Abstract
There are a number of dietary interventions capable of inhibiting mammary tumorigenesis however the effectiveness of dietary combinations is largely unexplored. Here we combined two interventions previously shown individually to inhibit mammary tumor development. The first was the use of the omega-3 fatty acid, eicosapentaenoic acid (EPA), and the second was the implementation of calorie restriction. MMTV-Her2/neu mice were used as a model for human breast cancers which over express Her2/neu. Six groups of mice were enrolled. Half were fed a control (Con) diet with 10.1% fat calories from soy oil, while the other half consumed a diet with 72% fat calories from EPA. Within each diet mice were further divided into ad libitum (AL), chronic calorie restricted (CCR) or intermittent calorie restricted (ICR) groups. Mammary tumor incidence was lowest in ICR-EPA (15%) and highest in AL-Con mice (87%) while AL-EPA, CCR-Con, CCR-EPA and ICR-Con groups had mammary tumor incidence rates of 63%, 47%, 40% and 59% respectively. Survival was effected similarly by the interventions. Consumption of EPA dramatically reduced serum leptin (P<0.02) and increased serum adiponectin in the AL-EPA mice compared to AL-Con mice (P<0.001). Both CCR and ICR decreased serum leptin and IGF-I compared to AL mice but not compared to each other. These results illustrate that mammary tumor inhibition is significantly increased when ICR and EPA are combined as compared to either intervention alone. This response may be related to alterations in the balance of serum growth factors and adipokines.
Keywords: Breast cancer, intermittent calorie restriction, eicosapentaenoic acid, Her2/neu and fish oil
Introduction
A variety of nutritional interventions have been considered for breast cancer inhibition (1). This has included altering the type of fat included in the diet. The potential effects of fat are seen when comparing women who consume traditional Nordic diets to those who migrate to countries with western diets (2). The incidence of breast cancer in women who have immigrated increases and can triple in a single generation (3). The consumption of fish in the diets of the women after they have immigrated is significantly decreased and this may be one of the mitigating factors in the increase of breast cancer in these women as compared to women who have not immigrated (4-5). Fish such as wild salmon, sardines and mackerel are all high in omega 3 (ω–3) long chain polyunsaturated fatty acids (LC-PUFAs). Human case controlled studies have reported an inverse relationship between the ratio of ω-3 LC-PUFAs and ω-6 LC-PUFAs and the incidence of breast cancer, and it has been proposed that the relative proportions of ω-3 and ω-6 LC-PUFAs in the diet are important for this effect (6).
A second area of nutrition under investigation for its role in breast cancer is calorie restriction. Chronic calorie restriction (CCR) has clearly been shown to reduce mammary tumor incidence in animal models. For example, a meta-analysis of 14 studies found that CCR in rodents resulted in 55% fewer tumors than controls regardless of what nutrient(s) were restricted (7). Another way to implement calorie restriction is in an intermittent fashion. In fact, intermittent caloric restriction (ICR) applied to rodents has been reported to protect in the development of mammary tumors to an even greater extent than CCR (8-9).
Animal models of breast cancer also support a role for fish oil influencing the development of breast cancer. For example, MMTV-Her2/neu transgenic mice fed fish oil instead of corn oil as 25% of energy, had a mammary tumor incidence of 57% compared to 87% for mice fed corn oil (10). In addition, when mice with MDA-MB-435 human breast cancer cell line xenografts were fed a diet of 18% fish oil and 5% corn oil they had significantly slower tumor growth and fewer metastases than mice fed 5% fish oil and 18% corn oil (11).
The mechanisms of action of fish oil and calorie restriction are under active investigation. The omega-3 fatty acid eicosapentaenoic acid (EPA) which is thought to be one of the main active factors in fish oil can reduce growth and increase apoptosis of some breast cancer cells in vitro (12-13). The serum levels of a number of growth factors are altered by both EPA and calorie restriction (14-15). Fish oil influences adipose tissue secretion of a specific group of growth factors known as adipokines (16). Adiponectin is one adipokine that circulates at high concentrations (2-20 ug/ml) in human serum and in contrast to most adipose secreted proteins, is negatively correlated with body weight, BMI, body fat and serum leptin in humans (17). With respect to breast cancer, reduced serum adiponectin levels have been reported for postmenopausal women with breast cancer (18) and premenopausal women with a higher risk of additional neoplastic events (19). Calorie restriction decreases serum levels of the adipokine leptin (20) which has been associated with increased growth of breast cancer cells in vitro (21). Calorie restriction also decreases the serum levels of Insulin-like Growth Factor 1 (IGF-I) which is involved in the growth of a number of different tumor types (22) and whose pathway is being targeted for anti-breast cancer interventions in the clinic (23). It may be that identification of the optimal levels of adiponectin, leptin and IGF-I is the key to breast cancer prevention (18, 24-25).
In humans, Her2/neu is over expressed in approximately 25-30% of breast cancer patients and is associated with aggressive, hormone-independent breast cancer (26). The use of drug combinations has proven to be more effective than the use of single agents for reducing tumor recurrence and prolonging lifespan in women with Her2-neu positive tumors (27). However, prevention is the ultimate goal and very little is known about whether combining potential interventions will be effective for this malignancy. The MMTV-Her2/neu mouse model over expresses Her2/neu in mammary tissues and has close parallels to this type of human breast cancer (28). In the following study we hypothesized that this model could be utilized to illustrate that combining two dietary interventions would lead to superior breast cancer inhibition compared to either intervention alone. Further this inhibition may be mediated by increased serum adiponectin and reduced serum leptin and IGF-I.
