Abstract
Background:
C-reactive protein (CRP), an acute-phase protein monitored as a marker of inflammatory status, has been identified as a major risk factor for various systemic diseases. It is a reliable marker to infectious burdens and/or inflammation. The aim of this study was to compare and evaluate the systemic levels of CRP in the serum sample of the patients with healthy gingiva, gingivitis, and chronic periodontitis.
Materials and Methods:
A total of 60 systemically healthy patients were selected and divided into three groups: Patients with healthy gingiva (Group A), patients with generalized gingivitis (Group B) and patients with chronic periodontitis (Group C). Peripheral blood was collected and high-sensitive (hs)-CRP levels were estimated in the serum samples by using the particle-enhanced turbidimetric immuno-assay technique using a commercially available kit.
Results:
The mean hs-CRP level in Group A recorded was 0.437 ± 0.216, Group B was 0.771 ± 0.384 and Group C was 2.285 ± 0.381. A significantly elevated hs-CRP level was found in Group C as compared with Group B and A (P < 0.05). However, a moderate, but statistically significant increase in the hs-CRP levels was observed in Group B as compared with Group A (P < 0.05). The percentage of patients with elevated levels of hs-CRP >2 mg/l was significantly higher in Group C.
Conclusion:
The patients with chronic periodontitis demonstrated a mean hs-CRP levels higher than the patients with gingivitis and with healthy gingiva. Furthermore, with the increasing inflammation, the hs-CRP levels increased proportionately.
Keywords: Acute-phase reaction, chronic periodontitis, gingivitis, high-sensitive C-reactive protein, particle-enhanced turbidimetric immuno-assay technique
INTRODUCTION
The local inflammatory response to pathogenic bacteria or bacterial products is characterized by infiltration of the periodontal tissues of inflammatory cells including polymorphonuclear neutrophils, macrophages, lymphocytes, and plasma cells.[1] The acute-phase response is a nonspecific process, initiated and coordinated by a large number of diverse inflammatory mediators that may occur in the initial host response to injuries, infections, ischemic necrosis or malignancy. C-reactive protein (CRP), fibrinogen, and acute-phase proteins are sensitive markers to evaluate the inflammatory status.[2]
C-reactive protein was discovered in Tillett and Francis laboratory in 1930 during the course of study of patients with Streptococcus pneumoniae infection.[3] Forty years later, Volanakis and Kaplan identified the specific ligand for CRP in the pneumococcal ‘C’ polysaccharide as phosphocholine, part of the teichoic acid of the pneumococcal cell wall. Although phosphocholine was the first defined ligand for CRP, a number of other ligands have since been identified. In addition to interacting with various ligands, CRP can activate the classical complement pathway, stimulate phagocytosis, and bind to immunoglobulin receptors (FcγR). In humans, plasma levels of CRP may rise rapidly and markedly, as much as 1000-fold or more, after an acute inflammatory stimulus, largely reflecting increased synthesis by hepatocytes.[4]
C-reactive protein is a phylogenetically highly conserved plasma protein, with homologs in vertebrates and many invertebrates that participate in the systemic response to inflammation. It is a pattern recognition molecule, binding to specific molecular configurations that are typically exposed during cell death or found on the surfaces of pathogens. Its rapid increase in synthesis within hours after tissue injury or infection suggests that it contributes to host defense and that it is part of the innate immune response.[4] It is produced in response to many forms of injury other than periodontitis, such as other infections, trauma and hypoxia, and it is regulated by cytokines such as interleukin-6 (IL-6), IL-1β, and tumor necrosis factor-α. CRP levels have an association with smoking, obesity, triglycerides, diabetes, and periodontal disease.[5] It is proposed that changes in cellular and molecular components of peripheral blood can be found in patients with periodontitis because of inflammatory changes of the periodontal tissues.[6]
Thus, the aim of this study was to compare and evaluate the systemic levels of CRP in the serum sample of the patients with healthy gingiva, gingivitis and chronic periodontitis and compare them with the periodontal clinical parameters.
