Abstract
A number of studies indicate that a growing list of cancers may be influenced by obesity. In obese individuals these cancers can be more frequent and more aggressive resulting in reduced survival. One of the most prominent and well characterized cancers in this regard is breast cancer. Obesity plays a complex role in breast cancer and is associated with increased inflammation, angiogenesis and alterations in serum levels of potential growth factors such as adiponectin, leptin and estrogen in the serum. Reduced levels of serum adiponectin have been reported in breast cancer patients compared to healthy controls, particularly in postmenopausal women. The role of serum leptin levels in breast cancer appears to be more complex. Some studies have shown leptin to be increased in women with breast cancer but other studies have found leptin to be decreased or unchanged. This may be due to a number of confounding issues. We and others propose that it may be the levels of adiponectin and leptin as well as the balance of adiponectin and leptin that are the critical factors in breast and other obesity related cancer tumorigenesis.
1. Introduction
There has been increasing interest in the role of body weight particularly overweight and/or obesity in association with cancer development as well as in its progression and prognosis. In general, the focus of the relationship has been body weight at the time of diagnosis as to whether overweight/obesity increases risk and how body weight status impacts prognosis with respect to disease free survival and/or mortality. With the increasing interest in this subject attempts have been made to evaluate body weight status prior to cancer diagnosis- but in humans this is a daunting task- relying either on recall or expensive prospective studies.
Breast cancer has been the most widely investigated malignancy for the evaluation of body weight’s impact as a risk factor due to the clear association of body fatness with increased postmenopausal breast cancer risk [1]. Estrogen has been implicated as the primary driving force of how elevated body weight could promote the development of this disease [2]. But how this finding would relate to cancers of other organs and to cancer in men in relationship to body weight leave open the possibility for additional causes. Other growth factors such as growth hormone, IGF-I and insulin which may be impacted by body weight status have also been implicated as mediators of the role of obesity in cancer [3-4]. Additional proteins synthesized in adipose tissue, adipokines, have also been considered to have a role in relationship of body weight and cancer, in particular leptin and adiponectin. These two proteins will be the focus of this manuscript particularly with regards their role in the development of breast/mammary cancer.
2. Leptin
2.1 Overview
The discovery in 1994 of leptin and shortly thereafter adiponectin eventually presented new possibilities to explain how body weight through body fat might directly impact carcinogenesis. Although leptin and adiponectin were identified at around the same time, initially the major focus was on leptin’s impact on both normal and abnormal physiologly. Leptin, a 16 kDa cytokine was discovered by positional cloning of the ob gene in 1994 [5]. Early investigations identified leptin as a regulator of body weight, food intake and energy balance acting through receptors in the hypothalamus. However it is now known to also affect fetal development, sex maturation, lactation, hematopoiesis and immune responses [6-14]. Circulating leptin levels are usually proportional to total adipose tissue mass, i.e. increased in obese and decreased in lean subjects [15-16]. Serum leptin levels are significantly higher in women than men even when adjusted for age, body mass index (BMI = weight (kg)÷height (m2 )) and total body fat [17-19].
Leptin exerts its cellular functions through transmembrane leptin receptors (ObR) [20]. A number of isoforms of ObR have been identified but the long isoform (Ob-Rl/Rb) contains an active intracellular signaling domain and has the ability to activate intracellular JAK2/STAT, Ras/ERK-1/2 and PI3-K/Akt/GSK3 pathways [21-23]. Short (Ob-Rs) isoforms lack major domains and mainly activate MAPK and have little effect on STAT activation [21, 24] although short forms of Ob-R may be involved in intra- and transcellular transport [25].
2.2 Leptin and Cancer
Leptin as a potential growth factor in malignancies was first addressed in a 1998 publication by Nakao et al identifying the presence of the leptin receptor in fresh human leukemic cells [26]. Another publication reported expression of leptin receptor isoforms in myeloid leukemia and in hematopoietic cells lines [27]. Additionally, it was reported that with increasing concentrations of leptin increased cell proliferation occurred and apoptosis was inhibited in these cell lines. The leptin receptor was also found to be expressed in adrenal tumor cells although the proliferation of the cells was not affected by additional of leptin [28]. In human neuroblastoma cell lines Ob-Rb mRNA was expressed [29] while in a rat glioblastoma cell line the short form of the leptin receptor as well as leptin were identified [30].