Methods and Materials
Mice and Diets
Pairs of MMTV-Her2/neu homozygous transgenic mice were obtained from Jackson Laboratories and bred. Female pups were then identified by genotyping and enrolled into the study. The diet is based on the AIN-93M diet (29) that was originally designed for long-term maintenance of rodents in aging and tumorigenesis studies. The diets were obtained from Harlan Laboratories, Inc. (Madison, WI). Our base diet contains 10.1% fat calories derived from soy oil. No phytoestrogens have been found in the AIN-93 diet (30). All diets contained t-butylhydroquinone (TBHQ) for stabilization of the EPA or to control for effects of this ingredient on the study mice. These are the same levels of t-butylhydroquinone per gram of fish oil as used for a prior study using the same MMTV-Her2/neu mice (10). All diets have 3.6 calories per gram. The intermittent restricted diets are fed at 50% and designed so that the animals consume the same amounts in calories of protein, fat, vitamins, minerals and TBHQ as the Ad Lib animals. The diets meet the recommended dietary linoleate requirements for mice of a minimum 0.68% of energy, and mice were monitored for signs of essential fatty acid deficiency such as hair loss, dermatitis and scaly skin during the course of the experiment as well as fatty livers postmortem (31). Diets were handled under low light conditions and stored at minus 20°C. The EPA diet contains 10.1% total fat calories with 7.25% fat calories from EPA and 2.85% fat calories from soy oil. The EPA was purchased as 90% Ethyl Ester from Sanmark LLC (Greensboro, NC) and incorporated into the appropriate diets by Harlan Laboratories. Table 1 shows the specific details of the diets. All procedures protocols and studies were approved by the University of Minnesota IACUC and the Hormel Institute Animal Facility which is AAALAC accredited.
Table 1.
Diet ingredients.
| Ad lib Con | Ad lib EPA | CCR Con | CCR EPA | ICR Con | ICR EPA | |
|---|---|---|---|---|---|---|
| Casein | 140.0 | 140.0 | 186.7 | 186.7 | 280.0 | 280.0 |
| L-Cystine | 1.8 | 1.8 | 2.4 | 2.4 | 3.6 | 3.6 |
| Corn Starch | 470.6 | 470.6 | 416.5 | 416.5 | 322.6 | 322.6 |
| Maltodextrose | 160.0 | 160.0 | 142.0 | 142.0 | 98.0 | 98.0 |
| Sucrose | 100.0 | 100.0 | 89.0 | 89.0 | 61.0 | 61.0 |
| Soy Oil | 40.0 | 11.3 | 53.3 | 15.1 | 80.0 | 22.6 |
| EPA | 28.7 | 38.3 | 57.4 | |||
| Cellulose | 40.0 | 40.0 | 46.6 | 46.6 | 59.58 | 59.58 |
| Mineral Mix | 35.0 | 35.0 | 46.7 | 46.7 | 70.0 | 70.0 |
| Vitamin Mix | 10.0 | 10.0 | 13.3 | 13.3 | 20.0 | 20.0 |
| Choline bitartrate | 2.5 | 2.5 | 3.3 | 3.3 | 5.0 | 5.0 |
| TBHQ (antioxidant) | 0.086 | 0.086 | 0.115 | 0.115 | 0.172 | 0.172 |
Study Design
Following weaning all MMTV-Her2/neu transgenic mice were fed daily the ad libitum-control diet - from 6-8 weeks of age to adapt them to the daily feeding regimen with powdered food. At 8 weeks of age one half of the mice were assigned to the EPA group and switched to the Ad Lib EPA diet for two weeks. At 10 weeks of age mice were divided into 6 groups. The mice assigned to the intermittent calorie restricted (ICR) groups were fed 50% of the average amount of food consumed by the corresponding Ad Lib group for three weeks and then 100% of the Ad Lib diet for 3 weeks for a total caloric restriction of 25%. The 6 week cycles of restriction and refeeding were maintained until the mice were 60 weeks of age or were euthanized due to mammary tumor (MT) burden. Chronic calorie restricted (CCR) groups were given 75% of the total calories that the AL age-matched groups consumed. To achieve these feeding regimens it was necessary to stagger the experimental groups at the beginning of the study such that the Ad Lib mice were the first mice enrolled in the study and the other groups' food allotments were based on how much the Ad Lib mice ate. Mice were weighed weekly and carefully examined for MTs. To facilitate accurate food intake measurements, mice were individually housed and food intakes were monitored daily. Food was placed in small glass cups placed inside larger cups to collect any spillage. This overall protocol is based on our earlier studies (9). When a MT on a mouse measured 7 × 7 mm the mouse's age was designated as the age of palpable MT detection. Some MTs were detected when a mouse was euthanized due to the experimental endpoint. The age of the mouse at the time of euthanization was then designated as the age of MT detection. The termination of the study was 60 weeks of age. However, mice with a mammary tumor 20mm in length were euthanized before 60 weeks. Criteria other than tumor size for early euthanasia included unexplained weight loss, not eating, open skin sores or other signs of distress. In order that all mice were in a similar postprandial state prior to euthanasia, we always fed the mice in the afternoon between 14:00-17:00 hours. The ICR mice were sacrificed during a restricted feeding period.