MATERIALS AND METHODS
In this study, 60 patients of age group 20-55 years with good systemic health diagnosed with healthy gingiva, gingivitis, and chronic periodontitis were selected. Diagnosis was made on the basis of measurement of gingival index (GI), plaque index (PI), gingival bleeding index (GBI), probing depth (PD) (in mm), clinical attachment level (CAL) (in mm), and high-sensitive-(hs)-CRP level in serum sample using particle-enhanced turbidimetric immuno-assay (PETIA) in patients visiting regular outpatient department of our institution. Patients so selected were equally divided into three groups:
Group A: Patients with healthy gingiva with no change in color, no signs of redness, edema, and no bleeding on probing
Group B: Patients with generalized gingivitis with moderate redness, edema, and presence of bleeding on probing
Group C: Patients with chronic generalized periodontitis with marked redness, edema and tendency of spontaneous bleeding on probing, at least 8 teeth with PD ≥ 5 mm, at least 8 teeth with CAL ≥ 5 mm and presence of alveolar bone loss (ABL) radiographically.
In this study, both males and females were included having a minimum of 20 teeth and with good systemic health. However, patients having any systemic diseases or with history of any periodontal surgery in past 6 months, pregnant and lactating females, smokers, any antibiotic or anti-inflammatory medication in past 6 months, any history of trauma and recent tooth extractions or any other infections and un-cooperative patients were excluded from the study. All the patients were informed about the purpose of the study prior to participation. A consent form, duly signed by each patient was obtained.
A total of 10 ml of peripheral blood was collected from the patients by venipuncture in the antecubetal fossa using tubes with ethylenediaminetetraacetic acid and was immediately sent for centrifugation at 2000 rpm for 5 min. Serum was separated carefully from the centrifuged sample and was then stored in freezer until analysis. It is preferable to test samples within 2-3 days or the serum sample can be stored best at –20°C if prolonged storage is desired.
Serum concentrations of CRP were quantified using a commercially available turbidimetric immuno-assay (Transasia Bio-Medicals Ltd., Erba Diagnostics, Mannheim, Germany) for hs determination of CRP. Diagnostic reagent kit is used for the in vitro detection of CRP in human serum by quantitative test.
Statistical analysis
All the values were expressed in the form of mean, standard deviation, and standard error of mean. The parameters were compared between groups using one-way analysis of variance. Unpaired t-test was used for intragroup comparison at same time that is, baseline. Karl-Pearson's correlation coefficient test was used to measure the correlation of two indices with each other in each group. The analysis was performed by the data analysis software through Microsoft excel 2003 and Statistical software (Statistical Package for Social Sciences or SPSS version 16 SPSS Inc., Chicago, IL, USA). The P < 0.05 indicated significant difference between the groups at 5% level of significance.
RESULTS
The mean values of GI, PI, GBI, PD, CAL, and hs-CRP levels of all the three groups have been shown in the Table 1 and Figure 1. There was a statistical difference between Group A and B, Group B and C, and Group A and C scores at 5% level of significance for all the parameters. The variation in hs-CRP values was correlated with variation changes in the different periodontal parameters, which were determined using Karl-Pearson's coefficient correlation test. The correlation was observed among all the clinical parameters in Group A, B, and C, respectively.
Table 1.
Mean values of GI, PI, GBI, PD, CAL and hs-CRP level of Group A, B and C
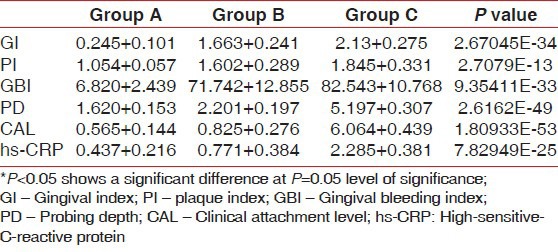
Figure 1.