Following these publications suggesting a role for leptin in tumor growth a number of studies were initiated to investigate the role of leptin in breast cancer. Initially, serum leptin levels did not appear to be consistently increased in women with breast cancer despite the fact that leptin levels were significantly higher in breast cancer tissue compared with normal breast tissue. However, most of these studies used small numbers of subjects, only premenopausal subjects or combined pre and postmenopausal women [31-37]. More recent studies have differentiated between peri and postmenopausal patients and found a dichotomy in the effects of leptin. When perimenopausal breast cancer patients were examined leptin was inversely associated with breast cancer risk [38]. However postmenopausal breast cancer patients with ER positive breast cancer had serum leptin levels significantly correlated with pathological tumor classification and TNM stage [39]. In a separate study leptin receptor expression in breast carcinoma was positively correlated with ER expression and tumor size.[40] Studies also indicate that the combined presence of the leptin receptor, Ob-Rb, in breast tumors and high serum leptin are associated with poor prognosis [34, 41] and over-expression of both leptin and leptin receptors in breast cancer tissue was associated with distant metastasis.[42]
Concurently with the human investigations in vitro studies characterized the effect of leptin on cellular proliferation of breast cancer cell lines. In particular, extensive evaluation of leptin as it might relate obesity to breast cancer was done. In 2002 three publications established the presence of the leptin receptor in MCF-7 and T47-D human breast cancer cells and reported that the addition of physiological levels of leptin increased cell proliferation [43-45]. Subsequent studies have examined additional breast cancer cell lines with respect to leptin and cell proliferation as well as determining effects of leptin on various cell signaling pathways [46-50]. In general it has been found that ER-positive MCF-7 and T47-D cells express high levels of Ob-Rb while the shorter forms are present in ER-negative MDA-MB-231 and MDA-MB-435 cell lines [50]. In addition, ObR and ER-α are coexpressed in some breast cancer cell lines [44-45].
In an attempt to determine how obesity may impact breast cancer we planned studies cross-breeding genetically obese mice with a transgenic mouse strain that develops mammary tumors. The rationale for choosing genetically obese mice was to avoid complications of data interpretation associated with feeding a high-fat diet to induce obesity. Further, since we wanted to apply the findings to human postmenopausal breast cancer we chose MMTV-TGF-α mice that develop hormone responsive mammary tumors in the second year of life [51]. At the time of undertaking these studies two of the most commonly used genetically obese mice were ob/ob and db/db. Both strains were crossed with the MMTV-TGF-α mice and followed for two years. As summarized in Table 1 in both studies the obese mice did not develop mammary tumors, in contrast their lean counterparts developed mammary tumors in the range of 50-69% incidence [52-53]. During the time these studies were underway it became widely recognized that ob/ob mice now termed LepobLepob were leptin-deficient [5] and db/db mice now termed LeprdbLeprdb were leptin receptor-deficient [20]. These two studies in conjunction with the in vitro experiments described above, provided strong evidence for a role of leptin in mammary tumor development. However, the role of the leptin receptor in the process of mammary tumorigenesis as evidenced from preclinical studies with rats is not clear. For example, when genetically obese Zucker rats which also have a leptin receptor defect were administered the chemical carcinogen, methyl nitrosourea, no mammary tumors developed [54] and we did not detect mammary tumors in obese Zucker rats following administration of DMBA another chemical carcinogen (Cleary, MP and Morton, R., unpublished data). However Hakkak and coworkers have reported that obese Zucker rats had greater susceptibility to DMBA than did lean rats [55]. These different results may be attributable to substrains of the Zucker rats.
