Abstract
Background:
The purpose of this prospective study was to report the long-term risks of rotator cuff tear enlargement and symptom progression associated with degenerative asymptomatic tears.
Methods:
Subjects with an asymptomatic rotator cuff tear in one shoulder and pain due to rotator cuff disease in the contralateral shoulder enrolled as part of a prospective longitudinal study. Two hundred and twenty-four subjects (118 initial full-thickness tears, fifty-six initial partial-thickness tears, and fifty controls) were followed for a median of 5.1 years. Validated functional shoulder scores were calculated (visual analog pain scale, American Shoulder and Elbow Surgeons [ASES], and simple shoulder test [SST] scores). Subjects were followed annually with shoulder ultrasonography and clinical evaluations.
Results:
Tear enlargement was seen in 49% of the shoulders, and the median time to enlargement was 2.8 years. The occurrence of tear-enlargement events was influenced by the severity of the final tear type, with enlargement of 61% of the full-thickness tears, 44% of the partial-thickness tears, and 14% of the controls (p < 0.05). Subject age and sex were not related to tear enlargement. One hundred subjects (46%) developed new pain. The final tear type was associated with a greater risk of pain development, with the new pain developing in 28% of the controls, 46% of the shoulders with a partial-thickness tear, and 50% of those with a full-thickness tear (p < 0.05). The presence of tear enlargement was associated with the onset of new pain (p < 0.05). Progressive degenerative changes of the supraspinatus muscle were associated with tear enlargement, with supraspinatus muscle degeneration increasing in 4% of the shoulders with a stable tear compared with 30% of the shoulders with tear enlargement (p < 0.05). Nine percent of the shoulders with a stable tear showed increased infraspinatus muscle degeneration compared with 28% of those in which the tear had enlarged (p = 0.07).
Conclusions:
This study demonstrates the progressive nature of degenerative rotator cuff disease. The risk of tear enlargement and progression of muscle degeneration is greater for shoulders with a full-thickness tear, and tear enlargement is associated with a greater risk of pain development across all tear types.
Level of Evidence:
Prognostic Level II. See Instructions for Authors for a complete description of levels of evidence.
Quantifying the risk and rate of rotator cuff tear progression is of fundamental importance for formulating appropriate surgical indications. Despite numerous reports pertaining to the prevalence of asymptomatic rotator cuff tears1-7, we are not aware of any long-term longitudinal studies defining the natural history of degenerative rotator cuff tears. Previous studies have suggested the progressive nature of degenerative rotator cuff tears in both asymptomatic and painful shoulders8-12; however, these studies were often retrospective with short-term follow-up.
The indications for the surgical treatment of rotator cuff tears have varied widely13. Early surgical intervention for painful tears is intended to prevent the tear enlargement and muscle degeneration that can occur with nonoperative treatment as well as the diminished potential for tendon healing with delayed surgery14-19. A more thorough understanding of the natural history of rotator cuff disease can increase the ability to identify patients and shoulders at risk for disease progression and refine surgical indications. Furthermore, patients can be more effectively counseled regarding the potential risks of nonoperative treatment. Although recent studies have improved our understanding of the evolution of rotator cuff disease8-11, many questions remain unanswered.
Accurate characterization of the natural history of degenerative cuff disease requires a well-controlled prospective longitudinal follow-up study design with well-defined end points, particularly since the factors related to tear progression and the relationships of tear progression to pain development have not been clearly defined. Asymptomatic degenerative rotator cuff tears represent an ideal cohort for studying the natural history of cuff disease, as clinical intervention is not necessitated by pain, and the recognized potential for later symptomatic change8,9 suggests an appropriate model. The purpose of this study was to report the risk of symptom development, tear size progression, and muscle degeneration in a cohort of subjects with an asymptomatic rotator cuff tear and analyze factors associated with these changes.
Materials and Methods
Our university institutional review board approved this study. This study cohort consisted of subjects with an asymptomatic rotator cuff tear who presented for evaluation of shoulder pain secondary to rotator cuff disease in the contralateral shoulder8. All subjects had physical findings in the painful shoulder that were consistent with rotator-cuff-based pain and nearly all had an ultrasound-proven cuff tear in the painful shoulder. Inclusion criteria were (1) bilateral shoulder ultrasonography performed to investigate unilateral shoulder pain, (2) painful rotator cuff disease in the symptomatic shoulder, (3) a rotator cuff tear in the asymptomatic shoulder at the time of study enrollment, and (4) no history of trauma to either shoulder and no traumatic episode throughout the study period. Control subjects with no ultrasound evidence of a rotator cuff tear in one shoulder and painful cuff disease in the contralateral shoulder were also enrolled during the same time period. Exclusion criteria were (1) any past or current pain in the “asymptomatic” shoulder, (2) continuous use of narcotic or nonsteroidal anti-inflammatory drugs (NSAIDs) in the three months prior to enrollment, (3) a traumatic episode affecting the asymptomatic shoulder, (4) inflammatory arthritis, (5) radiographic evidence of osteoarthritis in the asymptomatic shoulder, (6) upper-extremity weight-bearing demands, (7) a subscapularis tendon tear in the asymptomatic shoulder, and (8) a very small (<5 mm) partial-thickness tear in the asymptomatic shoulder.
