Abstract
Visceral leishmaniasis (VL) in Brazil is transmitted by the phlebotomine Lutzomyia longipalpis and in some midwestern regions by Lutzomyia cruzi. Studies of the phlebotomine fauna, feeding habits and natural infection rate by Leishmania contribute to increased understanding of the epidemiological chain of leishmaniases and their vectorial capacity. Collections were performed in Jaciara, state of Mato Grosso from 2010-2013, during which time 2,011 phlebotomines (23 species) were captured (68.70% Lu. cruzi and 20.52% Lutzomyia whitmani). Lu. cruzi females were identified by observing the shapes of the cibarium (a portion of the mouthpart) and spermatheca, from which samples were obtained for polymerase chain reaction to determine the rates of natural infection. Engorged phlebotomines were assessed to identify the blood-meal host by ELISA. A moderate correlation was discovered between the number of Lu. cruzi and the temperature and the minimum rate of infection was 6.10%. Twenty-two females were reactive to the antisera of bird (28%), dog (3.30%) and skunk (1.60%). We conclude that Lu. cruzi and Lu. whitmani have adapted to the urban environment in this region and that Lu. cruzi is the most likely vector of VL in Jaciara. Moreover, maintenance of Leishmania in the environment is likely aided by the presence of birds and domestic and synanthropic animals.
Keywords: visceral leishmaniasis, Lutzomyia sp, PCR, ELISA
Leishmaniases are diseases caused by parasites of the genus Leishmania, which are protozoans transmitted by phlebotomines that manifest clinically in three forms (cutaneous, mucocutaneous and visceral), depending on the parasite species. Visceral leishmaniasis (VL) is the most severe form, resulting in high mortality if it is not treated early, and it is caused by Leishmania infantum chagasi in the Americas (Harhay et al. 2011).
In Brazil, two species have been related to the transmission of VL, including Lutzomyia longipalpis and Lutzomyia cruzi, the former of which is considered the main vector and the latter of which appears in only some municipalities, where it most likely participates in the transmission cycle (Almeida et al. 2010). The species Lu. cruzi is morphologically related to Lu. longipalpis because the females of both are identical and the males can only be distinguished by small differences in genitalia (Young & Duncan 1994). Lu. cruzi has been detected in 24 municipalities in the state of Mato Grosso (MT) (Missawa & Lima 2006).
Identifying the natural infection rates of phlebotomines by Leishmania represents an important component of epidemiological studies of leishmaniases and their vectorial competence (Paiva et al. 2007). One technique involving dissection of the digestive system of the vector for direct research of the parasite has been commonly employed for this purpose; however, this method is laborious and time-consuming because it involves searching for the parasite in loco. Furthermore, in positive cases, the infection requires confirmation by in vitro Leishmania cultures, which are frequently prone to contamination or by the inoculation of laboratory animals because other flagellates are commonly found in the digestive tracts of these insect vectors (Michalsky et al. 2002). The development of molecular biology techniques used in association with polymerase chain reaction (PCR) has made the identification of even the smallest quantities of parasite genetic material possible, regardless of its stage and location within the digestive system of the vector insect (Oliveira-Pereira et al. 2006).
The eating habits of phlebotomines provide information on the identification of hosts, revealing the potential reservoirs of leishmaniases (Missawa et al. 2008), which may include rodents, marsupials, edentulous and canids in the wild (Ashford 2000) and domestic dogs in urban settings (Dantas-Torres 2007). The attraction that various wild and domestic animals exercise over the phlebotomines as food sources is an important factor contributing to the understanding of host-vector relationships in various environments, especially in dealing with the transmission of leishmaniases (Marassá et al. 2006).
Currently, 21 of the 27 states of Brazil, which are situated in all five Regions of the country, register the autochthonous transmission of VL (Tonini et al. 2012). According to information provided by the Informatics Department of the Unified Health Service (dtr2004.saude.gov.br/sinanweb/tabnet/dh?sinannet/leishvi/bases/leishvbrnet.def.), Brazil registered 3,348 cases of VL in 2012. In MT, 56 autochthonous cases were registered, with Rondonópolis and Jaciara together accounting for approximately 43% of these cases. In Jaciara, the first human case of VL occurred in 2003 and, between that date and 2013, 19 autochthonous cases with one death in 2011 were reported. In accordance with the Environmental Health Surveillance Department of the State Health Secretariat, surveys of canine serum have indicated high positive levels of contamination and entomological data have revealed the absence of Lu. longipalpis and frequent presence of Lu. cruzi in areas including those with registered cases of both human and canine VL.
The objective of this study was to investigate phlebotomine fauna and their natural infection rate and identify the animals that serve as feeding sources for the Lu. cruzi vector in the municipality of Jaciara.