Table 1.
| Study 1 | Study 2 | ||||
|---|---|---|---|---|---|
| Genotype | Number of mice |
Mammary tumor positive |
Genotype | Number of mice |
Mammary tumor positive |
| TGF-α/Lep+Lep+ lean |
38 | 50% | TGF-α/Lepr+Lepr+ lean |
40 | 69% |
| TGF-α/LepobLepob obese |
59 | 0% | TGF-α/LeprdbLeprdb obese |
43 | 0% |
Adapted from Cleary, M.P. et al. 2003 and Cleary, M.P. et al. 2004
To investigate the role of leptin and body weight in mammary tumor development of animals with normal leptin and leptin receptor genes we used a high–fat diet protocol to induce obesity in the MMTV-TGF-α mice. High-fat diet fed mice were separated by body weight status into Obesity-Prone and Obesity-Resistant groups as previously done in rats [56-57]. In earlier studies the animals in the middle weight category were removed from the experiment [58] but these mice were included in our study and termed Overweight. It was found that Obesity-Prone mice had mammary tumors detected at a significantly younger age compared to those that remained lean on the high fat diet [59]. Obesity-Prone mice also developed high-grade adenocarcinomas while mice in other weight categories were found to only have low-grade adenocarcinomas. In contrast, body weight did not impact tumor development in obese-MMTV-neu mice which develop ER-negative mammary tumors [60].
These findings were consistent with human studies that obesity is a risk factor for hormone-dependent breast cancer in postmenopausal women [61-63]. However, the two transgenic mouse strains were also on two different background strains. Thus, the next approach to assess the impact of body weight on mammary tumor development was to implant obese mice with either ER-positive, MCF-7, or ER-negative, MDA-MB-231, human breast cancer cells and monitor tumor growth [64]. The MCF-7 cells did not grow particularly well so it was not possible to evaluate the impact of body weight on ER-positive cells. As expected tumor weights were not associated with body weight in the mice inoculated with the ER-negative MDA-MB-231 cells. Serum leptin levels were three-fold higher in the Obesity-Prone mice compared to those of the Obesity-Resistant mice. However, measurements of growth-related proteins in the MDA-MD-231 mammary tumors were mostly impacted by consumption of a high fat diet not with serum leptin levels. For example, OB-Rb, BAX, BCL-2 and IGF-IR were all expressed to a greater extent in mice fed the high fat diet compared to those fed the low-fat control diet [64]. Due to the very small tumors that developed from the MCF-7 cells no examination of tumor protein levels were done.
Several recent studies have evaluated direct effects of leptin on tumorigenesis. For example, mice treated daily with 1μg/g body weight of leptin had significantly greater tumor weight following inoculation with melanoma cells than did control mice as well as mice treated with leptin and 9F8 which is a monoclonal blocking antibody for the human leptin receptor [65]. In another study leptin deficient mice treated with leptin increased tumor size using a carcinogen-induced colon polyp formation model [66]. The inverse of the above studies utilizing a leptin receptor antagonist to directly determine the role of leptin in tumor development has also been reported. The leptin receptor antagonist peptide, Allo-aca, inhibited leptin-induced proliferation of MDA-MB-231 cells in vitro. In addition, the antagonist significantly extended average survival time in an orthotopic mouse xenograft model utilizing MDA-MB-231 cells [67]. The use of pegylated leptin peptide receptor antagonist 2 also significantly reduced the tumor volume of both MDAMB-231 and MCF-7 orthotopic mouse xenografts [68]. These studies consolidate the importance of leptin in tumor growth although the exact mechanisms of action and how they impact human tumor development remain to be fully elucidated.
3. Adiponectin
3.1 Overview
Adiponectin was first identified in the mid 1990’s [69]. It is found at high concentrations (2-20 ug/ml) in human serum [70-75] and in contrast to most adipose secreted proteins, is negatively correlated with body weight, BMI, body fat and serum leptin in humans [76]. Low levels of adiponectin were implicated in pathological conditions such as insulin resistance, type 2 diabetes and coronary artery disease [77]. Adiponectin appears to have global effects on a number of different aspects of physiology and cell growth.