Sample Procurement and Analysis
To obtain blood mice were anesthetized at 7 weeks of age before the diets were implemented, during the middle of the study prior to palpable tumor formation at 25-28 weeks of age or at the end point when mice were 35-60 weeks of age. Approximately 100 ul of blood was obtained by orbital bleed. The blood was then allowed to clot, centrifuged and serum stored at −70 C. Quantikine ELISA Kits from R&D Systems (Minneapolis, MN) were utilized according to the manufacturer's instructions. All samples were analyzed in duplicate. At the time of euthanasia, mammary tumors were removed and weighed. A sample was placed in formalin and the remaining tissue frozen at −70°C. In addition, livers, spleens, kidneys, ovaries, hearts and lungs were removed, weighed and examined for abnormalities. Samples of the lungs and any abnormalities were processed for histopathological analysis. The remaining tissues were frozen at −70°C. Left and right parametrial and retroperitoneal fat deposits were removed, weighed and frozen. Samples were then analyzed in a blinded fashion.
Histological diagnosis
Part of the tumor tissue was fixed with formalin, embedded with paraffin, and stained with hematoxylin and eosin. Tumor histology was diagnosed by a trained pathologist and was graded based on a combination of the Scarff-Bloom-Richardson (SBR) system and the Black method that emphasizes nuclear grading and excludes consideration of tubules as a criterion. All tumors were either well differentiated (grade 1) or some differentiated (grade 2) breast cancer.”
Data Analysis
Mammary tumor incidence, age of mammary tumor detection and age of death were analyzed by ANOVA for general differences among the groups. When the ANOVA was significant, Neuman Keul's test was used to determine significance between specific groups.
Results
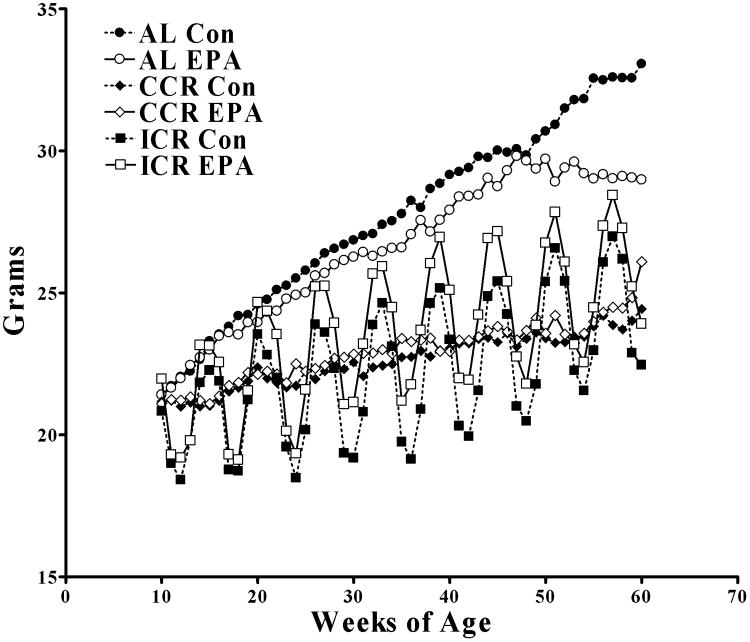
Ad Lib Control (Con) and Ad Lib-EPA groups ate similar amounts of food and as such the CCR-Con and CCR-EPA also ate similar amounts of food as did the ICR-Con and ICR-EPA groups. The AL-Con and AL-EPA mice gained weight at similar rates (Figure 1). The final weights of the AL-Con and AL-EPA were somewhat different but this was not statistically significant for the overall experiment due to the very small numbers of AL-Con mice that survived to the 60 week endpoint. The body weights of the CCR-Con and CCR-EPA were also similar to each other. The ICR-EPA mice were slightly heavier (p<0.05) than the ICR-Con mice despite consuming similar quantities of food.
Figure 1.
Body weights of mice over the course of the study. At 10 weeks of age the mice were divided into final groups and fed either AL Con (N=30), AL EPA (n=27), CCR Con (n=36), CCR EPA (n=30), ICR Con (n=29) or ICR EPA (N=26). Mice were weighed weekly and the weight in grams is shown along the y-axis with their age along the x-axis. The standard error of the mean for all points was so small that they are hidden by the symbols.
MT incidence was lowest in the ICR-EPA group (15.4%) and highest in the AL-Con group (86.7%) while the AL-EPA, CCR-Con, CCR-EPA and ICR-Con had MT incidence rats of 55.6%, 47.2%, 40% and 58.6%, respectively (Figure 2a). The reduction in MT incidence was accompanied by a delay in the identification of the first tumors in the groups although this was not significant. The main criteria for identification of a tumor was palpation of a tumor that was 7 mm × 7 mm or larger. However a few mice were only identified as tumor positive at the termination of the study and the age of MT palpation was considered to be 60 weeks for these mice. The number of mice with MTs identified at euthanasia for each groups was; AL-Con = 5, AL-EPA = 3, CCR-Con = 3, CCR-EPA = 2, ICR-Soy = 1 and ICR-EPA =0. Figure 2b shows a similar trend for the survival curves. The AL-Con group had the lowest percentage of mice surviving to 60 weeks of age with only 40.0% while the ICR-EPA had the highest percentage of mice surviving to 60 weeks of age with 88.5% (p<0.01). The AL-EPA, CCR-Con, CCR-EPA and ICR-Con survival percentages were 48.1%, 72.2%, 73.3% and 62.1%, respectively (Figure 2b). Mice with tumors were euthanized due to tumor burden (AL-Con = 18, AL-EPA = 11, CCR-Con = 13, CCR-EPA = 8, ICR-Soy = 11 and ICR-EPA =1), tumor necrosis (AL-Con = 0, AL-EPA = 1, CCR-Con = 0, CCR-EPA = 1, ICR-Soy = 0 and ICR-EPA =0) or end point of the study (AL-Con = 8, AL-EPA = 3, CCR-Con = 4, CCR-EPA = 3, ICR-Soy = 6 and ICR-EPA = 1). When examining the survival curves for effects of calorie restriction we found that the AL-Con was significantly different from the CCR-Con (p<0.003) and that the AL-EPA was significantly different from the CCR-EPA and the ICR-EPA (P<0.04 and p<0.001 respectively). When examining the survival curves for the effects of EPA we found that the ICR-Con was significantly different from the ICR-EPA (p<0.02).