MATERIALS AND METHODS
Study area - Jaciara, which is located 145 km from the state capital of Cuiabá, is situated in the southern region of the state at the coordinates 15°57’55’’S 54°58’06’’W at an altitude of 367 m above sea level. Its estimated population in 2012 was 25,927, with inhabitants living in an area of 1,654 km2 in size. This municipality lies within a savanna biome and has a hot, tropical, sub-humid climate with a four-month dry season occurring from May-August [Brazilian Institute of Geography and Statistic (ibge.gov.br/cidadesat/topwindow.htm?1)].
Phlebotomine captures - Captures were performed in accordance with the Manual for the Surveillance and Control of VL (MS/SVS 2006) using CDC-type light traps (Sudia & Chamberlain 1962) installed in peridomiciles at dusk (05:00 pm). These captures were conducted in the morning (06:00 am) on three consecutive days. Traps were installed in 10 dwellings in four suburbs (2 traps in São Sebastião, Jardim Aurora and Planalto and four traps in Santo Antônio) selected according to the register of human and canine cases from previous years. The collections were performed from July 2010-June 2011, from August-December 2011, in October and December 2012 and in February and March 2013 for a total of 63 captures. The captures carried out after the first 12 months were necessary to increase the number of engorged females. All males and females that were not Lu. cruzi were clarified and mounted on slides in Berlese liquid and identified in accordance with Young and Duncan’s (1994) taxonomic key, the shapes of the female cibarium (a region of the mouthpart) and spermatheca and the different structures forming the genitalia in males. The specific identification of Lu. cruzi was based on the characteristics of the male genitalia [4 foliaceous setae at the inner base of the coxite, not 6 as described by Mangabeira (1938)] because female Lu. longipalpis and Lu. cruzi are indistinguishable (Young & Duncan 1994). The abbreviations of the phlebotomine genera were made in accordance with Marcondes (2007).
Molecular tests - DNA extractions were performed according to Michalsky et al. (2002) and Souza et al. (2004) with the phenol/chloroform method and samples of up to 10 females from the same trap were pooled.
For the detection of constitutive sandfly genes (cacophony) we used a specific primer pair targeting the IVS6 region of sandflies of the genus Lutzomyia as follows: 5Llcac, 5’-GTGGCCGAACATAATGTTAG-3’ and 3Llcac, 5’-CCACGAACAAGTTCAACATC-3,’ as described by Michalsky et al. (2011).
PCR was performed using the primers 150 (sense), 5’-GGG(G/T)AGGGGCGTTCT(C/G)CGAA-3’ and 152 (antisense), 5’-(C/G)(C/G)(C/G)(A/T)CTAT(A/T)TTACACCAACCCC-3’, as described by Degrave et al. (1994), which amplified a 120-bp DNA fragment of a conserved region of the kDNA minicircle found in all species of Leishmania. The temperature and time conditions for amplification were as follows: initial denaturation at 94ºC for 4 min, followed by 30 cycles of 94ºC for 30 s, 60ºC for 30 s, 72ºC for 30 s and a final extension of 72ºC for 10 min. Next, the amplified product was dyed with GelRed and analysed by electrophoresis in 2% agar gel using a transilluminator (ultraviolet 300 nm). Strains of Leishmania braziliensis (MHOM/BR/75/M2903) were used as positive controls and master mix lacking DNA was used as a negative control according to Michalsky et al. (2011).
The pooled DNA samples positive for the genus Leishmania were submitted to PCR in accordance with Lachaud et al. (2002), using the primers RV1, 5’-CTTTTCTGGTCCCGCGGGTAGG-3’ and RV2, 5’-CCACCTGGCTATTTT ACACCA-3’, which amplified a 145-bp fragment specific to L. infantum chagasi. The time and temperature of amplification were the same for all Leishmania sp. For all tests, L. infantum chagasi (MHOM/BR/1974/PP75) reference samples were used as positive controls and master mix without DNA was employed as the negative control.
Because they were assessed in pools, the minimum infection rate of the phlebotomines was calculated using the following formula: minimum rate (MR) = number of positive pools x 100/total number of insects (Paiva et al. 2007).
Analysis of food source - Sixty-one Lu. cruzi females that were either engorged or contained some residual blood were dissected to expose their digestive tubes and were macerated in accordance with the ELISA protocol described by Burkot et al. (1981), as modified by Duarte (1997), using antisera from a bird, dog, skunk, primate and rodent. The choice of antisera was based on the animals observed in the environments where the collections had been made. Samples were diluted to 1:20 in carbonate-bicarbonate buffer (pH 9.6, 0.05 M; Sigma Chemical Co, USA) and added to a 96-well polystyrene microplate (NuncC, 442404, Maxisorp, Denmark). After incubation (at 37°C for 2 h), the plates were washed in phosphate buffered saline (PBS)/Tween 20 (0.05%) (Sigma Chemical Co). The next steps involved the addition of antisera (PBS/Tween 20 plus 1% skim milk; Molico-Nestle, Brazil) to the wells and the incubation of the microplate at 37°C for 30 min (goat anti-rabbit serum peroxidase conjugate; Sigma Chemical Co). Following a recommended wash step, the 1:20,000-diluted conjugate was added and after an additional incubation and wash, a developing buffer [citrate/phosphate, pH 5.0, 0.05 M hydrogen peroxide, 30 vol. (Merck Diagnostica, Brazil) and o-phenylenediamine (Sigma Immunochemical Co, USA)] was added. The reaction was stopped after 15 min by adding 50 μL of 1N sulphuric acid solution and measurements were obtained with an ELISA plate reader (USA) using 490-nm filters.