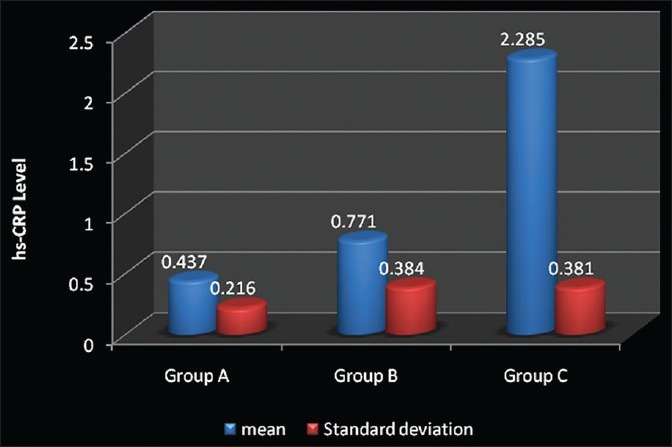
Comparison between the mean high-sensitive-C-reactive protein (in mg/l) for Group A, Group B and Group C
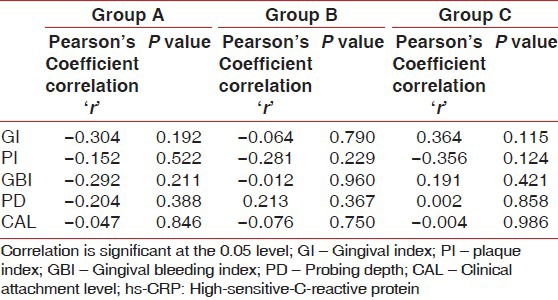
Gingival index and hs-CRP were found to have a weak negative correlation coefficient “r” of −0.304 and −0.064 in Group A and B, respectively [Figures 2 and 3]. While in Group C, a strongly positive and significant correlation coefficient “r” of 0.364 was found [Figure 4]. PI and hs-CRP were found to have a weak negative correlation coefficient “r” of −0.152 and −0.281 in Group A and B, respectively. Furthermore, a strong negative significant correlation coefficient “r” of −0.356 was found for Group C. However, GBI and hs-CRP were found to have a weak negative correlation coefficient “r” of −0.292 and −0.012 in Group A and B, respectively and a weak positive correlation coefficient “r” of 0.191 was found for Group C. In addition to this, a weak negative correlation coefficient “r” of − 0.204 for Group A, a weak positive correlation coefficient “r” of 0.213 and 0.002 in Group B and C, respectively was observed between PD and hs-CRP. Unlike all the other parameters, there was a weak negative correlation coefficient “r” of − 0.047, −0.076 and − 0.004 found between CAL and hs-CRP for Group A, B, and C, respectively [Table 2].
Figure 2.
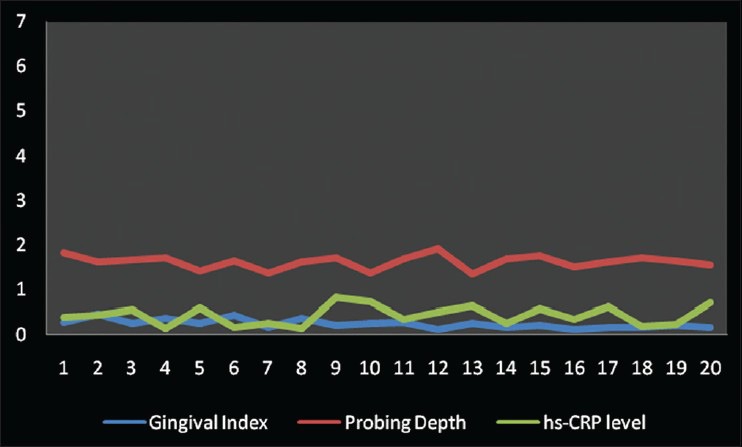
Correlation among gingival index, probing depth and high-sensitive-Creactive protein levels in Group A
Figure 3.