Cellular actions of adiponectin are mediated through two main adiponectin receptors, AdipoR1 and AdipoR2 [78] and there are two main forms of adiponectin, full-length and cleaved or globular adiponectin [79]. Full-legnth adiponectin binds with highest affinity to AdipoR2 [80] while globular adiponectin binds with highest affinity to AdipoR1 [78] . It is the combination of these interactions that result in multiple physiological effects attributable to adiponectin.
3.2 Adiponectin and Cancer
Interest in adiponectins potential involvement in tumorigenesis was not evident for a number of years. Then it was reported that lower serum adiponectin levels were found in women diagnosed with postmenopausal breast cancer compared to those without this disease [70, 72, 81]. Endometrial cancer has also been associated with reduced serum adiponectin levels [82-83]. Reduced serum adiponectin was detected in men diagnosed with prostate cancer compared to men with benign prostatic hyperplasia or healthy controls [84]. In another study adiponectin was not associated with the overall risk of prostate cancer but higher adiponectin levels were associated with lower grade cancers and with a lower risk of dying from prostate cancer [85]. In a nested case-control study diagnosis of colorectal cancer was associated with reduced serum adiponectin levels [86]. Reduced adiponectin levels have also been reported in patients with gastric cancer and were further related to increased tumor stage [87].
In ductal breast carcinoma in situ AdipoR1 expression was inversely correlated with tumor size [88]. Additionally, when adiponectin variants were utilized to classify patients as high, intermediate or low signalers it was found that compared with high signalers, intermediate signalers had a 4.16-fold increase in breast cancer risk (95% CI, 0.49-35.19), and low signalers had a 6.56-fold increase in breast cancer risk [89]. Other types of cancer also express adiponectin receptors and may be regulated by adiponectin. For example, weaker expression of adiponectin receptors AdipoR1 and AdipoR2 was found in prostate cancer compared to healthy prostate tissue [84]. One study of colorectal cancer risk found that AdipoR1 expression was negatively associated with nodal stage while AdipoR2 expression was positively associated with tumor, node and metastasis stage [90]. A second study of colorectal cancer found that both AdipoR1 and AdipoR2 expression levels were inversely related to cancer stage [91]. Not all tumors express AdipoR1 and AdipoR2 and it has been suggested that obesity related tumors more ubiquitously express the adiponectin receptors [92] and as such these types of tumors are more likely to be targets of adiponectin regulation.
As with leptin, in vitro studies have complemented and expanded knowledge of adiponectin’s affects. For example, antiproliferative responses of various cancer cell lines following the addition of adiponectin have been reported. This includes prostate [93-94], endometrial, [95], gastric [96] and colon [94] cell lines as well as a number of studies using human breast cancer cell lines. With respect to breast cancer cell lines MDA-MB-231 cells have been shown to respondto the addition of adiponectin with reduced proliferation as well as increased apoptosis [97-99]. Further, MCF-7 human breast cancer cell lines also have reduced proliferation in response to adiponectin [100-101]. A similar study using MCF-7 cells confirmed the antiproliferative effect of adiponectin and its effects on enhancing apoptosis [102]. When several different breast cancer cell lines were included in the same publication it was reported that MCF-7, T47-D and SK-BR-3 cells had reduced proliferation in response to adiponectin while no effects were found for MDA-MB-231 and MDA-MB-361 cell lines [103]. However in a separate study when MDA-MB-231 and MDA-ERα7 ( MDA-MB-231 cell line transfected with ERalpha) cells were treated with either adiponectin or globular adiponectin and leptin there was a reduction in proliferation of MDA-MB-231 and MDA-ERα7 cells as compared to cells treated with leptin alone [104] suggesting that adiponectin was able to block the proliferative effects of leptin in these cell lines. In addition it was reported that adiponectin blocked effects of IGF-I stimulated proliferation. The mechanism involved increased phosphorylation of AMPKalpha and decreased activated Akt. Adiponectin also increased intracellular levels of cAMP and the activity of protein kinase-A (PKA) [102]. Overall these findings demonstrate the potential for adiponectin to block the cell proliferation actions of multiple different types of growth factors that may be responsible for proliferation of breast cancer. However, there are still aspects of cell characteristics which may alter this response and remain to be determined.