Statistical analysis - The accumulated frequency and percentage were calculated for each species (those with > 50% were classified as constant, those with between 10-49% as common and those with < 10% as rare). For the calculation of species diversity, Shannon-Weiner’s diversity index (H’) was used according to the following equation: H’ = -Ʃpi(log pi). The relationship between the climatic data [National Notifiable Diseases Surveillance System (sisam.cptec.inpe.br.)] and the density of Lu. cruzi was calculated using the model adopted by Vilela et al. (2011) via Pearson’s correlation index (r) and only the data collected regularly between July 2010-June 2011 were used. All analyses were conducted using Microsoft Excel 2010 and are described in the Tables and Figures.
RESULTS
At the end of the collections carried out in 21 states, 2,011 phlebotomines belonging to 23 species had been captured. A total of 1,354 of these were male and 657 were female. Lu. cruzi predominated, representing 68.70% of the specimens collected, followed by Lu. whitmani, comprising 20.52%, and Lutzomyia sordellii, amounting to 5.16% (Table I). These species, together with Brumptomyia brumpti, were considered constant because their absolute frequency was above 50%. The majority of the species (11) were classified as rare, as they were only registered for one or two of the captures. Among the species identified, with the exception of Lu. cruzi, the observed leishmaniasis vectors included Lutzomyia flaviscutellata, Lu. whitmani and Lutzomyia antunesi, which were involved in the transmission of Leishmania causing cutaneous leishmaniasis.
TABLE I. Species of phlebotomines captured in an urban area of the municipality of Jaciara, state of Mato Grosso, 2010-2013.
| Species | ♂ | ♀ | Total | Accumulated frequency | % |
|---|---|---|---|---|---|
| Brumptomyia brumpti | 19 | 30 | 49 | 61.90 | 2.43 |
| Lutzomyia acanthopharynx | 0 | 2 | 2 | 9.52 | 0.10 |
| Lutzomyia antunesi | 2 | 1 | 3 | 14.28 | 0.15 |
| Lutzomyia carrerai carrerai | 0 | 2 | 2 | 9.52 | 0.10 |
| Lutzomyia christenseni | 0 | 1 | 1 | 4.70 | 0.05 |
| Lutzomyia cruzi | 976 | 406 | 1,382 | 100 | 68.70 |
| Lutzomyia davisi | 0 | 1 | 1 | 4.70 | 0.05 |
| Lutzomyia evandroi | 0 | 4 | 4 | 19.40 | 0.20 |
| Lutzomyia flaviscutellata | 0 | 1 | 1 | 4.70 | 0.05 |
| Lutzomyia hermanlenti | 3 | 1 | 4 | 19.04 | 0.20 |
| Lutzomyia lenti | 1 | 4 | 5 | 14.28 | 0.25 |
| Lutzomyia longipennis | 1 | 0 | 1 | 4.70 | 0.05 |
| Lutzomyia microps | 0 | 1 | 1 | 4.70 | 0.05 |
| Lutzomyia punctigeniculata | 1 | 4 | 5 | 19.40 | 0.25 |
| Lutzomyia quinquifer | 1 | 0 | 1 | 4.70 | 0.05 |
| Lutzomyia sallesi | 8 | 6 | 14 | 38.09 | 0.70 |
| Lutzomyia saulensis | 2 | 1 | 3 | 14.28 | 0.15 |
| Lutzomyia sordellii | 42 | 62 | 104 | 90.40 | 5.16 |
| Lutzomyia teratodes | 1 | 5 | 6 | 23.80 | 0.30 |
| Lutzomyia termitophila | 2 | 5 | 7 | 28.50 | 0.34 |
| Lutzomyia walkeri | 1 | 0 | 1 | 4.70 | 0.05 |
| Lutzomyia whitmani | 294 | 119 | 413 | 90.40 | 20.52 |
| Lutzomyia yuillii yuillii | 0 | 1 | 1 | 4.70 | 0.05 |
|
| |||||
| Total | 1,354 | 657 | 2,011 | - | 100 |
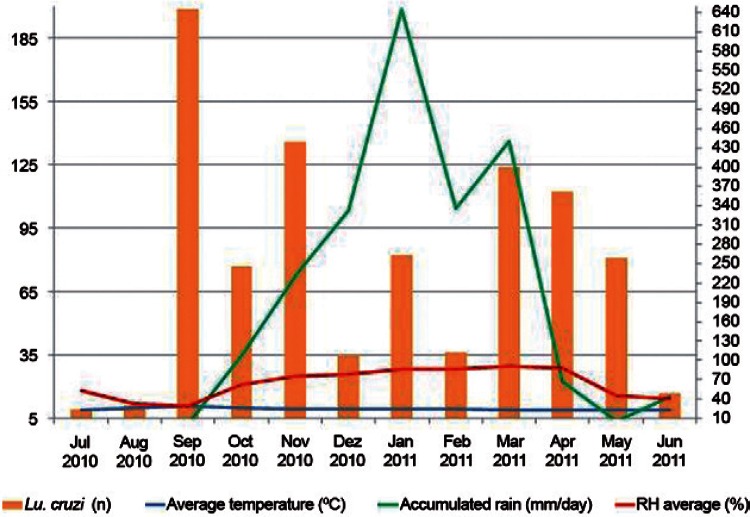
Monthly captures were performed regularly for one year (from July 2010-June 2011), resulting in 1,240 phlebotomines captured, 919 of which (74%) were identified as Lu. cruzi. Analysis of the monthly distribution of Lu. cruzi demonstrated an absence of any correlation with the variables of accumulated rainfall (r = 0.07) and relative humidity (r = 0.08) and a moderate correlation with average temperature (r = 0.4) (Fig. 1).