Study Protocol
Subjects were enrolled from the clinical practices of three surgeons over a thirty-month period. A priori power calculations were performed to determine the sample size needed for acceptably precise estimated rates of tear enlargement and pain development in shoulders with an asymptomatic tear. With an enrollment of 265 subjects, a 30% dropout rate (185 subjects providing data), and the assumption that 39% of the subjects would experience tear enlargement during the follow-up periord20, the 95% confidence bounds around the estimated enlargement rate ranged from 32% to 46%, meaning that the estimates would be within 7% of the true value. With these assumptions, the sample size provided enlargement estimates within 8.5% and 11.5% of the true value for each group, respectively. Assuming that 51% of the subjects would develop pain, the target sample size would again achieve 95% bounds with 7% of the true rate (44% to 58%).
A trained research nurse performed a comprehensive physical examination of the asymptomatic shoulder consisting of goniometric assessment of its active range of motion. Assessment of isometric shoulder elevation strength (90° elevation in the scapular plane) and external rotation strength (with the shoulder adducted and in neutral rotation) was performed with an IsoBex dynamometer (Medical Device Solutions, Oberburg, Switzerland), with the final measurement the average of three repetitions.
Patient questionnaires consisting of a visual analog scale (VAS) ranging from 0 to 10 to rate average daily pain with use of whole integers, the American Shoulder and Elbow Surgeons (ASES) score, and the simple shoulder test (SST) with the score normalized to 100 points were completed. The patient was assessed for shoulder pain at study enrollment and during longitudinal follow-up, with the definition of pain consisting of any of the following: (1) shoulder pain ≥3 on the 10-point scale lasting six weeks or longer, (2) pain greater than that experienced as a part of daily living, (3) pain requiring narcotic or NSAID medications, (4) pain prompting evaluation by a physician, and (5) night pain affecting sleep. Annual (plus or minus three months) examinations were performed with all components of the initial evaluation repeated, including ultrasonography. Subjects were asked to contact the study coordinator if new shoulder pain developed between visits, and this prompted immediate repeat evaluation. Subjects were retained for ongoing evaluation regardless of pain development or tear enlargement.
Shoulder Ultrasonography and Tear Enlargement Criteria
Each subject underwent a standardized shoulder ultrasound study (see Appendix)21,22. The maximum anteroposterior dimension of the tear was measured on transverse views (perpendicular to the long axis of the cuff) and designated as the tear width. The maximum degree of retraction was measured on longitudinal views (parallel to the long axis of the cuff) and designated as the tear length. Tear length was measured from the tendon defect to the lateral edge of the normal tendon footprint on the greater tuberosity. In this study, “tear type” refers to the control, partial-thickness-tear, or full-thickness-tear category.
Tear enlargement was determined on the basis of sequential annual ultrasound reports. A full-thickness cuff tear was considered to have enlarged if its size had increased by ≥5 mm in any dimension compared with baseline. Five millimeters was chosen to account for the inherent variability in the accuracy of ultrasonography22 and to represent clinically meaningful enlargement. If the change in tear dimension met the criterion for enlargement but was a borderline value, subsequent ultrasound studies were reviewed to confirm that the size change was consistent with that seen on the previous ultrasound study. If the subsequent value did not confirm an increase from baseline values, the tear was not considered to have enlarged. Once a tear was confirmed to have enlarged, the new tear size was set as the new baseline for future comparisons. A partial-thickness tear was considered to have enlarged when it had converted to a full-thickness defect, defined as a complete disruption of tendon continuity at the insertion. In the control shoulders, enlargement was defined as the development of a partial or full-thickness defect of at least 5 mm in any dimension. Tear-size values were also analyzed to identify a decrease in size over time. A tear was considered to be smaller when a decrease of ≥5 mm in any dimension was identified and was corroborated on subsequent ultrasound examinations. Five subjects who had a massive tear that was too large to accurately measure at baseline were excluded from the tear-enlargement analysis.
Tear enlargement was defined as a change in the tear type or an increase in the size from baseline values. Once a tear progressed into a more advanced category (from control to partial or from partial to full), that tear was included in the more advanced group (now defined as the final tear type) for further monitoring of pain development (Table I).
TABLE I.
Baseline and Final Tear Types in the Study Cohort (N = 219)*
| Tear Type at Baseline |
||||
| Control | Partial-Thickness | Full-Thickness | Total | |
| Final tear type | ||||
| Control | 36 | 0 | 0 | 36 |
| Partial-thickness | 9 | 45 | 0 | 54 |
| Full-thickness | 5 | 11 | 113 | 129 |
| Total | 50 | 56 | 113 | 219 |
Baseline tear types were reclassified to a new, final tear type when a control was converted to a partial or full-thickness tear or a partial-thickness tear was converted to a full-thickness tear during the follow-up period.
