.
** Olympus, Germany.
*** Olympus, Germany.
**** ProgRes image analyzer™, Jenoptik, Laser Optik system, Version 2.7.0, Germany.
***** Wyko NT™ 1100 optical profilometer, Germany.
****** FEI 2000, Japan.
******* JFC – 1101, JEOL, Tokyo, Japan.
Source of Support: No external funding, apart from the support of the author's institution, was available for this study
Conflict of Interest: None declared.
REFERENCES
- 1.Kishida M, Sato S, Ito K. Comparison of the effects of various periodontal rotary instruments on surface characteristics of root surface. J Oral Sci. 2004;46:1–8. doi: 10.2334/josnusd.46.1. [DOI] [PubMed] [Google Scholar]
- 2.Research, Science and Therapy Committee of the American Academy of Periodontology. Treatment of plaque-induced gingivitis, chronic periodontitis and other clinical conditions. J Periodontol. 2001;72:1790–800. doi: 10.1902/jop.2001.72.12.1790. [DOI] [PubMed] [Google Scholar]
- 3.Badersten A, Nilveus R, Egelberg J. Effect of nonsurgical periodontal therapy. I. Moderately advanced periodontitis. J Clin Periodontol. 1981;8:57–72. doi: 10.1111/j.1600-051x.1981.tb02024.x. [DOI] [PubMed] [Google Scholar]
- 4.Lindhe J, Westfelt E, Nyman S, Socransky SS, Haffajee AD. Long-term effect of surgical/non-surgical treatment of periodontal disease. J Clin Periodontol. 1984;11:448–58. doi: 10.1111/j.1600-051x.1984.tb01344.x. [DOI] [PubMed] [Google Scholar]
- 5.Fujikawa K, O’Leary TJ, Kafrawy AH. The effect of retained subgingival calculus on healing after flap surgery. J Periodontol. 1988;59:170–5. doi: 10.1902/jop.1988.59.3.170. [DOI] [PubMed] [Google Scholar]
- 6.Breininger DR, O’Leary TJ, Blumenshine RV. Comparative effectiveness of ultrasonic and hand scaling for the removal of subgingival plaque and calculus. J Periodontol. 1987;58:9–18. doi: 10.1902/jop.1987.58.1.9. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan SA, Robertson PB. Calculus removal by scaling/root planing with and without surgical access. J Periodontol. 1987;58:159–63. doi: 10.1902/jop.1987.58.3.159. [DOI] [PubMed] [Google Scholar]
- 8.Kepic TJ, O’Leary TJ, Kafrawy AH. Total calculus removal: An attainable objective? J Periodontol. 1990;61:16–20. doi: 10.1902/jop.1990.61.1.16. [DOI] [PubMed] [Google Scholar]
- 9.Sherman PR, Hutchens LH, Jr, Jewson LG. The effectiveness of subgingival scaling and root planing. II. Clinical responses related to residual calculus. J Periodontol. 1990;61:9–15. doi: 10.1902/jop.1990.61.1.9. [DOI] [PubMed] [Google Scholar]
- 10.Dragoo MR. A clinical evaluation of hand and ultrasonic instruments on subgingival debridement. Part I. With unmodified and modified ultrasonic inserts. Int J Periodontics Restorative Dent. 1992;12:310–23. [PubMed] [Google Scholar]
- 11.Cross-Poline GN, Stach DJ, Newman SM. Effects of curette and ultrasonics on root surfaces. Am J Dent. 1995;8:131–3. [PubMed] [Google Scholar]
- 12.Jepsen S, Ayna M, Hedderich J, Eberhard J. Significant influence of scaler tip design on root substance loss resulting from ultrasonic scaling: A laserprofilometric in vitro study. J Clin Periodontol. 2004;31:1003–6. doi: 10.1111/j.1600-051X.2004.00601.x. [DOI] [PubMed] [Google Scholar]
- 13.Schmidlin PR, Beuchat M, Busslinger A, Lehmann B, Lutz F. Tooth substance loss resulting from mechanical, sonic and ultrasonic root instrumentation assessed by liquid scintillation. J Clin Periodontol. 2001;28:1058–66. doi: 10.1034/j.1600-051x.2001.281111.x. [DOI] [PubMed] [Google Scholar]