Preclinical studies with various mouse models have also been used to evaluate adiponectins impact on tumor development. Adiponectin knockout mice that were fed a choline deficient L-amino acid-defined diet to induce nonalchoholic steatohepatitis had increased tumor formation compared to wild-type mice [105]. Adiponectin knockout mice were also used to investigate the effects of the chemical carcinogen azoxymethane on colon carcinogenesis [106]. The knockout mice fed a high-fat diet had increased number of polyps, larger tumor size and reduced survival rate compared to wild-type mice. In a study using haploinsufficient MMTV-PyV-mT mice that had 50% lower serum adiponectin levels compared to wild-type mice both male and female haploinsufficient mice had shortened tumor latency and increased tumor weights compared to MMTVPyV-mT mice [107]. However, the opposite results were obtained when mice lacked all adiponectin. For example, Denzel et al [108] crossed APN-KO mice which lack adiponectin with MMTV-PyV-mT transgenic mice and reported that the APN-KO/MMTV-PyV-mT mice had delayed mammary tumor development and death compared to wild-type MMTV-PyV-mT mice. Further tumor growth was reduced and metastasis was lower. A second study using this model had similar results with respect to reduction in tumor growth [109]. It was also reported that angiogenesis was reduced in the knockout mice. These results suggest that adiponectin may have different roles in different tumors and/or that additional undefined genetic differences may impact the function of adiponectin in tumorigenesis.
Another approach to determine the role of adiponectin in tumorigenesis would be to treat animals with adiponectin and assess its impact on tumor development. However, due to the large amounts of adiponectin needed to attain physiological relevant levels this has only rarely been attempted. In one case APCMin/+ mice, a model for colon cancer, were treated with 1.5 mg/kg of adiponectin in PBS once a week from 6-15 weeks of age [110]. At 16 weeks of age, one week after the last injection, serum adiponectin levels were increased ~15% (not significant) in treated mice and the number of polyps and mean diameter of polyps were reduced. In a xenograft study using a gastric cancer cell line mice were treated with 5 or 50 μg adiponectin per mouse daily for ~3 weeks [96]. A slight but significant decrease in tumor growth was found for the lower adiponectin level and the higher level resulted in an 80% decrease in tumor volume. In a xenograft model of liver cancer adenoviral adiponectin treatment was used which resulted in a doubling of serum adiponectin levels [111]. Treated mice had reduced tumor volume and reduced rates of metastasis to the lung.
4. The Adiponectin Leptin Ratio
The fact that adiponectin is associated with reduced risk and leptin with increased risk of various cancers provides the perfect stage for a yin and yang situation. Interestingly, an increased leptin:adiponectin or conversely a reduced adiponectin:leptin ratio has been associated with the diagnosis of cancer. This has included several studies of breast cancer [74-75] although only Chen et al actually calculated the ratio based on individual measurements of the subjects. A reduced adiponectin:leptin ratio has also been associated with the diagnosis of postmenopausal endometrial cancer [112]. and an inverse association of adiponectin with colorectal adenomas was found at the higher two tertiles of serum leptin [113].
In vitro studies have also addressed the issue of the ratio and/or the interaction of adiponectin and leptin and its effect on cancer related processes. For example, in breast cancer cell lines higher ratios of adiponectin:leptin are associated with reduced cell proliferation [104, 114]. Similar effects using prostate cancer cell lines have been reported [115-116]. Recently, adiponectin was shown to directly interfere with the oncogenic actions of leptins proliferative actions in human hepatic cancer cells [117].
Studies using preclinical animal models have further addressed the issue of the interactions of adiponectin and lepin on tumorigenesis. For example, mice fed a high fat diet (45% fat calories) had elevated serum leptin and lower adiponectin than did mice fed lower fat level AIN-93G diet and had a higher rate of spontaneous metastasis of the Lewis lung carcinoma than the low fat diet mice [118]. The actual ratio of adiponectin to leptin was not calculated but using the means of the two adipokines there was almost a 5 fold reduction in the adiponectin:leptin ratio in the high-fat fed mice. In another study mice consumed 60% fat by calorie diet, and were injected with PAN02 cells. The heaviest mice were identified as overweight and found to have significantly larger tumor weights than low-fat fed mice [119]. The overweight mice also had significantly higher serum leptin levels with no effect on adiponectin levels with the resulting adiponectin:leptin ratio calculation lower in the overweight mice. It is unfortunate that the results for the high-fat diet mice that did not become overweight were not presented as this would address whether consumption of the high-fat diet and/or body weight was the deciding factor affecting tumor growth.