Statistical Analysis
Categorical baseline characteristics were compared across baseline tear groups with use of the Fisher exact test. Continuous baseline characteristics were compared with analysis of variance followed by Tukey-adjusted post-hoc pairwise comparisons between tear groups. Variables that were not normally distributed were rank-transformed prior to analysis. To account for different durations of follow-up, separate univariable Cox proportional hazard regression models were used to determine if the risk factors for new pain development or degenerative rotator cuff muscle changes were associated with the time between study entry and the occurrence of tear enlargement. Subjects with a stable tear who underwent surgery were censored at the time of surgery, and subjects with a stable tear who were lost to follow-up were censored at their final visit. Hazard regression analysis was used to examine the association of demographic variables, tear type, new pain development, and progression of muscle degeneration with tear enlargement. The reference group for the hazard regression analysis for each categorical risk factor was the group of patients without the risk factor. Hazard regression analysis for continuous risk factors is expressed for a one-unit increase in the risk factor.
Without regard for the temporal relationship of events, the Cochran-Armitage trend test was used to determine if advancing category of final tear type was associated with new pain development. A Cox model was used to determine if the occurrence of tear enlargement was associated with the time between study entry and new pain development.
Kaplan-Meier survival curves were generated to describe the annual rate at which (1) tear enlargement occurred according to tear type and (2) new pain occurred. Annual survival rates are reported for the time between study entry and the outcome at each follow-up point through year five.
For patients who developed pain before the occurrence of tear enlargement, an unpaired t test or the Wilcoxon signed rank test was performed to determine if a change in function occurred between baseline and the first occurrence of pain.
Summary data for normally distributed variables are presented as the mean and standard deviation (SD), and data for variables that are not normally distributed are presented as the median and interquartile range (IQR). Categorical variables are presented as the number of subjects and the percentage of the specified group. P values of <0.05 were considered significant.
Source of Funding
The study was funded by the National Institutes of Health (R01 AR051026).
Results
Baseline Demographics
Two hundred and fifty subjects were enrolled, and twenty-six of them were lost to follow-up or later excluded during the study. Of the 224 subjects who remained, 118 (53%) had a full-thickness tear, fifty-six (25%) had a partial-thickness tears, and fifty (22%) were control subjects. The age and sex distributions were similar among the baseline tear types (Table II). At baseline, the full-thickness tears were significantly larger than the partial-thickness tears (median, 11.0 mm versus 6.0 mm, p < 0.05) and significantly larger in area (p < 0.05); however, the difference in tear width did not reach significance (median, 10.0 mm versus 9.0 mm, p = 0.052). A greater proportion of full-thickness tears were associated with degenerative changes in the supraspinatus (p < 0.05) and infraspinatus (p < 0.05) muscles. Baseline SST (p < 0.05) and ASES (p < 0.05) scores worsened with advancing tear type; however, no significant differences were seen in baseline pain scores or strength among the tear groups.
TABLE II.
Demographics and Baseline Characteristics of the Study Cohort
| P Value |
||||||||
| Variable (Baseline) | Entire Cohort (N = 224*) | Control (N = 50*) | Partial-Thickness Tear (N = 56*) | Full-Thickness Tear (N = 118*) | Comparison Across Tear Groups | Control Vs. Full-Thickness | Partial Vs. Full-Thickness | Control Vs. Partial-Thickness |
| Age† (yr) | 62.0 ± 10 | 60.7 ± 10 | 59.4 ± 10 | 63.8 ± 9 | ||||
| Female sex (no. [%]) | 92 (41%) | 22 (44%) | 26 (46%) | 44 (37%) | ||||
| Dominant side asymptomatic (no. [%]) | 81 (36%) | 14 (28%) | 17 (30%) | 50 (42%) | ||||
| Full-thickness tear size (no.) | 31 small, 74 medium, 10 large, 3 massive | |||||||
| Tear length‡ (mm) | Not applic. | Not applic. | 6.0 (2.5) | 11.0 (8.0), n = 114 | <0.05#** | |||
| Tear width‡ (mm) | Not applic. | Not applic. | 9.0 (4.0) | 10.0 (7.0), n = 113 | 0.052#** | |||
| Tear area‡ (mm2) | Not applic. | Not applic. | 64.0 (49) | 108 (164), n = 111 | <0.05#** | |||
| Muscle degeneration§ (no. [%]) | ||||||||
| Supraspinatus | 13/162 (8%) | 1/46 (2%) | 0/36 (0%) | 12/80 (15%) | <0.05†† | <0.05 | <0.05 | |
| Infraspinatus | 11/163 (7%) | 1/46 (2%) | 0/37 (0%) | 10/80 (13%) | <0.05†† | 0.055 | <0.05 | |
| Supraspinatus or infraspinatus | 16/162 (10%) | 1/46 (2%) | 0/36 (0%) | 15/80 (19%) | <0.05†† | <0.05 | <0.05 | |
| VAS pain score‡ | 1.0 (0) | 1.0 (0) | 1.0 (0) | 1.0 (0) | 0.30#** | |||
| ASES score‡ (points) | 98.3 (10), n = 223 | 100 (0) | 98.3 (10) | 96.3 (12) | <0.05#** | <0.05 | ||
| SST score‡ (points) | 91.7 (27), n = 218 | 100 (0) | 91.7 (33) | 87.1 (33) | <0.05#** | <0.05 | 0.05 | |
| Elevation strength† (N) | 55.8 ± 28, n = 115 | 58.3 ± 27 | 55.4 ± 28 | 53.3 ± 29 | 0.71# | |||
| External rotation strength† (N) | 68.8 ± 32, n = 220 | 70.7 ± 26 | 73.5 ± 33 | 65.7 ± 34 | 0.30# | |||
When data were available for less than the entire cohort, the sample size (n) or the denominator is also provided.