Figure 2.
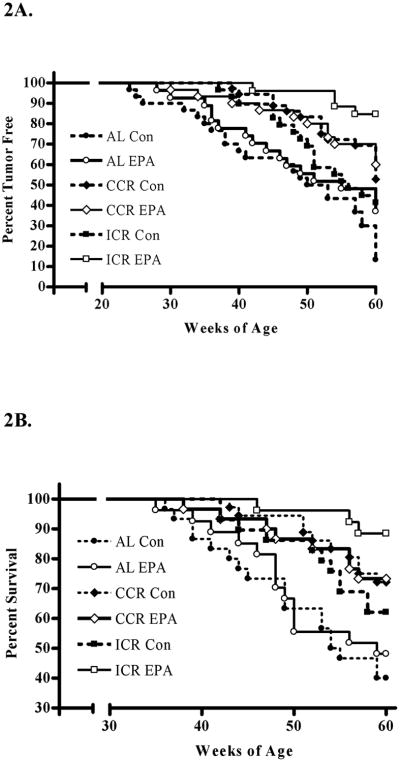
Disease free and survival curves of MMTV-Her2/neu mice in response to dietary interventions. Mice were either fed AL, CCR or ICR using diets with 10.1% fat calories from soy oil (Con) or with 2.85% fat from soy oil and 7.25% fat from eicosapentaenoic acid (EPA). A) Tumor formation was determined by palpation of a 7mm tumor. Percentage of tumor free mice is shown along the y-axis and the age of the mice is shown along the x-axis. B) Percent of surviving mice is shown along the y-axis and age of the mice is shown along the x-axis. Termination was determined by mammary tumor size or associated endpoints.
An overview of the experimental results is provided in Table 2. We examined the amounts of food that the mice consumed over the course of the experiment. When the control mice from each dietary restriction regimen were compared to the EPA fed mice there were no significant differences. The percentage of tumor free mice was significantly lower in the AL-Con group when compared to all other groups and the percentage of tumor free mice was significantly higher in the ICR-EPA group as compared to all other groups over the course of the 60 week study. The ICR-EPA group had 22 of 26 mice remaining tumor free during the 60 week study while only 4 of 30 mice from the AL-Con groups remained tumor free. The average age of the mice in weeks when a tumor was palpated was earliest in the AL fed groups. The fact that the experiment ended when the mice were 60 weeks of age influenced the average age of survival. However, the average survival until terminal end point was longest in the ICR-EPA group at 59.2 weeks and shortest in the AL-Con group at 52.6 (P<0.0002). The average number of tumors per mouse that developed at least one tumor and the total tumor burden per animal was not significantly different between any of the groups despite the fact that the average tumor burden was 0.98 grams for ICR-EPA mice and 1.78 grams for AL-Con mice. This was due to the very small number of mice with mammary tumors in the ICR-EPA group. All tumors were breast cancer and not benign tumors although they were low grades that were well differentiated (grade 1) or with some differentiation (grade 2). No significant differences in tumor grade between the groups were found. There was a single metastasis in the AL-Con group.
Table 2.
Comparison of tumor characteristics among the groups.