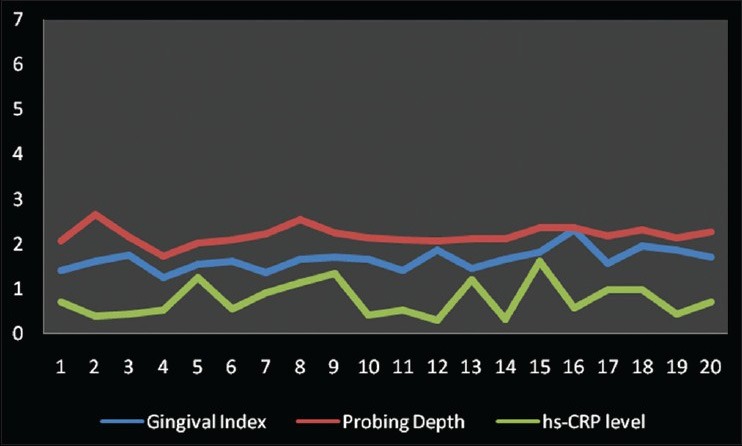
Correlation among gingival index, probing depth and high-sensitive-Creactive protein levels in Group B
Figure 4.
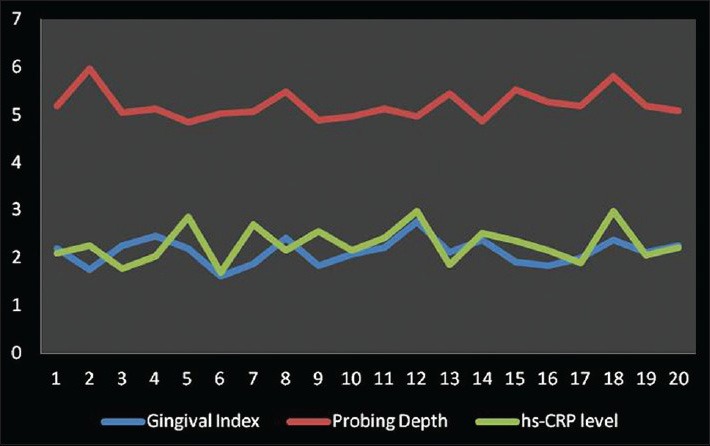
Correlation among gingival index, probing depth and high-sensitive-Creactive protein levels in Group C
Table 2.
Karl-pearson's correlation coefficient among the hs-CRP level with different parameters in Group A, B and C
DISCUSSION
It has been shown that inflammation plays a role in the pathogenesis of cardiovascular diseases (CVD).[7] Epidemiological studies have also implicated periodontitis as a risk factor for the development of CVD.[8,9] CRP represents an emerging and reliable marker of the acute-phase response to infectious burdens and/or inflammation. As a consequence of its kinetics, it best describes the inflammatory status of the individual.[10] Race/ethnicity has also been found to affect the levels of CRP.[11]
In a very early study, it was apparent from the results that CRP appears in the serum of patients with some forms of inflammatory oral diseases.[12] Recent evidence has indicated that patients with severe periodontitis have increased serum levels of CRP, when compared with unaffected control population.[5] However, they fall short in indicating that periodontitis was the cause for the observed serum CRP levels as CRP levels fluctuate with various confounding factors such as aging, high blood pressure, alcohol use, smoking, low levels of physical activity, chronic fatigue, coffee consumption, elevated triglycerides, insulin-resistance diabetes, taking estrogen, eating a high-protein diet, suffering from sleep disturbances, and depression.[13]
In this study, we have used the high-sensitivity CRP assay kit to determine the values of CRP as low as possible (i.e. <1 mg/l). In the past, turbidimetric immuno-assay has been used, but with the detection limit of >3 mg/l. However, in this study, we have used a PETIA kit with the detection limit of even <1 mg/l. This technique provides the quantitative immuno-assay and is regarded as the most rapid, sophisticated, accurate and sensitive method of detecting, and measuring CRP. Furthermore, the extended upper assay limit decreases the need for retesting the post-diluted samples.[14] The added advantage of this technique is that it is less expensive as compared with the ELISA or nephelometry techniques.[15]
The results of our study were in accordance with the findings of the earlier studies.[1,16] In another study, a median CRP of 2 mg/l for periodontitis patients, 0 mg/l among controls was estimated.[17] However, a study has reported the highest CRP values in patients with a generalized form of periodontitis (median 1.45 mg/l); for patients with a more localized form of periodontal disease, the median CRP value was 1.30 mg/l, while healthy controls presented with a median of 0.90 mg/l.[9]
In this study, GI has shown a positive correlation with the levels of CRP in chronic periodontitis group as compared with the gingivitis group and the healthy controls suggesting that increase in the microbial colonization leads to the increased levels of CRP in serum. Furthermore, GBI has shown a positive correlation with the CRP levels in chronic periodontitis group as compared with the gingivitis group and the healthy controls, which proves that the severity and the extent of the infection causes the rise in the levels of CRP. While PI has shown a negative correlation with the CRP levels in all the three groups. Along with this, CRP levels increased with the increase in the PD and CAL in all the patients of chronic periodontitis group as compared with the healthy controls. This is in accordance with the studies conducted in past where they found a positive correlation between CRP and periodontitis patients with probing pocket depth of ≥4 mm.[18,19]