- 14.Ewen SJ, Gwinnett AJ. A scanning electron microscopic study of teeth following periodontal instrumentation. J Periodontol. 1977;48:92–7. doi: 10.1902/jop.1977.48.2.92. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg RM, Ash MM., Jr The effect of root roughness on plaque accumulation and gingival inflammation. J Periodontol. 1974;45:146–50. doi: 10.1902/jop.1974.45.3.146. [DOI] [PubMed] [Google Scholar]
- 16.Santos FA, Pochapski MT, Leal PC, Gimenes-Sakima PP, Marcantonio E., Jr Comparative study on the effect of ultrasonic instruments on the root surfaces in vivo. Clin Oral Invest. 2008;12:143–50. doi: 10.1007/s00784-007-0167-3. [DOI] [PubMed] [Google Scholar]
- 17.Busslinger A, Lampe K, Beuchat M, Lehmann B. A comparative in vitro study of a magnetostrictive and a piezoelectric ultrasonic scaling instrument. J Clin Periodontol. 2001;28:642–9. doi: 10.1034/j.1600-051x.2001.028007642.x. [DOI] [PubMed] [Google Scholar]
- 18.Eberhard J, Ehlers H, Falk W, Acil Y, Albers HK, Jepsen S. Efficacy of subgingival calculus removal with Er: YAG laser compared to the mechanical debridement: An in situ study. J Clin Periodontol. 2003;30:511–8. doi: 10.1034/j.1600-051x.2003.00052.x. [DOI] [PubMed] [Google Scholar]
- 19.Huerzeler MB, Einsele FT, Leupolz M, Kerkhecker U, Strub JR. The effectiveness of different root debridement modalities in open flap surgery. J Clin Periodontol. 1998;25:202–8. doi: 10.1111/j.1600-051x.1998.tb02429.x. [DOI] [PubMed] [Google Scholar]
- 20.Yukna RA, Scott JB, Aichelmann-Reidy ME, LeBlanc DM, Mayer ET. Clinical evaluation of the speed and effectiveness of subgingival calculus removal on single-rooted teeth with diamond-coated ultrasonic tips. J Periodontol. 1997;68:436–42. doi: 10.1902/jop.1997.68.5.436. [DOI] [PubMed] [Google Scholar]
- 21.Kocher T, Langenbeck M, Ruhling A, Plagmann HC. Subgingival polishing with a teflon-coated sonic scaler insert in comparison to conventional instruments as assessed on extracted teeth. (I) Residual deposits. J Clin Periodontol. 2000;27:243–9. doi: 10.1034/j.1600-051x.2000.027004243.x. [DOI] [PubMed] [Google Scholar]
- 22.Bye FL, Ghilzon RS, Caffesse RG. Root surface roughness after the use of different modes of instrumentation. Int J Periodontics Restorative Dent. 1986;5:37–47. [PubMed] [Google Scholar]
- 23.Schlageter L, Rateitschak–Pluss EM, Schwarz JP. Root surface smoothness or roughness following open flap debridement. An in vivo study. J Clin Periodontol. 1996;23:460–4. doi: 10.1111/j.1600-051x.1996.tb00575.x. [DOI] [PubMed] [Google Scholar]
- 24.Vastardis S, Yukna RA, Rice DA, Mercante D. Root surface removal and resultant surface texture with diamond-coated ultrasonic inserts: An in vitro and SEM study. J Clin Periodontol. 2005;32:467–73. doi: 10.1111/j.1600-051X.2005.00705.x. [DOI] [PubMed] [Google Scholar]
- 25.Schmidlin PR, Beuchat M, Busslinger A, Lehmann B, Lutz F. Tooth substance loss resulting from mechanical, sonic and ultrasonic root instrumentation assessed by liquid scintillation. J Clin Periodontol. 2001;28:1058–66. doi: 10.1034/j.1600-051x.2001.281111.x. [DOI] [PubMed] [Google Scholar]
- 26.Volkinbdrg JW, Green E, Armitage GC. The nature of root surfaces after curette, cavitron and alpha-sonic instrumentation. J Periodontal Res. 1976;11:374–81. doi: 10.1111/j.1600-0765.1976.tb00092.x. [DOI] [PubMed] [Google Scholar]