The values are given as the mean and standard deviation.
The values are given as the median with the interquartile range in parentheses.
Fatty infiltration was identified by rating architecture and echogenicity on a 0 to 2-point scale and summing the two scores. Values of >0 indicate the presence of fatty infiltration.
Analysis of variance was used to compare the tear-type groups and, when the overall model was significant (p < 0.05), Tukey-adjusted pairwise comparisons were performed.
The analysis was performed with use of rank-transformed data.
The analysis was performed with the Fisher exact test.
Tear Progression
The median duration of follow-up was 5.1 years (range, 0.3 to 10.0 years). The median duration of study inclusion was longer for the tears that enlarged (5.9 years) than for the stable tears (4.9 years, p < 0.05). Tear progression was seen in 49% of the shoulders, and the median time to enlargement was 2.8 years. The risk of tear enlargement was significantly influenced by the severity of the final tear type (Table III), with tear-enlargement rates of 61% in the full-thickness-tear group, 44% in the partial-thickness-tear group, and 14% in the control group (p < 0.05). Cox regression analysis showed the risk of enlargement in the full-thickness-tear group to be 4.2 times greater than that in the control group and 1.5 times larger than that in the partial-thickness-tear group. Three partial-thickness and six full-thickness tears decreased in size compared with baseline. Four of these tears (all full-thickness) later enlarged compared with the original values.
TABLE III.
Risk Factors for Tear Progression*
| Risk Factor | No Enlargement (N = 111) | Enlargement (N = 108) | P Value from Cox Regression for Enlargement† |
| Tear type‡ | P < 0.05 Full vs. control: p < 0.05, HR = 4.17 Partial vs. control: p < 0.05, HR = 2.73 Full vs. partial: p = 0.07, HR = 1.53 |
||
| Control | 31 (86%) | 5 (14%), time to enlargement: median, 2.2 yr (IQR, 1.7) | |
| Partial-thickness | 30 (56%) | 24 (44%), time to enlargement: median, 3.3 yr (IQR, 3.1) | |
| Full-thickness | 50 (39%) | 79 (61%), time to enlargement: median, 2.3 yr (IQR, 3.7) | |
| Mean baseline age (±SD) (yr) | 62.4 ± 10 | 61.4 ± 10 | |
| Sex‡ | HR = 0.99 (0.97-1.01)§ | ||
| Male | 61 (48%) | 67 (52%) | |
| Female | 50 (55%) | 41 (45%) | |
| Study side dominant‡ | P < 0.05, HR = 1.53 | ||
| No | 82 (58%) | 59 (42%) | |
| Yes | 29 (37%) | 49 (63%) | |
| Smoking status‡ | Previous vs. never: HR = 0.97 (0.65-1.45)§ Current vs. never: HR = 0.90 (0.39-2.09)§ |
||
| Never | 59 (48%) | 63 (52%) | |
| Previous | 41 (51%) | 39 (49%) | |
| Current | 11 (65%) | 6 (35%) |
Five massive tears were excluded from the analysis because they were too large for enlargement to be documented accurately.
HR = hazard ratio. The numbers in parentheses after the hazard ratios represent the 95% confidence intervals for the hazard ratios.
The values are given as the number of subjects with the percentage of the risk-factor group in parentheses.
A p value was not assigned because of a lack of power analysis for the variable.
If the study shoulder was the dominant extremity, there was a greater likelihood of tear enlargement (63% in dominant shoulders compared with 42% in nondominant shoulders, p < 0.05). We found no clinically relevant differences in age, sex, or smoking status between stable and enlarged tears.
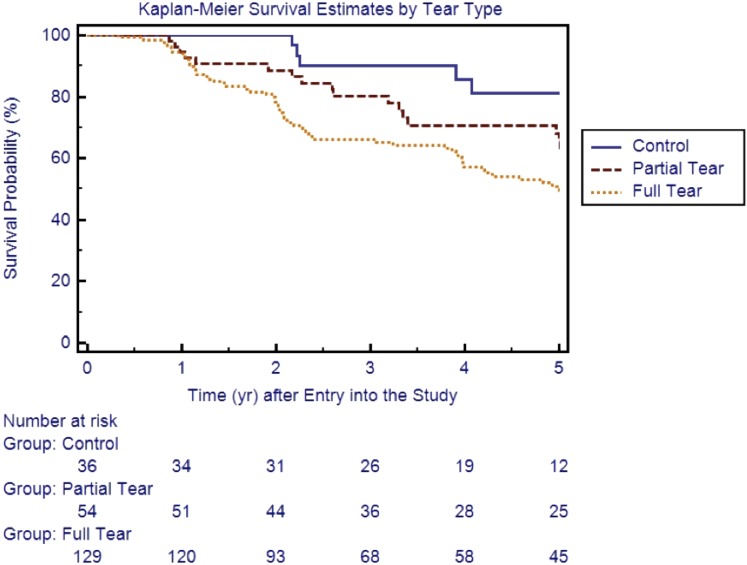
Survivorship Analysis
The median time to enlargement was 2.2, 3.3, and 2.3 years for the control, partial-thickness, and full-thickness groups, respectively. Figure 1 shows the five-year survivorship of the three groups with tear enlargement as the end point. Survivorship was 100% at one and two years, 90% at three years, 86% at four years, and 81% at five years in the control group. The yearly survivorship values (for years one through five) were 94%, 89%, 80%, 71%, and 65% in the partial-thickness group and 95%, 78%, 66%, 57%, and 50% in the full-thickness group.