| Group | Ave Daily Food Intake (grams) | Tumor Free | % Tumor Free | Week to Palpable Tumory | Ave Survivalz | Tumors Per Animaly | Tumor Wt Per Animaly (grams) | % Grade 1 Tumors | % Grade 2 Tumors |
|---|---|---|---|---|---|---|---|---|---|
| AL Con | 4.17 | 4/30a | 13.3a | 46.1a | 52.6a | 1.46 | 1.78 | 87.5 | 12.5 |
| AL EPA | 4.18 | 12/27b | 44.4b | 44.5a,b | 53.4a,b | 1.71 | 1.73 | 82.4 | 17.6 |
| CCR Con | 3.13 | 19/36b | 52.8b | 52.4b,c | 57.9b,c | 1.59 | 1.52 | 58.3 | 41.7 |
| CCR EPA | 3.14 | 18/30b | 60.0b | 48.7b,c | 57.2b,c | 1.27 | 1.48 | 75.0 | 25.0 |
| ICR Con | 3.13 | 12/29b | 41.4b | 49.1b | 56.6a,b,c | 1.58 | 1.44 | 81.3 | 18.7 |
| ICR EPA | 3.14 | 22/26c | 84.6c | 51.8c | 59.2c | 1.25 | 0.98 | 75.0 | 25.0 |
tumor bearing animals only;
all animals
Significance<0.05 between groups is denoted by superscripts
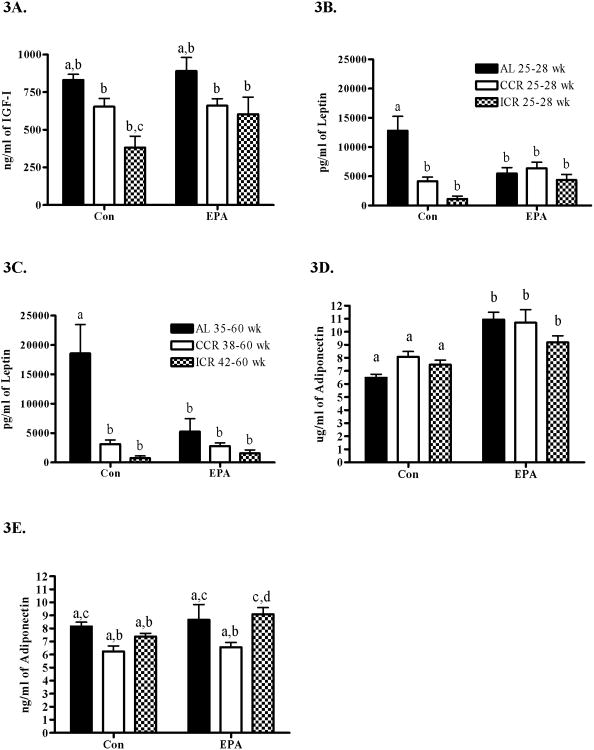
To better understand the effects of the interventions we examined serum for alterations in growth factors and adipokines. Figure 3A shows that serum IGF-I was significantly reduced by calorie restriction with the ICR-Con being significantly different than the AL-Con (P<0.01) and the AL-EPA (P<0.001). The presence of EPA in the diet did not significantly change the levels of IGF-I. We found that serum leptin was significantly higher in the AL-Con mice compared to the corresponding calorie restricted mice (AL-Con > CCR-Con > ICR-Con). The presence of EPA in the diet significantly reduced the levels of leptin in the AL-EPA mice compared to the AL-Con mice (P<0.02) but combining EPA with either CCR or ICR did not result in any significant changes verses the CCR-Con or ICR-Con groups. This was true regardless of whether the sera were obtained during the middle of the study at 25-28 weeks of age (Figure 3B) before the appearance of palpable tumors or at the terminal end points of the study when the mice were 35-60 weeks of age (Figure 3C). The levels of adiponectin in the AL-Con, CCR-Con and ICR-Con mice compared to the mice fed EPA were significantly lower (Overall ANOVA P<0.0001) but dietary restriction did not significantly change the adiponectin levels at the study midpoint (Figure 3D). The levels of serum adiponectin at the endpoint (Figure 3E) were more similar between the groups although there were significant differences between some groups (Overall ANOVA P<0.0036).
Figure 3.
Serum growth factors and adipokines in relationship to calorie restriction and dietary fat. The AL fed animals are shown with filled bars. The CCR fed animals are shown with open bars and the ICR fed animals are shown with checked bars. A) IGF-I was measured in serum obtained at euthanasia and analyzed in duplicate by ELISA from 6-7 mice per group. B) Leptin was obtained midstudy (25-28 weeks of age) or C) at the terminal endpoint which was determined by mammary tumor size or associated endpoints. Serum was analyzed for leptin by ELISA in duplicate from 6-12 mice per group. D) Adiponectin from serum obtained midstudy (25-28 weeks of age) or E) at the terminal endpoint which was determined by mammary tumor size or associated endpoints. Serum was analyzed for adiponectin by ELISA in duplicate from 6-12 mice per group. The diets are shown at the bottom of each graph and the calorie restriction protocol is identified in the legend to the right of the graph. Bars represent standard error of the mean and letters above the bars show significant differences.
Discussion
The goals of this project were to determine if two nutritional interventions that individually protect against mammary tumor development would provide even greater protection when combined and to identify potential mechanisms of action. The first nutritional intervention was alteration of the ratio of ω-3 to ω-6 fatty acids in the diet by addition to the ω-3 fatty acid EPA. The second nutritional intervention was calorie restriction. Highlights of our findings include that ICR in combination with EPA, resulted in significantly increased survival and tumor free periods compared to all other treatments (Figure 2). This may be related to the alteration of serum growth regulatory molecules. The first dietary intervention, addition of the ω-3 fatty acid EPA, was able to alter serum levels of adiponectin (Figure 3D and 3E) and the second intervention, calorie restriction, was able to alter levels of serum IGF-I and leptin (Figure 3A, 3B and 3C).
When we evaluated each intervention alone we found that the addition of EPA to the diets of the AL fed animals resulted in a trend toward increased times to survival and longevity as well as a significant difference in the absolute percentage of animals that developed tumors as compared to the AL control fed animals (Table 2). EPA may be acting in part through both leptin and adiponectin. The addition of EPA to the diet resulted in a significant decrease in serum leptin in all groups as compared to the AL-Con mice regardless of whether the serum was obtained before or after palpable tumors had arisen (Figures 3B and 3C). Leptin is positively correlated with total body fat and is present in the serum of almost all humans. Leptin has been implicated as a growth promoter in breast cancer (32). One dramatic example is that mice deficient in leptin, LepobLepob, or with nonfunctioning leptin receptors, LeprdbLeprdb, did not develop transgene-induced mammary tumors (33-34). Some human studies indicate that the presence of the leptin receptor, Ob-Rb, in breast cancer (35) and high serum leptin are associated with poor prognosis (36). Calorie restriction is well documented to result in a reduction in serum leptin in humans (37) and a decrease in tumorigenesis in animal studies (7, 38). Therefore it is interesting that the addition of EPA to the diet was able to mimic the effects of calorie restriction on serum leptin (Figures 3B and 3C).