Several studies have examined the adiponectin:leptin ratio in association with mammary tumorigenesis. In mice with goldthioglucose-induced obesity there was no effect on serum adiponectin while leptin levels were increased significantly resulting in a substantial decrease in the adiponectin:leptin ratio however mammary tumor development from inoculation of T47-D cells was not affected [120]. Approaching this issue from a somewhat different perspective we asked “If a low adiponectin:leptin ratio is a factor in development and/or progression of cancer what is the potential role of an elevated adiponectin:leptin ratio in cancer prevention?” We have consistently found that intermittent calorie restriction leads to reduced mammary tumorigenesis in MMTV-TGF-α mice compared to ad libitum fed as well as chronic calorie restricted mice [121-124]. This intervention is characterized by three week periods of 50% calorie restriction followed by three week periods of refeeding. Although adiponectin levels were not impacted by either chronic or intermittent calorie restriction, periods of 50% calorie restriction were characterized by significant reductions in serum leptin levels as well as an increase in the adiponectin:leptin ratio [124-125]. We did not find that the ratio was related to the presence of tumors in individual mice but the remarkable and consistent reduction of tumor incidence in the mice subjected to intermittent calorie restriction was clearly associated with an elevated adiponectin:leptin ratio. The other interesting thing noticed was that when the adipokine values were followed over the course of the study from 10-82 weeks of age ad libitum fed mice had a substantial decrease in the adiponectin:leptin ratio but the intermittent restricted mice did not 61.
5. Conclusions
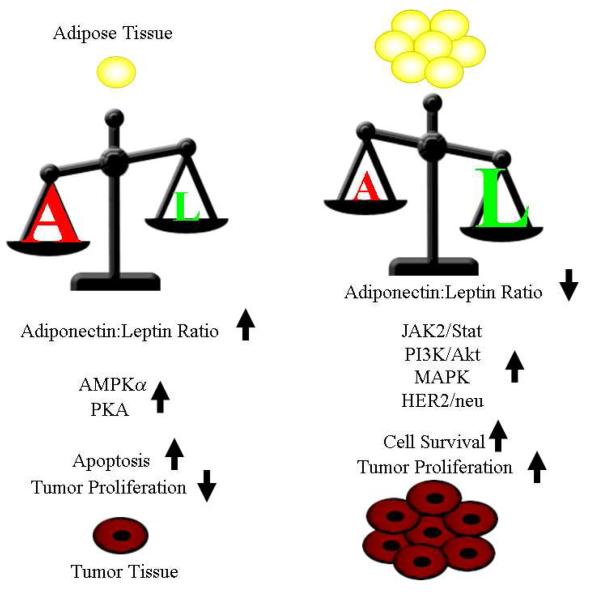
Adiponectin and leptin are both synthesized in adipose tissue and serum levels are modulated by body weight/fat and dietary factors, however body fatness appears to impact the levels of leptin and adiponectin in opposing manners. Leptin increases with increasing body fat while the levels of serum adiponectin decrease with increasing (Figure 1). This results in a higher adiponectin:leptin ratio in normal weight individuals as compared to overweight or obese individuals. In vitro studies suggest that STAT3, MAPK, PI3K/Akt and HER2/neu signaling in leptin-treated breast cancer cells are important in promoting cell survival and proliferation [43, 46, 126-128]. Conversely, adiponectin is able to block Akt and increase the activity of AMPKalpha and PKA resulting in increased apoptosis and decreased proliferation [102-103]. Recent studies of serum leptin levels indicate that it may have opposite indications for perimenopausal verses post menopausal breast cancer. Higher serum adiponectin levels are associated with lower breast cancer risk and/or reduced cancer cell proliferation regardless of menopausal status. The serum adiponectin:leptin ratio may be the key to understanding the physiological effects of these two adipokines and initial investigations indicate that a high adiponectin to leptin ratio is indicative of a positive risk profile compared to a low adiponectin to leptin ratio although further study is needed to evaluate the role of the ratio in perimenopausal verses post menopausal breast cancer patients.