- 27.Flemmig TF, Petersilka GJ, Mehl A, Hickel R, Klaiber B. The effect of working parameters on root substance removal using a piezoelectric ultrasonic scaler in vitro. J Clin Periodontol. 1998;25:158–63. doi: 10.1111/j.1600-051x.1998.tb02422.x. [DOI] [PubMed] [Google Scholar]
- 28.Adriaens PA, De Boever JA, Loesche WJ. Bacterial invasion in root cementum and radicular dentin of periodontally diseased teeth in humans. J Periodontol. 1988;59:222–30. doi: 10.1902/jop.1988.59.4.222. [DOI] [PubMed] [Google Scholar]
- 29.Oberholzer R, Rateitschak KH. Root cleaning or root smoothing. An in vivo study. J Clin Periodontol. 1996;23:326–30. doi: 10.1111/j.1600-051x.1996.tb00553.x. [DOI] [PubMed] [Google Scholar]
- 30.Jones SJ, Lozdan J, Boyde A. Tooth surfaces treated in situ with periodontal instruments. Scanning electron microscopic studies. Br Dent J. 1972;132:57–64. doi: 10.1038/sj.bdj.4802798. [DOI] [PubMed] [Google Scholar]
- 31.Leknes KN, Lie T, Wikesjo UM, Bogle GC, Selvig KA. Influence of tooth instrumentation roughness on subgingival microbial colonisation. J Periodontol. 1994;65:303–8. doi: 10.1902/jop.1994.65.4.303. [DOI] [PubMed] [Google Scholar]
- 32.Quirynen M, Bollen CM. The influence of surface roughness and surface free energy on supragingival plaque formation in man. J Clin Periodontol. 1995;22:1–14. doi: 10.1111/j.1600-051x.1995.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 33.Corbet EF, Vaughan AJ, Kieser J. The periodontally involved root surface. J Clin Periodontol. 1993;20:402–10. doi: 10.1111/j.1600-051x.1993.tb00380.x. [DOI] [PubMed] [Google Scholar]
- 34.Khatiblou FA, Ghodssi A. Root surface smoothness or roughness in periodontal treatment. A clinical study. J Periodontol. 1983;54:365–7. doi: 10.1902/jop.1983.54.6.365. [DOI] [PubMed] [Google Scholar]
- 35.Rylander H, Lindhe J. Cause related periodontal therapy. In: Lindhe J, Karring T, Lang NP, editors. Clinical periodontology and implant dentistry. 4th ed. Copenhagen: Blackwell Munksgaard; 2003. pp. 432–46. [Google Scholar]
- 36.Rabbani GM, Ash MM, Caffesse RG. The effectiveness of subgingival scaling and root planing in calculus removal. J Periodontol. 1981;52:119–23. doi: 10.1902/jop.1981.52.3.119. [DOI] [PubMed] [Google Scholar]
- 37.Dibart S, Capri D, Casavecchia P, Nunn M, Skobe Z. Comparison of the effectiveness of scaling and root planing in vivo using hand versus rotary instruments. Int J Periodontics Restorative Dent. 2004;24:370–7. [PubMed] [Google Scholar]
- 38.Kishida M, Sato S, Ito K. Effects of a new ultrasonic scaler on fibroblast attachment to root surfaces: A Scanning electron microscopy analysis. J Periodontol Res. 2004;39:111–9. doi: 10.1111/j.1600-0765.2004.00715.x. [DOI] [PubMed] [Google Scholar]
- 39.Wirthin MR, Hancock EB. Biologic preparation of diseased root surfaces. J Periodontol. 1980;51:291–7. doi: 10.1902/jop.1980.51.5.291. [DOI] [PubMed] [Google Scholar]
- 40.Wirthin MR, Hancock EB. Chemical treatment of diseased root surfaces in vitro. J Periodontol. 1981;52:694–6. doi: 10.1902/jop.1981.52.11.694. [DOI] [PubMed] [Google Scholar]
- 41.Kenji T, Noriyuki A, Shigetaka H, Hiroshi M, Koichi I, Sedai M. Cell attachment and growth of the cultured cells to the smooth and rough surfaces of the dentin surface. J Jpn Soc Periodontol. 1987;29:859–69. [Google Scholar]
- 42.Nyman S, Sarhed G, Ericsson I, Gottlov J, Karring T. Role of ‘diseased’ root cementum in healing following treatment of periodontal disease. An experimental study in the dog. J Periodontol Res. 1986;21:496–503. doi: 10.1111/j.1600-0765.1986.tb01485.x. [DOI] [PubMed] [Google Scholar]