Adiponectin was significantly increased by EPA at the middle time point (Figure 3D) and to a lesser degree at the endpoint of the study (Figure 3E). Increased serum adiponectin has been associated with decreased risk of breast cancer, particularly in post-menopausal women (39). Adiponectin has been shown to be negatively correlated with body fat (17) in humans and it was originally assumed that the amount of body fat was the factor regulating how much adiponectin was in the serum. However, this study as well as others (14) indicates that regulation of serum adiponectin may actually be related to dietary components such as EPA.
When we evaluated the effects of calorie restriction in the absence of EPA, both by chronically or intermittently restricting calories, we found significant differences in tumorigenesis between the restricted groups and the ad libitum fed groups. The AL-Con mice had significantly reduced tumor free and survival times compared to the CCR-Con mice (P<0.01) as well as significantly more tumor bearing mice than either the CCR-Con (P<0.05) or the ICR-Con (P<0.05) groups. We did not find any significant differences between the CCR-Con and the ICR-Con groups. Previously in a different transgenic mouse model we found that the ICR diet regimen inhibited mammary tumorigenesis to a greater extent than the CCR diet regimen (8-9). It may be that Her2/neu over expressing mammary tumors in mice are less responsive to the ICR diet regimen than the tumors in mice that over express TGF-α It has become evident that breast cancer is composed of many different types of cancer that respond differently to treatment (40). Evaluation of the clinical meaning of the differences between the Her2/neu over expressing tumors compared to the TGF-α over expressing tumors may help elucidate the possible role of different diet regimens as they pertain to specific types of breast cancer.
How CCR and ICR are able to inhibit mammary tumorigenesis as compared to AL feeding and each other is probably multifaceted. We found that ICR was able to decrease serum IGF-I compared to AL fed mice but that there was not a significant difference between CCR and ICR (Figure 3A) groups. We also found that both CCR and ICR interventions were able to reduce levels of serum leptin as compared to AL feeding but not as compared to each other (Figure 3B and 3C). These results parallel with the MT inhibition that we found and illustrate the correlation in this model between levels of serum IGF-I and leptin with mammary tumor inhibition.
Calorie restriction and weight loss either through conventional dietary restriction or bariatric surgery have been reported to be linked to breast cancer inhibition in humans (41-42). The effects of ICR verses CCR in humans have not been well investigated. However, Harvie et al. found that serum leptin levels were reduced by approximately 40% in women who underwent either chronic or intermittent calorie restriction for six months to attain an overall 25% reduction in caloric intake while adiponectin increased by 10% in the intermittent group versus 1% in the chronic group although this was not a significant difference (43). There was no significant effect on the adiponectin:leptin ratio. A study of the effects of anorexia nervosa on the incidence of breast cancer has been published (44). It was found that in the anorexia nervosa group there was a 53% reduction in breast cancer incidence with a 23% reduction in nulliparous women and a 76% reduction in incidence in parous women compared to the general population. These results suggest that both CCR and ICR may be beneficial in reducing breast cancer risk in humans.
Finally we evaluated the combined effects of both CCR and ICR with EPA. The addition of EPA to the CCR fed mice did not result in a significant difference in MT development as compared to the CCR control mice. However, addition of EPA to the ICR protocol resulted in significant differences in the percentage of tumor free mice, the average tumor free time, and survival compared to the ICR-Con mice with soy oil as the fat source (Table 2). These results suggest an additive or possibly even a synergistic effect when ICR was combined with EPA for the inhibition of breast cancer. EPA alone was able to significantly decrease serum leptin and increase serum adiponectin levels as compared to AL mice.
ICR was able to decrease serum IGF-I and serum leptin as compared to the AL fed mice. It seems likely that alteration of these growth regulators is part of the mechanism by which the ICR-EPA mice were able to inhibit mammary tumorigenesis. However, there was also significant inhibition of mammary tumorigenesis in the ICR-EPA mice as compared to the CCR-EPA mice but without any differences in the serum levels of IGF-I, leptin or adiponectin. This suggests that additional mechanisms of action are most likely influencing the ICR-EPA to CCR-EPA differences in mammary tumor inhibition.
In summary, this project was performed to characterize a novel combination of dietary interventions for inhibition of breast cancer and to examine the role of growth regulators in breast cancer inhibition. The work provides research to help evaluate their use of fatty acids and calorie restriction for breast cancer prevention on which to base future investigations. The best inhibition was achieved by the combination of ICR with EPA. On a practical note, it may be easier for some humans to tolerate short periods of caloric restriction and substitutions of EPA for a portion of their fat intake than a lifetime of chronic calorie restriction and a low fat diet.
Acknowledgments
Grant Support: The Susan G. Komen for the Cure® grant KG081178 and The Hormel Foundation provided financial support for this work.
Financial Support: Susan G. Komen for the Cure® grant KG081178 and The Hormel Foundation.
Footnotes
Disclosure of Potential Conflicts of Interest: There are no potential conflicts of interest.