Figure 1.
Actions of high verses low ratios of adiponectin to leptin. The effects of a small amount of adipose tissue are illustrated on the left half of the figure. The effects of large amounts of adipose tissue are illustrated on the right half of the figure. Levels of adiponectin as shown as an A and amounts of leptin are shown as an L with the relative size of each letter and the position of the scale representing the effects of ratio of adiponectin to leptin.
Highlights.
▶ Adipose tissue secretes factors capable of regulating tumorigenesis
▶ Leptin positively correlates with fatness and can be a growth factor for cancer
▶ Adiponectin negatively correlates with fatness and can inhibit the growth of cancer
▶ The balance of leptin and adiponectin may be a critical dictator of tumorigenesis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].World Cancer Research Fund / American Institute for Cancer Research . Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Vol. 2007. AICR; Washington, DC: 2007. [Google Scholar]
- [2].Cleary MP, Grossmann ME. Minireview: Obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150:2537–2542. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Perks CM, Holly JM. Hormonal mechanisms underlying the relationship between obesity and breast cancer. Endocrinol Metab Clin North Am. 2011;40:485–507. vii. doi: 10.1016/j.ecl.2011.05.010. [DOI] [PubMed] [Google Scholar]
- [4].Sung MK, Yeon JY, Park SY, Park JH, Choi MS. Obesity-induced metabolic stresses in breast and colon cancer. Annals of the New York Academy of Sciences. 2011;1229:61–68. doi: 10.1111/j.1749-6632.2011.06094.x. [DOI] [PubMed] [Google Scholar]
- [5].Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- [6].Mantzoros CS, Magkos F, Brinkoetter M, Sienkiewicz E, Dardeno TA, Kim SY, Hamnvik OP, Koniaris A. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab. 2011;301:E567–584. doi: 10.1152/ajpendo.00315.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Scherer T, Buettner C. Yin and Yang of hypothalamic insulin and leptin signaling in regulating white adipose tissue metabolism. Rev Endocr Metab Disord. 2011;12:235–243. doi: 10.1007/s11154-011-9190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Schindler TH, Cardenas J, Prior JO, Facta AD, Kreissl MC, Zhang XL, Sayre J, Dahlbom M, Licinio J, Schelbert HR. Relationship between increasing body weight, insulin resistance, inflammation, adipocytokine leptin, and coronary circulatory function. Journal of the American College of Cardiology. 2006;47:1188–1195. doi: 10.1016/j.jacc.2005.10.062. [DOI] [PubMed] [Google Scholar]
- [9].Wauters M, Considine RV, Van Gaal LF. Human leptin: from an adipocyte hormone to an endocrine mediator. European journal of endocrinology / European Federation of Endocrine Societies. 2000;143:293–311. doi: 10.1530/eje.0.1430293. [DOI] [PubMed] [Google Scholar]
- [10].Bonnet M, Delavaud C, Laud K, Gourdou I, Leroux C, Djiane J, Chilliard Y. Mammary leptin synthesis, milk leptin and their putative physiological roles. Reprod Nutr Dev. 2002;42:399–413. doi: 10.1051/rnd:2002034. [DOI] [PubMed] [Google Scholar]
- [11].Brann DW, Wade MF, Dhandapani KM, Mahesh VB, Buchanan CD. Leptin and reproduction. Steroids. 2002;67:95–104. doi: 10.1016/s0039-128x(01)00138-6. [DOI] [PubMed] [Google Scholar]