Fig. 1. : number of Lutzomyia cruzi accumulated rainfall, average relative humidity (RH) and the average monthly average temperature, municipality of Jaciara, state of Mato Grosso, from July 2010-June 2011.
Of the four districts investigated, Santo Antônio registered the greatest number of specimens, with 1,028 individuals; however, despite having the greatest species richness, it exhibited the lowest H’ (Table II).
TABLE II. Number and diversity index (H’) of species by suburb investigated in the municipality of Jaciara, state of Mato Grosso, 2010-2013.
| Suburb | Species (n) | Phlebotomines (n) | H’ |
|---|---|---|---|
| São Sebastião | 14 | 708 | 1.30 |
| Santo Antônio | 17 | 1,028 | 0.75 |
| Jardim Aurora | 7 | 172 | 0.85 |
| Planalto | 11 | 104 | 1.58 |
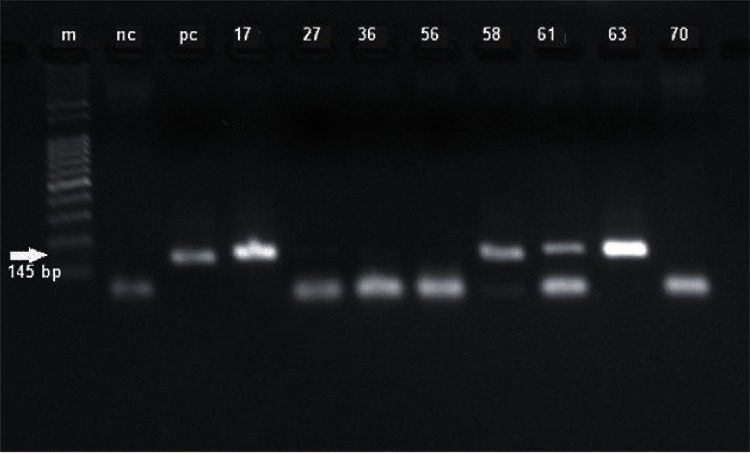
A total of 229 Lu. cruzi females, which were divided into 105 pools, were submitted to PCR testing for Leishmania. Fourteen pools were observed to possess the characteristic DNA band of Leishmania (120 bp), which was later confirmed to be L. infantum chagasi (145 bp) (Fig. 2), indicating the natural infection of Lu. cruzi at a MR of 6.1%. The districts of Santo Antônio and São Sebastião presented with the greatest numbers of positive samples (6 each).
Fig. 2. : products of amplification for Leishmania chagasi undertaken in females of Lutzomyia cruzi. m: marker of 100 bp; nc: negative control; pc: positive control (MHOM/BR/1974/PP75); 17, 27, 36, 58, 61, 63: positive samples of phlebotomines.
Analysis of blood obtained from the digestive tubes of the 61 engorged females assessed by ELISA indicated that only 22 were reactive, including 17 to bird antiserum, two for dog and one for skunk. Mixed profiles were observed in two samples (bird and skunk; dog and skunk), suggesting that those females had fed on both hosts. There was no reaction to primate or rodent antiserum (Table III).