In some studies relatively high levels of CRP was observed as compared to our study both in chronic periodontitis and healthy patients. This discrepancy could be caused by investigation of more severe cases of periodontitis in their study.[20,21]
Alveolar bone loss is not always related to current inflammation in the periodontium; however, it represents the degree of periodontal destruction directly. It was observed that ABL around posterior teeth was associated with elevated level of CRP.[22] Furthermore, it was found that the hs-CRP level was above 10.0 mg/l in all the patients in which evidence of significant ABL was present indicating periodontitis.[23] Thus, the level of CRP tends to increase with the periodontal destruction marked by ABL.
Greatest common factor (GCF) can also be used to assess the CRP level,[24] but failed to show any relation between GCF hs-CRP and the clinical parameters. The possible reasons for this may be that the CRP levels increase in serum within 24-48 h following acute tissue damage and decrease with the resolution of inflammation. Unlike GCF, serum CRP can be measured easily because of its long plasma half-life (12-18 h). Another possibility is that very small amount of GCF is obtained, which might be difficult for its quantitative assessment.
Some confounding factors such as age, gender, presence of systemic diseases, smoking, pregnancy, any antimicrobial treatment, etc., may influence the result of the study. The hidden factors such as genetics and undiagnosed conditions were not taken into cognizance in this study.
The potential effect of smoking has also been illustrated, and it was found that a median CRP concentration of nonsmoking patients with periodontitis was 2 mg/l, compared to a median of 0 mg/l for nonsmoking matched controls and a median CRP of around 2 mg/l regardless of their periodontal status.[17]
In this study, low levels of CRP were observed in the gingivitis group as compared with the chronic periodontitis group. This observation was in accordance with the previous study, where the levels of CRP were significantly lower in the gingivitis patients as compared with the periodontitis patients.[20] The hypothetical explanation for this might be that only the long-term chronic effects of periodontitis reflected as ABL and noticeable on radiographs are explanatory to elevated serum hs-CRP values, whereas current clinical evidence of gingivitis and/or an elevated proportion of increased PDs may be a current low-grade expression of inflammation not directly reflected by elevated hs-CRP values.
While further research is needed to elucidate the causal mechanisms that are responsible for this observed association between oral conditions and systemic CRP, there are three main implications to be drawn from the current findings. First, periodontal disease needs to be viewed more broadly in terms of systemic inflammation, either as a consequence of an underlying hyper-inflammatory trait or as a factor contributing to systemic inflammation. Second, there is a complementary need to recognize that “apparently healthy” individuals may nonetheless have extensive oral disease and raised CRP levels. Hence, oral disease assessments and systemic inflammatory response need to be incorporated into the signs and symptoms that define patients’ overall health status.