Fig. 1.
Kaplan-Meier annual survival curves for tear enlargement by final tear type.
Symptom Progression
One hundred shoulders (46%) developed new pain (Table IV). The median time to pain development was 2.6 years. In the entire cohort, the annual survivorship values (for years one through five) with the development of new pain as the end point were 94%, 82%, 72%, 63%, and 56%. A greater risk for pain development was associated with a more advanced final tear type (p < 0.05). Twenty-eight percent of the controls, 46% of the patients with a partial-thickness tear, and 50% of those with a full-thickness tear developed new pain.
TABLE IV.
Association Between Final Tear Type and the Development of Pain
| Final Tear Type* |
||||
| Variable | Control (N = 36) | Partial-Thickness (N = 54) | Full-Thickness (N = 129) | P Value from Cochran-Armitage Trend Test |
| New pain | 10 (28%) | 25 (46%) | 65 (50%) | <0.05 |
| Remained asymptomatic (ref.) | 26 (72%) | 29 (54%) | 64 (50%) | |
The values are given as the number of subjects with the percentage of the tear-type group in parentheses.
Tear enlargement was a significant risk factor for the development of shoulder pain (p < 0.05) (Table V). The tear enlarged in 38% of the shoulders that remained asymptomatic compared with 63% of those that developed pain. Cox regression analysis demonstrated a 1.66 times higher prevalence of tear enlargement in shoulders that developed pain compared with shoulders that remained asymptomatic. There was a 1.69 times higher prevalence of new pain in the shoulders in which the tear enlarged compared with the shoulders with a stable tear (p < 0.05). Of the sixty-three shoulders in which the tear enlarged and became painful, forty-one became painful at or before the time of the initial enlargement and twenty-two, at a later time point.
TABLE V.
Association Between Development of New Pain and Tear Enlargement
| Variable | No Enlargement* (N = 111) | Enlargement* (N = 108) | P Value from Cox Regression for Enlargement† |
| New pain | 37 (37%) | 63 (63%): 41 at/before 1st enlargement, 22 after 1st enlargement | P < 0.05, HR = 1.66 |
| Remained asymptomatic (ref.) | 74 (62%) | 45 (38%) |
The values are given as the number of subjects with the percentage of the pain group in parentheses.
HR = hazard ratio.
Muscle Degenerative Changes
Complete assessment of muscle degenerative changes was done for 159 shoulders. Tear enlargement was significantly associated with progressive degenerative changes in the supraspinatus muscle (Table VI). Four percent of the stable tears were associated with an increase in supraspinatus degeneration (of at least one grade) compared with 30% of the tears that enlarged (p < 0.05). Nine percent of the shoulders with a stable tear showed an increase in infraspinatus degeneration compared with 28% of the shoulders with tear enlargement (p = 0.07).
TABLE VI.
Association Between Degenerative Rotator Cuff Muscle Changes and Tear Enlargement
| Variable | No Enlargement* (N = 77) | Enlargement* (N = 82) | P Value from Cox Regression for Enlargement† |
| Increased muscle degeneration | |||
| Supraspinatus | <0.05, HR = 2.00 | ||
| No (ref.) | 74 (96%) | 57 (70%) | |
| Yes | 3 (4%) | 25 (30%) | |
| Infraspinatus | 0.07 | ||
| No (ref.) | 70 (91%) | 59 (72%) | |
| Yes | 7 (9%) | 23 (28%) | |
| Supraspinatus or infraspinatus | <0.05, HR = 1.91 | ||
| No (ref.) | 69 (90%) | 47 (57%) | |
| Yes | 8 (10%) | 35 (43%) |
The values are given as the number of subjects with the percentage of the no-enlargement or enlargement group in parentheses.
HR = hazard ratio.
Shoulder Function
Nearly all shoulders with new pain showed a significant decline in function from baseline values (Table VII). The median VAS pain score increased by 3.0 points (p < 0.05), and the median ASES and SST scores decreased by 31.9 (p < 0.05) and 14.8 (p < 0.05) points, respectively.
TABLE VII.