References
- 1.Tirona MT, Sehgal R, Ballester O. Prevention of breast cancer (Part II): risk reduction strategies. Cancer Invest. 2010;28:1070–7. doi: 10.3109/07357907.2010.512593. [DOI] [PubMed] [Google Scholar]
- 2.Kolonel LN, Altshuler D, Henderson BE. The multiethnic cohort study: exploring genes, lifestyle and cancer risk. Nature reviews. 2004;4:519–27. doi: 10.1038/nrc1389. [DOI] [PubMed] [Google Scholar]
- 3.Nelson NJ. Migrant studies aid the search for factors linked to breast cancer risk. J Natl Cancer Inst. 2006;98:436–8. doi: 10.1093/jnci/djj147. [DOI] [PubMed] [Google Scholar]
- 4.Dyerberg J, Bang HO, Hjorne N. Fatty acid composition of the plasma lipids in Greenland Eskimos. Am J Clin Nutr. 1975;28:958–66. doi: 10.1093/ajcn/28.9.958. [DOI] [PubMed] [Google Scholar]
- 5.Buell P. Changing incidence of breast cancer in Japanese-American women. J Natl Cancer Inst. 1973;51:1479–83. doi: 10.1093/jnci/51.5.1479. [DOI] [PubMed] [Google Scholar]
- 6.Simonsen N, van't Veer P, Strain JJ, et al. Adipose tissue omega-3 and omega-6 fatty acid content and breast cancer in the EURAMIC study. European Community Multicenter Study on Antioxidants, Myocardial Infarction, and Breast Cancer. Am J Epidemiol. 1998;147:342–52. doi: 10.1093/oxfordjournals.aje.a009456. [DOI] [PubMed] [Google Scholar]
- 7.Dirx MJ, Zeegers MP, Dagnelie PC, van den Bogaard T, van den Brandt PA. Energy restriction and the risk of spontaneous mammary tumors in mice: a meta-analysis. Int J Cancer. 2003;106:766–70. doi: 10.1002/ijc.11277. [DOI] [PubMed] [Google Scholar]
- 8.Cleary MP, Jacobson MK, Phillips FC, Getzin SC, Grande JP, Maihle NJ. Weight-cycling decreases incidence and increases latency of mammary tumors to a greater extent than does chronic caloric restriction in mouse mammary tumor virus-transforming growth factor-alpha female mice. Cancer Epidemiol Biomarkers Prev. 2002;11:836–43. [PubMed] [Google Scholar]
- 9.Cleary MP, Hu X, Grossmann ME, et al. Prevention of mammary tumorigenesis by intermittent caloric restriction: does caloric intake during refeeding modulate the response? Exp Biol Med (Maywood) 2007;232:70–80. [PubMed] [Google Scholar]
- 10.Yee LD, Young DC, Rosol TJ, Vanbuskirk AM, Clinton SK. Dietary (n-3) polyunsaturated fatty acids inhibit HER-2/neu-induced breast cancer in mice independently of the PPARgamma ligand rosiglitazone. J Nutr. 2005;135:983–8. doi: 10.1093/jn/135.5.983. [DOI] [PubMed] [Google Scholar]
- 11.Rose DP, Connolly JM. Effects of dietary omega-3 fatty acids on human breast cancer growth and metastases in nude mice. J Natl Cancer Inst. 1993;85:1743–7. doi: 10.1093/jnci/85.21.1743. [DOI] [PubMed] [Google Scholar]
- 12.Schley PD, Jijon HB, Robinson LE, Field CJ. Mechanisms of omega-3 fatty acid-induced growth inhibition in MDA-MB-231 human breast cancer cells. Breast Cancer Res Treat. 2005;92:187–95. doi: 10.1007/s10549-005-2415-z. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto D, Kiyozuka Y, Adachi Y, Takada H, Hioki K, Tsubura A. Synergistic action of apoptosis induced by eicosapentaenoic acid and TNP-470 on human breast cancer cells. Breast Cancer Res Treat. 1999;55:149–60. doi: 10.1023/a:1006283131240. [DOI] [PubMed] [Google Scholar]
- 14.Siriwardhana N, Kalupahana NS, Moustaid-Moussa N. Health benefits of n-3 polyunsaturated fatty acids: eicosapentaenoic acid and docosahexaenoic acid. Adv Food Nutr Res. 2012;65:211–22. doi: 10.1016/B978-0-12-416003-3.00013-5. [DOI] [PubMed] [Google Scholar]
- 15.Harvie M, Howell A. Energy restriction and the prevention of breast cancer. The Proceedings of the Nutrition Society. 2012;71:263–75. doi: 10.1017/S0029665112000195. [DOI] [PubMed] [Google Scholar]
- 16.Rossi AS, Lombardo YB, Lacorte JM, et al. Dietary fish oil positively regulates plasma leptin and adiponectin levels in sucrose-fed, insulin-resistant rats. American journal of physiology. 2005;289:R486–R94. doi: 10.1152/ajpregu.00846.2004. [DOI] [PubMed] [Google Scholar]
- 17.Ryan AS, Berman DM, Nicklas BJ, et al. Plasma adiponectin and leptin levels, body composition, and glucose utilization in adult women with wide ranges of age and obesity. Diabetes Care. 2003;26:2383–8. doi: 10.2337/diacare.26.8.2383. [DOI] [PubMed] [Google Scholar]
- 18.Grossmann ME, Ray A, Nkhata KJ, et al. Obesity and breast cancer: status of leptin and adiponectin in pathological processes. Cancer Metastasis Rev. 2010;29:641–53. doi: 10.1007/s10555-010-9252-1. [DOI] [PubMed] [Google Scholar]