Clinical relevance
Not many studies have been conducted to estimate the level of CRP in gingivitis patients; an attempt was made to assess and compare the levels of hs-CRP with patients of healthy gingiva and chronic periodontitis and correlate it with all the clinical parameters considered. The elevated inflammatory factors may increase inflammatory activity in atherosclerotic lesions and potentially increasing the risk for cardiovascular events. Therefore, it could be concluded that inflammatory condition of the gingiva that is, gingivitis might result in elevated levels of CRP, but this level might increase to a much higher level if accompanied by ABL.
CONCLUSION
Through this study, it could be concluded that the level of CRP increases subsequently with the severity of the periodontal disease. However, it failed to prove the rise in the CRP levels with the increase in the severity of the gingival disease that is, gingivitis. Hence, patients with the periodontal infection have an elevated level of CRP and are at a greater risk of future CHD.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, De Nardin E. Periodontal infections contribute to elevated systemic C-reactive protein level. J Periodontol. 2001;72:1221–7. doi: 10.1902/jop.2000.72.9.1221. [DOI] [PubMed] [Google Scholar]
- 2.Keles GC, Cetinkaya BO, Simsek SB, Koprulu D, Kahraman H. The role of periodontal disease on acute phase proteins in patients with coronary heart disease and diabetes. Turk J Med Sci. 2007;37:39–44. [Google Scholar]
- 3.Tillett WS, Francis T. Serological reactions in pneumonia with a non-protein somatic fraction of pneumococcus. J Exp Med. 1930;52:561–71. doi: 10.1084/jem.52.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black S, Kushner I, Samols D. C-reactive Protein. J Biol Chem. 2004;279:48487–90. doi: 10.1074/jbc.R400025200. [DOI] [PubMed] [Google Scholar]
- 5.Gomes-Filho IS, Freitas Coelho JM, da Cruz SS, Passos JS, Teixeira de Freitas CO, Aragão Farias NS, et al. Chronic periodontitis and C-reactive protein levels. J Periodontol. 2011;82:969–78. doi: 10.1902/jop.2010.100511. [DOI] [PubMed] [Google Scholar]
- 6.López R, Baelum V, Hedegaard CJ, Bendtzen K. Serum levels of C-reactive protein in adolescents with periodontitis. J Periodontol. 2011;82:543–9. doi: 10.1902/jop.2010.100334. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation. 1998;97:425–8. doi: 10.1161/01.cir.97.5.425. [DOI] [PubMed] [Google Scholar]
- 8.Wu T, Trevisan M, Genco RJ, Falkner KL, Dorn JP, Sempos CT. Examination of the relation between periodontal health status and cardiovascular risk factors: Serum total and high density lipoprotein cholesterol, C-reactive protein, and plasma fibrinogen. Am J Epidemiol. 2000;151:273–82. doi: 10.1093/oxfordjournals.aje.a010203. [DOI] [PubMed] [Google Scholar]
- 9.Loos BG, Craandijk J, Hoek FJ, Wertheim-van Dillen PM, van der Velden U. Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. J Periodontol. 2000;71:1528–34. doi: 10.1902/jop.2000.71.10.1528. [DOI] [PubMed] [Google Scholar]
- 10.Gani DK, Lakshmi D, Krishnan R, Emmadi P. Evaluation of C-reactive protein and interleukin-6 in the peripheral blood of patients with chronic periodontitis. J Indian Soc Periodontol. 2009;13:69–74. doi: 10.4103/0972-124X.55840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun XJ, Meng HX, Shi D, Xu L, Zhang L, Chen ZB, et al. Elevation of C-reactive protein and interleukin-6 in plasma of patients with aggressive periodontitis. J Periodontal Res. 2009;44:311–6. doi: 10.1111/j.1600-0765.2008.01131.x. [DOI] [PubMed] [Google Scholar]