Changes in Function Between Baseline and the Onset of New Pain
| Visit* | Entire Cohort (N = 80: 10 Controls, 20 Partial-Thickness Tears, 50 Full-Thickness Tears) | P Value† |
| VAS score for pain (0 = no pain, 10 = incapacitating pain)‡ | <0.05§ | |
| Baseline | 1.0 (0.0) | |
| 1st occurrence of pain | 5.0 (3.0) | |
| Delta | 3.0 (3.0) | |
| SST‡ | <0.05 | |
| Baseline | 83.3 (34.8) | |
| 1st occurrence of pain | 69.7 (33.3) | |
| Delta | −14.8 (33.2) | |
| ASES‡ | <0.05 | |
| Baseline | 96.5 (11.7) | |
| 1st occurrence of pain | 60.0 (26.5) | |
| Delta | −31.9 (27.0) | |
| External rotation strength# (N) | <0.05 | |
| Baseline | 68.4 ± 34 | |
| 1st occurrence of pain | 58.2 ± 30 | |
| Delta | −10.2 ± 25 | |
| Elevation strength# (N) | <0.05 | |
| Baseline | 55.4 ± 26 | |
| 1st occurrence of pain | 33.3 ± 20 | |
| Delta | −22.1 ± 18 | |
| Forward elevation—active# (deg) | <0.05§ | |
| Baseline | 154 ± 14 | |
| 1st occurrence of pain | 145 ± 20 | |
| Delta | −9.2 ± 25 | |
| External rotation at 90°—active# (deg) | <0.05 | |
| Baseline | 91.1 ± 12 | |
| 1st occurrence of pain | 82.2 ± 13 | |
| Delta | −8.9 ± 16 | |
| Internal rotation in abduction—active# (deg) | 0.24 | |
| Baseline | 65.9 ± 20 | |
| 1st occurrence of pain | 62.7 ± 17 | |
| Delta | −3.2 ± 24 | |
| External rotation with arm at side—active# (deg) | <0.05 | |
| Baseline | 72.4 ± 18 | |
| 1st occurrence of pain | 62.8 ± 14 | |
| Delta | −9.6 ± 23 |
Delta for each patient was calculated by subtracting the baseline value from the value at the first occurrence of pain. The average time between these visits was 987 ± 705 days (range, 161 to 2881 days).
The p values were derived by comparing delta with zero in paired t tests unless otherwise indicated.
The values are given as the median with the interquartile range in parentheses.
The p value was derived with the Wilcoxon signed rank test.
The values are given as the mean and standard deviation.
Discussion
The results of this prospective longitudinal study demonstrated a high risk of rotator cuff tear enlargement over a relatively short time period. The most clinically relevant relationship was between a greater risk of tear progression and a more severe tear type. Full-thickness tears were 4.2 times and 1.5 times more likely to enlarge than controls and partial-thickness tears, respectively. Our data suggest that activity level may influence tear progression given the higher rate of enlargement seen in dominant shoulders. We found a greater risk of pain development in shoulders in which the tear enlarged compared with those in which the tear remained stable.
Natural history studies are important for defining the risks and rates of disease progression over time, and they allow better assessment of the appropriate timing of intervention and identification of specific at-risk groups in which early intervention may be most beneficial. Natural history information about degenerative rotator cuff disease is lacking in comparison with the large volume of data dedicated to the outcomes of various treatments, usually surgical intervention. Repeated longitudinal assessments provide clearer estimates of the timeline for tear progression and allow closer monitoring of symptom progression and subsequent changes in shoulder function.
The rate of progression of asymptomatic cuff tears in this study is similar to rates in two previous studies of conservatively treated painful cuff tears10,11. Using ultrasound and similar definitions of tear enlargement, Safran et al. showed that 49% of painful full-thickness cuff tears enlarged after a mean of twenty-nine months of follow-up11. Maman et al. examined tear progression with magnetic resonance imaging (MRI) and noted that 48% of painful tears reexamined at more than eighteen months had enlarged compared with 19% of tears followed for less than eighteen months10. Similar to our findings, the risk of progression was lower for partial-thickness tears than for full-thickness tears10. The increased risk of progression of full-thickness tears highlights the need for either close surveillance or surgical intervention for painful shoulders in this higher-risk group.
The relatively low prevalence of muscle degeneration in our study may be a reflection of the small size of the typical full-thickness tear in this cohort. Nevertheless, the results demonstrated that progressive muscle degeneration is associated with progression of even smaller tears. In two previous studies, no correlation was seen between enlargement of painful tears and progression of muscle degeneration, although trends were evident10,12. In both series the number of subjects, particularly those who developed atrophy, was small. It is likely that our findings differ because of our larger cohort, longer follow-up period, and prospective study design. The progression of muscle degeneration is a very relevant consideration when counseling subjects with an enlarging rotator cuff tear as both factors are associated with lower rates of tendon healing following surgery.
The onset of pain was associated with a clinically meaningful decline in shoulder function based on established minimal clinically important differences for shoulder pain and the ASES score; however, the decrease in the SST score was just below the established minimal clinically important difference for patients with rotator cuff disease23. A more severe tear type was associated with a higher risk of pain development. We chose to analyze the risk of pain development on the basis of the final tear type to more accurately reflect how these risks change as a tear evolves. In our previous study of this cohort, 23% of the subjects developed new pain, which was related to tear progression8. In the present study, we showed that tear progression was a significant risk factor for pain development, noting an even stronger relationship than was seen in our earlier study. These findings are similar to those in a recent study by Moosmayer et al., in which 36% of the subjects with an asymptomatic full-thickness tear developed pain over the course of short-term follow-up9. They also noted a greater progression of tear size in shoulders that became painful. The temporal relationship between enlargement and pain development warrants further analysis as pain occurred after the identification of initial tear enlargement in 35% of our patients who had both enlargement and pain development. We believe that the collective findings of these studies emphasize that symptom progression should be carefully evaluated as a possible clinical sign of tear progression.