- 19.Macis D, Gandini S, Guerrieri-Gonzaga A, et al. Prognostic effect of circulating adiponectin in a randomized 2 × 2 trial of low-dose tamoxifen and fenretinide in premenopausal women at risk for breast cancer. J Clin Oncol. 2012;30:151–7. doi: 10.1200/JCO.2011.35.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wadden TA, Considine RV, Foster GD, Anderson DA, Sarwer DB, Caro JS. Short- and long-term changes in serum leptin dieting obese women: effects of caloric restriction and weight loss. J Clin Endocrinol Metab. 1998;83:214–8. doi: 10.1210/jcem.83.1.4494. [DOI] [PubMed] [Google Scholar]
- 21.Dieudonne MN, Machinal-Quelin F, Serazin-Leroy V, Leneveu MC, Pecquery R, Giudicelli Y. Leptin mediates a proliferative response in human MCF7 breast cancer cells. Biochem Biophys Res Commun. 2002;293:622–8. doi: 10.1016/S0006-291X(02)00205-X. [DOI] [PubMed] [Google Scholar]
- 22.Weroha SJ, Haluska P. The insulin-like growth factor system in cancer. Endocrinol Metab Clin North Am. 2012;41:335–50. vi. doi: 10.1016/j.ecl.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sachdev D. Targeting the type I insulin-like growth factor system for breast cancer therapy. Curr Drug Targets. 2010;11:1121–32. doi: 10.2174/138945010792006816. [DOI] [PubMed] [Google Scholar]
- 24.Chen DC, Chung YF, Yeh YT, et al. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett. 2006;237:109–14. doi: 10.1016/j.canlet.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 25.Mohanraj L, Oh Y. Targeting IGF-I, IGFBPs and IGF-I receptor system in cancer: the current and future in breast cancer therapy. Recent Pat Anticancer Drug Discov. 2011;6:166–77. doi: 10.2174/157489211795328512. [DOI] [PubMed] [Google Scholar]
- 26.Gradishar WJ. HER2 therapy--an abundance of riches. N Engl J Med. 2012;366:176–8. doi: 10.1056/NEJMe1113641. [DOI] [PubMed] [Google Scholar]
- 27.Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2012;9:16–32. doi: 10.1038/nrclinonc.2011.177. [DOI] [PubMed] [Google Scholar]
- 28.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Molecular and cellular biology. 1992;12:954–61. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 30.Thigpen JE, Setchell KD, Goelz MF, Forsythe DB. The phytoestrogen content of rodent diets. Environmental health perspectives. 1999;107:A182–3. doi: 10.1289/ehp.107-1566530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Council NR, editor. Nutrient Requirements of Laboratory Animals. Washington, DC: National Academy Press; 1995. [Google Scholar]
- 32.Vona-Davis L, Rose DP. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocrine-related cancer. 2007;14:189–206. doi: 10.1677/ERC-06-0068. [DOI] [PubMed] [Google Scholar]
- 33.Cleary MP, Phillips FC, Getzin SC, et al. Genetically obese MMTV-TGF-alpha/Lep(ob)Lep(ob) female mice do not develop mammary tumors. Breast Cancer Res Treat. 2003;77:205–15. doi: 10.1023/a:1021891825399. [DOI] [PubMed] [Google Scholar]
- 34.Cleary MP, Juneja SC, Phillips FC, Hu X, Grande JP, Maihle NJ. Leptin receptor-deficient MMTV-TGF-alpha/Lepr(db)Lepr(db) female mice do not develop oncogene-induced mammary tumors. Exp Biol Med (Maywood) 2004;229:182–93. doi: 10.1177/153537020422900207. [DOI] [PubMed] [Google Scholar]
- 35.Garofalo C, Koda M, Cascio S, et al. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin Cancer Res. 2006;12:1447–53. doi: 10.1158/1078-0432.CCR-05-1913. [DOI] [PubMed] [Google Scholar]
- 36.Miyoshi Y, Funahashi T, Tanaka S, et al. High expression of leptin receptor mRNA in breast cancer tissue predicts poor prognosis for patients with high, but not low, serum leptin levels. Int J Cancer. 2006;118:1414–9. doi: 10.1002/ijc.21543. [DOI] [PubMed] [Google Scholar]
- 37.Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 38.Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nature medicine. 1995;1:1311–4. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 39.Miyoshi Y, Funahashi T, Kihara S, et al. Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res. 2003;9:5699–704. [PubMed] [Google Scholar]
- 40.Perou CM, Borresen-Dale AL. Systems biology and genomics of breast cancer. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCawley GM, Ferriss JS, Geffel D, Northup CJ, Modesitt SC. Cancer in obese women: potential protective impact of bariatric surgery. Journal of the American College of Surgeons. 2009;208:1093–8. doi: 10.1016/j.jamcollsurg.2009.01.045. [DOI] [PubMed] [Google Scholar]
- 42.Howell A, Chapman M, Harvie M. Energy restriction for breast cancer prevention. Recent Results Cancer Res. 2009;181:97–111. doi: 10.1007/978-3-540-69297-3_11. [DOI] [PubMed] [Google Scholar]
- 43.Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. International journal of obesity (2005) 2011;35:714–27. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michels KB, Ekbom A. Caloric restriction and incidence of breast cancer. Jama. 2004;291:1226–30. doi: 10.1001/jama.291.10.1226. [DOI] [PubMed] [Google Scholar]