TABLE III. Feeding habits by ELISA test in engorged females of Lutzomyia cruzi collected in the municipality of Jaciara, state of Mato Grosso, from 2011-2013.
| Antisera | Absolute (n) | Relative (%) |
|---|---|---|
| Bird | 17 | 27.90 |
| Dog | 2 | 3.30 |
| Skunk | 1 | 1.60 |
| Human | 0 | 0 |
| Rodent | 0 | 0 |
| Bird and skunk | 1 | 1.60 |
| Dog and skunk | 1 | 1.60 |
| Not reactive | 39 | 64 |
|
| ||
| Total | 61 | 100 |
DISCUSSION
The proximity of the city of Jaciara to the vegetation of its natural surroundings may explain the diversity of the phlebotomines found in this study despite the predominance of only two species, Lu. cruzi and Lu. whitmani, which together accounted for approximately 90% of the phlebotomines collected. The predominance of Lu. cruzi in the areas investigated demonstrates its adaptation to these environments, corroborating the findings of Missawa and Lima (2006), who discovered a greater predominance of Lu. cruzi in municipalities containing areas of marsh and savanna, the latter being their preferred environment.
Studies have suggested that Lu. whitmani is the most important vector of anthroponotic cutaneous leishmaniasis in Brazil in association with L. braziliensis. This species is present in various Brazilian biomes and has adapted to different climatic conditions. In addition, this species can survive in the intra and peridomiciliary environments of impacted areas (Vilela et al. 2011). Despite the high density of Lu. whitmani, according to the Environmental Surveillance of Jaciara, there is no record of the transmission of cutaneous leishmaniasis in urban areas, which has consistently been reported in rural, forested areas.
Despite the finding that the greatest number of phlebotomines was present in Santo Antônio, it cannot be confirmed that this location contains the greatest density of these insects because twice as many traps were placed there compared to other locations. This greater number of traps was due to the larger size of the district, the existence of various residences with an appearance suggestive of the presence of the vector and the various cases of canine VL registered there in the years immediately prior to this research project. Considering the number of phlebotomines in relation to the number of traps installed, São Sebastião (2 traps) presented with the greatest number of individuals.
Michalsky et al. (2009) reported that many authors have shown a clear relationship between abiotic factors and phlebotomine population density due to the interference of these factors with their biological cycles and associated modifications of their locations of reproduction. Variable population densities of Lu. longipalpis were observed in Montes Claros, state of Minas Gerais, which exhibited an increase in density every other month independent of season, probably due to the peculiar weather conditions of this municipality, where the climate variables followed an almost constant seasonal cycle.
Although Fig. 1 shows an increase in the population density of Lu. cruzi before and after the rainy season, in this study, only temperature exhibited a moderate correlation with the density of Lu. cruzi. Specifically, a tendency towards the abundance of these insects during periods of high temperature was observed. For a more precise analysis, a larger amount of data should be assessed, requiring testing over longer periods of time.
The comparison between species richness and diversity, such as that performed among the suburbs investigated in Jaciara, showed that the Santo Antônio presented a greater richness (number of species), but a lower diversity, which was due to the great disparity between species’ frequencies with an absolute predominance of Lu. cruzi.
One important step towards the verification of a particular Leishmania vector is the determination of the occurrence of naturally infected phlebotomines (Killick-Kendrick & Ward 1981). Studies of the rate of natural infection in phlebotomines in various regions of the country have revealed low values, even in areas of widespread transmission. Studies of different populations of Lu. longipalpis performed using distinct techniques have found the following minimal rates of infection: 2.6% in Antônio João, state of Mato Grosso do Sul (MS) (Nascimento et al. 2007), 1.1% in Teresina, state of Piauí (PI) (Silva et al. 2007), 1.25% in São Luís, state of Maranhão, in an area of ancient colonisation and 0.25% in an area of recent colonisation (Soares et al. 2010), and 3.5% in Missiones, Argentina (Acardi et al. 2010).
Few studies have assessed Lu. cruzi, although it has been suggested that this species is a vector of L. infantum chagasi. For example, the study by Santos et al. (1998) reported a MR of infection of 0.4% in Corumbá and Ladário, MS and in 2008 Pita-Pereira et al. reported a 1.5% rate of infection of Lu. cruzi at that same locality. The infection rate of 6.1% obtained in the present study was higher than that which has been previously reported; however, it is in accordance with that reported by Missawa et al. (2011), who found a positive reaction in one of three pools of Lu. cruzi females collected from the same municipality.
Studies of food sources have demonstrated that phlebotomines are very diverse with regard to their choice of host. Analyses of Luyzomyia intermedia females carried out in Mesquita, state of Rio de Janeiro using the precipitin technique have found that 39.8% are reactive for rodents, followed by 23.7% for birds, 20.4% for dogs and 16% for humans (Afonso et al. 2005). In Várzea Grande, Lu. longipalpis was shown to react to the antisera of birds (30.8%), rodents (21.2%), humans (13.5%) and dogs (4.8%) using the precipitin technique (Missawa et al. 2008). In the municipality of Teresina, PI, out of 58 Lu. longipalpis females analysed using PCR/FTA, 41 had fed on chicken and two on dogs as a food source and the food sources of 15 could not be identified (Sant’Anna et al. 2008). In a study of Lu. longipalpis obtained from Jequié, state of Bahia, Sobral and Massapé, state of Ceará and Teresina, PI, Afonso et al. (2012) detected positive reactions in various animals, predominantly birds (36% in Jequié, 67% in Teresina, 29% in Sobral and 51% in Massapê), using the ELISA method. Chagas et al. (2007) studied the haematophagous behaviour of Lu. cruzi bred in the laboratory and found that the females fed more easily on and preferred humans to hamsters when both sources of food were offered simultaneously. A previous study of the eating habits of Lu. cruzi in Jaciara revealed that of 22 positive females, 18 used birds as a blood source. Although chickens do not act as reservoirs of Leishmania, they can be important in the maintenance of populations of Lutzomyia by attracting reservoir mammals or by acting as sentinels for these insects in the area investigated (Alexander et al. 2002, Soares et al. 2013).