Our study had several limitations. Our subjects had painful rotator cuff disease in the contralateral shoulder; therefore, findings may differ from those in subjects with unilateral disease. Nevertheless, we believe that the results of this study may represent the natural history of symptomatic cuff disease given the recognized bilateral nature of tears; our cohort was a true at-risk population. Another limitation is that the chronicity of the tears was unknown and it is possible that unknown differences in disease duration can influence the rates of tear progression and symptom development. Also, we did not analyze the potential relationships of other variables, such as occupation, medical comorbidities, and the tear size in the contralateral shoulder, to the risks of tear progression and pain development.
The evaluation of muscle degeneration may have been biased as muscle evaluation was not performed in the earlier part of the study. We began collecting muscle degeneration data after ultrasound findings were validated through a comparison with MRI findings24. Despite this shortcoming, we were able to clearly demonstrate progression of supraspinatus degeneration and a trend toward progression of infraspinatus degeneration in association with full-thickness tears that enlarged.
The strengths of this study are primarily related to its design, as it involved a large cohort of patients followed prospectively at well-defined time intervals to allow better definition of time-dependent tear progression. Use of a control group similar to the study group was an important reference with which to compare risks of tear progression in shoulders with tears. This study included a standardized and validated annual evaluation performed by trained research nurses, and radiologists conducted all ultrasound examinations to minimize observer bias. Strict definitions of tear enlargement and symptom development were designated prior to study initiation. Finally, categorization of final tear type better characterized the risks of tear progression and pain development as an asymptomatic tear evolves over time.
In conclusion, this prospective, longitudinal study demonstrated the progressive nature of asymptomatic degenerative rotator cuff tears in subjects with a contralateral symptomatic rotator cuff tear. The risk of enlargement is greater for more advanced tears, and tear enlargement is associated with a greater risk of cuff muscle degeneration and of pain development. New pain may be a clinical sign of tear progression.
Appendix
A detailed description of the ultrasound studies of the rotator cuff tears and the evaluation of the fatty degeneration of the rotator cuff muscles is available with the online version of this article as a data supplement at jbjs.org.
Footnotes
Investigation performed at the Shoulder and Elbow Service, Department of Orthopaedic Surgery, Washington University, St. Louis, Missouri
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. In addition, one or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. Also, one or more of the authors has had another relationship, or has engaged in another activity, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Moosmayer S, Smith HJ, Tariq R, Larmo A. Prevalence and characteristics of asymptomatic tears of the rotator cuff: an ultrasonographic and clinical study. J Bone Joint Surg Br. 2009February;91(2):196-200. [DOI] [PubMed] [Google Scholar]
- 2.Kim HM, Teefey SA, Zelig A, Galatz LM, Keener JD, Yamaguchi K. Shoulder strength in asymptomatic individuals with intact compared with torn rotator cuffs. J Bone Joint Surg Am. 2009February;91(2):289-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamaguchi K, Ditsios K, Middleton WD, Hildebolt CF, Galatz LM, Teefey SA. The demographic and morphological features of rotator cuff disease. A comparison of asymptomatic and symptomatic shoulders. J Bone Joint Surg Am. 2006August;88(8):1699-704. [DOI] [PubMed] [Google Scholar]
- 4.Schibany N, Zehetgruber H, Kainberger F, Wurnig C, Ba-Ssalamah A, Herneth AM, Lang T, Gruber D, Breitenseher MJ. Rotator cuff tears in asymptomatic individuals: a clinical and ultrasonographic screening study. Eur J Radiol. 2004September;51(3):263-8. [DOI] [PubMed] [Google Scholar]
- 5.Tempelhof S, Rupp S, Seil R. Age-related prevalence of rotator cuff tears in asymptomatic shoulders. J Shoulder Elbow Surg. 1999Jul-Aug;8(4):296-9. [DOI] [PubMed] [Google Scholar]
- 6.Milgrom C, Schaffler M, Gilbert S, van Holsbeeck M. Rotator-cuff changes in asymptomatic adults. The effect of age, hand dominance and gender. J Bone Joint Surg Br. 1995March;77(2):296-8. [PubMed] [Google Scholar]