- 12.Boucher NE, Jr, Hanrahan JJ, Kihara FY. Occurrence of C-reactive protein in oral disease. J Dent Res. 1967;46:624. doi: 10.1177/00220345670460033001. [DOI] [PubMed] [Google Scholar]
- 13.Graziani F, Cei S, Tonetti M, Paolantonio M, Serio R, Sammartino G, et al. Systemic inflammation following non-surgical and surgical periodontal therapy. J Clin Periodontol. 2010;37:848–54. doi: 10.1111/j.1600-051X.2010.01585.x. [DOI] [PubMed] [Google Scholar]
- 14.Wei TQ, Kramer S, Chu VP, Hudson D, Kilgore D, Salyer S, et al. An improved automated immunoassay for C-reactive protein on the Dimension clinical chemistry system. J Autom Methods Manag Chem. 2000;22:125–31. doi: 10.1155/S1463924600000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hengst JM. The role of C-reactive protein in the evaluation and management of infants with suspected sepsis. Adv Neonatal Care. 2003;3:3–13. doi: 10.1053/adnc.2003.50010. [DOI] [PubMed] [Google Scholar]
- 16.Slade GD, Offenbacher S, Beck JD, Heiss G, Pankow JS. Acute-phase inflammatory response to periodontal disease in the US population. J Dent Res. 2000;79:49–57. doi: 10.1177/00220345000790010701. [DOI] [PubMed] [Google Scholar]
- 17.Fredriksson MI, Figueredo CM, Gustafsson A, Bergström KG, Asman BE. Effect of periodontitis and smoking on blood leukocytes and acute-phase proteins. J Periodontol. 1999;70:1355–60. doi: 10.1902/jop.1999.70.11.1355. [DOI] [PubMed] [Google Scholar]
- 18.Ide M, McPartlin D, Coward PY, Crook M, Lumb P, Wilson RF. Effect of treatment of chronic periodontitis on levels of serum markers of acute-phase inflammatory and vascular responses. J Clin Periodontol. 2003;30:334–40. doi: 10.1034/j.1600-051x.2003.00282.x. [DOI] [PubMed] [Google Scholar]
- 19.D’Aiuto F, Ready D, Tonetti MS. Periodontal disease and C-reactive protein-associated cardiovascular risk. J Periodontal Res. 2004;39:236–41. doi: 10.1111/j.1600-0765.2004.00731.x. [DOI] [PubMed] [Google Scholar]
- 20.Ebersole JL, Machen RL, Steffen MJ, Willmann DE. Systemic acute-phase reactants, C-reactive protein and haptoglobin, in adult periodontitis. Clin Exp Immunol. 1997;107:347–52. doi: 10.1111/j.1365-2249.1997.270-ce1162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slade GD, Ghezzi EM, Heiss G, Beck JD, Riche E, Offenbacher S. Relationship between periodontal disease and C-reactive protein among adults in the Atherosclerosis Risk in Communities study. Arch Intern Med. 2003;163:1172–9. doi: 10.1001/archinte.163.10.1172. [DOI] [PubMed] [Google Scholar]
- 22.Saito T, Murakami M, Shimazaki Y, Oobayashi K, Matsumoto S, Koga T. Association between alveolar bone loss and elevated serum C-reactive protein in Japanese men. J Periodontol. 2003;74:1741–6. doi: 10.1902/jop.2003.74.12.1741. [DOI] [PubMed] [Google Scholar]
- 23.Persson GR, Pettersson T, Ohlsson O, Renvert S. High-sensitivity serum C-reactive protein levels in subjects with or without myocardial infarction or periodontitis. J Clin Periodontol. 2005;32:219–24. doi: 10.1111/j.1600-051X.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- 24.Tüter G, Kurtis B, Serdar M. Evaluation of gingival crevicular fluid and serum levels of high-sensitivity C-reactive protein in chronic periodontitis patients with or without coronary artery disease. J Periodontol. 2007;78:2319–24. doi: 10.1902/jop.2007.070150. [DOI] [PubMed] [Google Scholar]