The reaction to skunk antiserum observed in this study indicates that synanthropic animals can act as a link between domestic and wild transmission cycles, thus increasing the risk of canine infection by 2.6-fold, which has previously been reported by Missawa et al. (2008). Although this municipality registered high levels of positive reactions to canine antiserum, the presence of dog blood was detected in few females, which is probably due to competition with birds (chickens), which are normally bred in larger numbers than other domestic animals when present in peridomiciles.
Despite the low number of reactive samples, our findings suggest that there is a preference with regard to the type of host; however, further studies should be designed to study a greater number of specimens. These results may have been due to the advanced digestion of the blood ingested, which was possibly a result of the time lapse between the capture and separation of the females, the presence of a small quantity of blood or the feeding on a host that was not evaluated in this study.
The high densities and frequencies of Lu. cruzi and Lu. whitmani collected from the urban zone of Jaciara support the adaptation of these species to anthropic environments and suggest that both are present in urban regions of Jaciara. Moreover, the high rate of infection associated with the presence of synanthropic animals as blood sources for these insects in the peridomicile indicates the participation of Lu. cruzi in the transmission of VL in Jaciara.
Footnotes
Financial support: CNPq (Edital Universal 2011)
REFERENCES
- Acardi SA, Liotta DJ, Santini MS, Romagosa CM, Salomón OD. Detection of Leishmania infantum in naturally infected Lutzomyia longipalpis (Diptera: Psychodidae: Phlebotominae) and Canis familiaris in Misiones, Argentina: the first report of a PCR-RFLP and sequencing-based confirmation assay. Mem Inst Oswaldo Cruz. 2010;105:796–799. doi: 10.1590/s0074-02762010000600011. [DOI] [PubMed] [Google Scholar]
- Afonso MMS, Duarte R, Miranda JC, Caranha L, Rangel EF. Studies on the feeding habits of Lutzomyia (Lutzomyia) longipalpis (Lutz & Neiva, 1912) (Diptera: Psychodidae: Phlebotominae) populations from endemic areas of American visceral leishmaniasis in northeastern Brazil. J Trop Med. 2012;2012:1–5. doi: 10.1155/2012/858657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afonso MMS, Gomes AC, Meneses CRV, Rangel EF. Studies on the feeding habits of Lutzomyia (N.) intermedia (Diptera: Psychodidae), vector of cutaneous leishmaniasis in Brazil. Cad Saude Publica. 2005;21:1816–1820. doi: 10.1590/s0102-311x2005000600030. [DOI] [PubMed] [Google Scholar]
- Alexander B, Carvalho RL, McCallum H, Pereira MO. Role of the domestic chicken (Gallus gallus) in the epidemiology of urban visceral leishmaniasis in Brazil. Emerg Infect Dis. 2002;8:1480–1485. doi: 10.3201/eid0812.010485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida PS, Nascimento JC, Ferreira AD, Minzão LD, Portes F, Miranda AM, Faccenda O, Filho JDA. Espécies de flebotomíneos (Diptera: Psychodidae) coletadas em ambiente urbano em municípios com transmissão de leishmaniose visceral do estado de Mato Grosso do Sul, Brasil. Rev Bras Entomol. 2010;54:304–310. [Google Scholar]
- Ashford RW. The leishmaniasis as emerging and reemerging zoonoses. Int J Parasitol. 2000;30:1269–1281. doi: 10.1016/s0020-7519(00)00136-3. [DOI] [PubMed] [Google Scholar]
- Burkot TR, Goodman WG, DeFoliart GR. Identification of mosquito blood meals by enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 1981;30:1336–1341. doi: 10.4269/ajtmh.1981.30.1336. [DOI] [PubMed] [Google Scholar]
- Chagas AC, Medeiros JF, Justiniano SCB, Pessoa FAC. Haematophagic behavior in laboratory of Lutzomyia cruzi (Mangabeira) (Diptera: Psychodidae) in relation to three mammalian blood sources in Manaus, Brazil. Acta Amaz. 2007;37:127–132. [Google Scholar]