- 7.Sher JS, Uribe JW, Posada A, Murphy BJ, Zlatkin MB. Abnormal findings on magnetic resonance images of asymptomatic shoulders. J Bone Joint Surg Am. 1995January;77(1):10-5. [DOI] [PubMed] [Google Scholar]
- 8.Mall NA, Kim HM, Keener JD, Steger-May K, Teefey SA, Middleton WD, Stobbs G, Yamaguchi K. Symptomatic progression of asymptomatic rotator cuff tears: a prospective study of clinical and sonographic variables. J Bone Joint Surg Am. 2010November17;92(16):2623-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moosmayer S, Tariq R, Stiris M, Smith HJ. The natural history of asymptomatic rotator cuff tears: a three-year follow-up of fifty cases. J Bone Joint Surg Am. 2013July17;95(14):1249-55. [DOI] [PubMed] [Google Scholar]
- 10.Maman E, Harris C, White L, Tomlinson G, Shashank M, Boynton E. Outcome of nonoperative treatment of symptomatic rotator cuff tears monitored by magnetic resonance imaging. J Bone Joint Surg Am. 2009August;91(8):1898-906. [DOI] [PubMed] [Google Scholar]
- 11.Safran O, Schroeder J, Bloom R, Weil Y, Milgrom C. Natural history of nonoperatively treated symptomatic rotator cuff tears in patients 60 years old or younger. Am J Sports Med. 2011April;39(4):710-4 Epub 2011 Feb 10. [DOI] [PubMed] [Google Scholar]
- 12.Fucentese SF, von Roll AL, Pfirrmann CW, Gerber C, Jost B. Evolution of nonoperatively treated symptomatic isolated full-thickness supraspinatus tears. J Bone Joint Surg Am. 2012May2;94(9):801-8. [DOI] [PubMed] [Google Scholar]
- 13.Dunn WR, Schackman BR, Walsh C, Lyman S, Jones EC, Warren RF, Marx RG. Variation in orthopaedic surgeons’ perceptions about the indications for rotator cuff surgery. J Bone Joint Surg Am. 2005September;87(9):1978-84. [DOI] [PubMed] [Google Scholar]
- 14.Goutallier D, Postel JM, Gleyze P, Leguilloux P, Van Driessche S. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elbow Surg. 2003Nov-Dec;12(6):550-4. [DOI] [PubMed] [Google Scholar]
- 15.Liem D, Lichtenberg S, Magosch P, Habermeyer P. Magnetic resonance imaging of arthroscopic supraspinatus tendon repair. J Bone Joint Surg Am. 2007August;89(8):1770-6. [DOI] [PubMed] [Google Scholar]
- 16.Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007May;35(5):719-28 Epub 2007 Mar 2. [DOI] [PubMed] [Google Scholar]
- 17.Fuchs B, Gilbart MK, Hodler J, Gerber C. Clinical and structural results of open repair of an isolated one-tendon tear of the rotator cuff. J Bone Joint Surg Am. 2006February;88(2):309-16. [DOI] [PubMed] [Google Scholar]
- 18.Gerber C, Schneeberger AG, Hoppeler H, Meyer DC. Correlation of atrophy and fatty infiltration on strength and integrity of rotator cuff repairs: a study in thirteen patients. J Shoulder Elbow Surg. 2007Nov-Dec;16(6):691-6 Epub 2007 Oct 10. [DOI] [PubMed] [Google Scholar]
- 19.Tashjian RZ, Hollins AM, Kim HM, Teefey SA, Middleton WD, Steger-May K, Galatz LM, Yamaguchi K. Factors affecting healing rates after arthroscopic double-row rotator cuff repair. Am J Sports Med. 2010December;38(12):2435-42 Epub 2010 Oct 28. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi K, Tetro AM, Blam O, Evanoff BA, Teefey SA, Middleton WD. Natural history of asymptomatic rotator cuff tears: a longitudinal analysis of asymptomatic tears detected sonographically. J Shoulder Elbow Surg. 2001May-Jun;10(3):199-203. [DOI] [PubMed] [Google Scholar]
- 21.Teefey SA, Hasan SA, Middleton WD, Patel M, Wright RW, Yamaguchi K. Ultrasonography of the rotator cuff. A comparison of ultrasonographic and arthroscopic findings in one hundred consecutive cases. J Bone Joint Surg Am. 2000April;82(4):498-504. [PubMed] [Google Scholar]
- 22.Teefey SA, Rubin DA, Middleton WD, Hildebolt CF, Leibold RA, Yamaguchi K. Detection and quantification of rotator cuff tears. Comparison of ultrasonographic, magnetic resonance imaging, and arthroscopic findings in seventy-one consecutive cases. J Bone Joint Surg Am. 2004April;86(4):708-16. [PubMed] [Google Scholar]
- 23.Tashjian RZ, Deloach J, Green A, Porucznik CA, Powell AP. Minimal clinically important differences in ASES and simple shoulder test scores after nonoperative treatment of rotator cuff disease. J Bone Joint Surg Am. 2010February;92(2):296-303. [DOI] [PubMed] [Google Scholar]
- 24.Wall LB, Teefey SA, Middleton WD, Dahiya N, Steger-May K, Kim HM, Wessell D, Yamaguchi K. Diagnostic performance and reliability of ultrasonography for fatty degeneration of the rotator cuff muscles. J Bone Joint Surg Am. 2012June20;94(12):e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strobel K, Hodler J, Meyer DC, Pfirrmann CW, Pirkl C, Zanetti M. Fatty atrophy of supraspinatus and infraspinatus muscles: accuracy of US. Radiology. 2005November;237(2):584-9 Epub 2005 Sep 28. [DOI] [PubMed] [Google Scholar]