- Dantas-Torres T. The role of dogs as reservoirs of Leishmania parasites with emphasis on Leishmania (Leishmania) infantum and Leishmania (Viannia) braziliensis. Vet Parasitol. 2007;149:139–146. doi: 10.1016/j.vetpar.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Degrave W, Fernandes O, Campbell B, Bozza M, Lopez U. Use of molecular probes and PCR for detection and typing of Leishmania: a Mini Review. Mem Inst Oswaldo Cruz. 1994;89:463–469. doi: 10.1590/s0074-02761994000300032. [DOI] [PubMed] [Google Scholar]
- Duarte R. Ensaio imunoenzimático ELISA para identificação experimental de fontes alimentares em Panstrongilus megistus (Burmeister 1835) (Hemiptera: Reduviidae) Instituto Oswaldo Cruz/Fiocruz; Rio de Janeiro: 1997. 103 PhD Thesis. [Google Scholar]
- Harhay MO, Olliaro PL, Costa DL, Costa CHN. Urban parasitology: visceral leishmaniasis in Brazil. Trends Parasitol. 2011;27:403–409. doi: 10.1016/j.pt.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Killick-kendrick R, Ward RD. Ecology of Leishmania. Parasitology. 1981;82:143–152. [Google Scholar]
- Lachaud L, Marchergui-Hammmi S, Chabbert E, Dereure J, Dedet JP, Bastien P. Comparasion of six PCR methods using peripheral blood for detection on (of) canine visceral leishmaniasis. J Clin Microbiol. 2002;40:210–215. doi: 10.1128/JCM.40.1.210-215.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marassá AM, Aschenbrenner C, Galati EAB, Nunes VLB. Identificação do sangue ingerido por Lutzomyia (Lutzomyia) longipalpis (Lutz & Neiva, 1912) e Lutzomyia (Lutzomyia) almerioi (Galati & Nunes, 1999) pela técnica imunoenzimática do ELISA de captura no sistema avidina-biotina. Rev Bras Med Trop. 2006;39:183–186. doi: 10.1590/s0037-86822006000200010. [DOI] [PubMed] [Google Scholar]
- Marcondes CB. A proposal of generic and subgeneric abbreviation of phlebotomines sandflies (Diptera: Psychodidae: Phlebo- tominae) of the world. Entomological News. 2007;118:351–356. [Google Scholar]
- Michalsky EM, Fortes-Dias CL, França-Silva JC, Rocha MF, Barata RA, Dias ES. Association of Lutzomyia longipalpis (Diptera: Psychodidae) population density with climate variables in Montes Claros, an area of American visceral leishmaniasis transmission in the state of Minas Gerais, Brazil. Mem Inst Oswaldo Cruz. 2009;104:1191–1193. doi: 10.1590/s0074-02762009000800020. [DOI] [PubMed] [Google Scholar]
- Michalsky EM, Fortes-Dias CL, Pimenta PEP, Secundino NFC, Dias ES. Assessment of PCR in the detection of Leishmania spp in experimentally infected individual phlebotomine sandflies (Diptera: Psychodidae: Phlebotominae). Rev Inst Med Trop Sao Paulo. 2002;44:255–259. doi: 10.1590/s0036-46652002000500004. [DOI] [PubMed] [Google Scholar]
- Michalsky EM, Guedes KS, Silva FOL, França-Silva JO, Fortes-Dias CL, Barata RA, Dias ES. Infecção natural de Lutzomyia (Lutzomyia) longipalpis (Diptera: Psychodidae) por Leishmania infantum chagasi em flebotomíneos capturados no município de Janaúba, estado de Minas Gerais, Brasil. Rev Soc Bras Med Trop. 2011;44:58–62. doi: 10.1590/s0037-86822011000100014. [DOI] [PubMed] [Google Scholar]
- Missawa NA, Lima GBM. Distribuição espacial de Lutzomyia longipalpis (Lutz e Neiva, 1912) e Lutzomyia cruzi (Mangabeira, 1938) no estado de Mato Grosso. Rev Soc Bras Med Trop. 2006;39:337–340. doi: 10.1590/s0037-86822006000400004. [DOI] [PubMed] [Google Scholar]
- Missawa NA, Lorosa ES, Dias ES. Preferência alimentar de Lutzomyia longipalpis (Lutz & Neiva, 1912) em área de transmissão de leishmaniose visceral em Mato Grosso. Rev Soc Bras Med Trop. 2008;41:265–268. doi: 10.1590/s0037-86822008000400008. [DOI] [PubMed] [Google Scholar]
- Missawa NA, Veloso MAE, Maciel GBML, Michalsky EM, Dias ES. Evidência de transmissão de leishmaniose visceral por Lutzomyia cruzi no município de Jaciara, estado de Mato Grosso, Brasil. Rev Soc Bras Med Trop. 2011;44:76–78. doi: 10.1590/s0037-86822011000100017. [DOI] [PubMed] [Google Scholar]
- MS/SVS - Ministério da Saúde/Secretaria de Vigilância em Saúde Manual de vigilância e controle da leishmaniose visceral. 2. MS/SVS; Brasília: 2006. 122 [Google Scholar]
- Nascimento JC, Paiva BR, Malafronte RS, Fernandes WD, Galati EAB. Natural infection of phlebotomines (Diptera: Psychodidae) in a visceral-leishmaniasis focus in Mato Grosso do Sul, Brazil. Rev Inst Med Trop Sao Paulo. 2007;49:119–122. doi: 10.1590/s0036-46652007000200011. [DOI] [PubMed] [Google Scholar]
- Oliveira-Pereira YN, Rebêlo JMM, Moraes JLP, Pereira SRF. Diagnóstico molecular da taxa de infecção natural de flebotomíneos (Psychodidae, Lutzomyia) por Leishmania sp. na Amazônia maranhense. Rev Soc Bras Med Trop. 2006;39:540–543. doi: 10.1590/s0037-86822006000600005. [DOI] [PubMed] [Google Scholar]
- Paiva BR, Secundino NFC, Pimenta PFP, Galati EAB, Júnior HFA, Malafronte RS. Padronização de condições para detecção de DNA de Leishmania spp em flebotomíneos (Diptera: Psychodidae) pela reação em cadeia da polimerase. Cad Saude Publica. 2007;23:87–94. doi: 10.1590/s0102-311x2007000100010. [DOI] [PubMed] [Google Scholar]
- Pita-Pereira D, Cardoso MAB, Alves CR, Brazil RP, Britto C. Detection of natural infection in Lutzomyia cruzi and Lutzomyia forattinii (Diptera: Psychodidae: Phlebotominae) by Leishmania infantum chagasi in an endemic area of visceral leishmaniasis in Brazil using a PCR multiplex assay. Acta Tropica. 2008;107:66–69. doi: 10.1016/j.actatropica.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Sant’Anna MRV, Jones NG, Hindlley JA, Mendes-Sousa AF, Dillon RJ, Cavalcante RR, Alexander B, Bates PA. Blood meal identification and parasite detection in laboratory-fed and field-captured Lutzomyia longipalpis by PCR using FTA databasing paper. Acta Tropica. 2008;107:230–237. doi: 10.1016/j.actatropica.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos SO, Arias JR, Ribeiro AA, Hoffmann MP, Freitas RA, Malacco MAF. Incrimination of Lutzomyia (Lutzomyia) cruzi as a vector of American visceral leishmaniasis. Med Vet Entomol. 1998;12:315–317. doi: 10.1046/j.1365-2915.1998.00104.x. [DOI] [PubMed] [Google Scholar]
- Silva JGD, Werneck GL, Cruz MSP, Costa CHN, Mendonça IL. Infecção natural de Lutzomyia longipalpis por Leishmania sp. em Teresina, Piauí, Brasil. Cad Saude Publica. 2007;23:1715–1720. doi: 10.1590/s0102-311x2007000700024. [DOI] [PubMed] [Google Scholar]
- Soares BR, Souza APA, Prates DB, Oliveira CL, Barral JCM, Neto, Barral A. Seroconversion of sentinela chickens as a biomarker for monitoring exposure to visceral leishmaniasis. Scientific Reports. 2013;3:1–6. doi: 10.1038/srep02352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares MRA, Carvalho CC, Silva LA, Lima MSCS, Barral AMP, Rebêlo JMM, Pereira SRF. Análise molecular da infecção natural de Lutzomyia longipalpis em área endêmica de leishmaniose visceral no Brasil. Cad Saude Publica. 2010;26:2409–2413. doi: 10.1590/s0102-311x2010001200019. [DOI] [PubMed] [Google Scholar]
- Souza CM, Pessanha JE, Barata RA, Monteiro EM, Costa DC, Dias ES. Study on phlebotomine sand fly (Diptera: Psychodidae) fauna in Belo Horizonte, state of Minas Gerais, Brazil. Mem Inst Oswaldo Cruz. 2004;99:795–803. doi: 10.1590/s0074-02762004000800003. [DOI] [PubMed] [Google Scholar]
- Sudia WA, Chamberlain RW. Battery-operated light trap: an improved model. Mosq News. 1962;22:126–129. [PubMed] [Google Scholar]
- Tonini MAL, Lemos EM, Reis AB, Coura-Vital W, Dias ES, Dietze R. First description of autochthonous canine visceral leishmaniasis in the metropolitan region of Vitória, state of Espírito Santo, Brazil. Rev Bras Med Trop. 2012;45:754–756. doi: 10.1590/s0037-86822012000600019. [DOI] [PubMed] [Google Scholar]
- Vilela MA, Azevedo CG, Carvalho BM, Rangel EF. Phlebotomine fauna (Diptera: Psychodidae) and putative vectors of leishmaniasis in impacted area by hydroeletric plant, state of Tocantins, Brazil. Plos ONE. 2011;6:1–7. doi: 10.1371/journal.pone.0027721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young DG, Duncan MA. Guide to the identification and geographic distribution of Lutzomyia sandflies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae) Mem Am Entomol Inst. 1994;54:1–881. [Google Scholar]