- [12].Neville MC, McFadden TB, Forsyth I. Hormonal regulation of mammary differentiation and milk secretion. Journal of mammary gland biology and neoplasia. 2002;7:49–66. doi: 10.1023/a:1015770423167. [DOI] [PubMed] [Google Scholar]
- [13].Goumenou AG, Matalliotakis IM, Koumantakis GE, Panidis DK. The role of leptin in fertility. Eur J Obstet Gynecol Reprod Biol. 2003;106:118–124. doi: 10.1016/s0301-2115(02)00359-7. [DOI] [PubMed] [Google Scholar]
- [14].Gomez R, Conde J, Scotece M, Gomez-Reino JJ, Lago F, Gualillo O. What’s new in our understanding of the role of adipokines in rheumatic diseases? Nat Rev Rheumatol. 2011;7:528–536. doi: 10.1038/nrrheum.2011.107. [DOI] [PubMed] [Google Scholar]
- [15].Maffei M, Fei H, Lee GH, Dani C, Leroy P, Zhang Y, Proenca R, Negrel R, Ailhaud G, Friedman JM. Increased expression in adipocytes of ob RNA in mice with lesions of the hypothalamus and with mutations at the db locus. Proc Natl Acad Sci U S A. 1995;92:6957–6960. doi: 10.1073/pnas.92.15.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- [17].Ostlund RE, Jr., Yang JW, Klein S, Gingerich R. Relation between plasma leptin concentration and body fat, gender, diet, age, and metabolic covariates. J Clin Endocrinol Metab. 1996;81:3909–3913. doi: 10.1210/jcem.81.11.8923837. [DOI] [PubMed] [Google Scholar]
- [18].Havel PJ, Kasim-Karakas S, Dubuc GR, Mueller W, Phinney SD. Gender differences in plasma leptin concentrations. Nature medicine. 1996;2:949–950. doi: 10.1038/nm0996-949b. [DOI] [PubMed] [Google Scholar]
- [19].Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, Leibel RL. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J Clin Endocrinol Metab. 1996;81:3424–3427. doi: 10.1210/jcem.81.9.8784109. [DOI] [PubMed] [Google Scholar]
- [20].Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor. OB-R, Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- [21].Bjorbaek C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. The Journal of biological chemistry. 1997;272:32686–32695. doi: 10.1074/jbc.272.51.32686. [DOI] [PubMed] [Google Scholar]
- [22].Sweeney G. Leptin signalling. Cell Signal. 2002;14:655–663. doi: 10.1016/s0898-6568(02)00006-2. [DOI] [PubMed] [Google Scholar]
- [23].Zabeau L, Lavens D, Peelman F, Eyckerman S, Vandekerckhove J, Tavernier J. The ins and outs of leptin receptor activation. FEBS Lett. 2003;546:45–50. doi: 10.1016/s0014-5793(03)00440-x. [DOI] [PubMed] [Google Scholar]
- [24].Yamashita T, Murakami T, Otani S, Kuwajima M, Shima K. Leptin receptor signal transduction: OBRa and OBRb of fa type. Biochem Biophys Res Commun. 1998;246:752–759. doi: 10.1006/bbrc.1998.8689. [DOI] [PubMed] [Google Scholar]
- [25].Hileman SM, Tornoe J, Flier JS, Bjorbaek C. Transcellular transport of leptin by the short leptin receptor isoform ObRa in Madin-Darby Canine Kidney cells. Endocrinology. 2000;141:1955–1961. doi: 10.1210/endo.141.6.7450. [DOI] [PubMed] [Google Scholar]
- [26].Nakao T, Hino M, Yamane T, Nishizawa Y, Morii H, Tatsumi N. Expression of the leptin receptor in human leukaemic blast cells. Br J Haematol. 1998;102:740–745. doi: 10.1046/j.1365-2141.1998.00843.x. [DOI] [PubMed] [Google Scholar]
- [27].Konopleva M, Mikhail A, Estrov Z, Zhao S, Harris D, Sanchez-Williams G, Kornblau SM, Dong J, Kliche KO, Jiang S, Snodgrass HR, Estey EH, Andreeff M. Expression and function of leptin receptor isoforms in myeloid leukemia and myelodysplastic syndromes: proliferative and anti-apoptotic activities. Blood. 1999;93:1668–1676. [PubMed] [Google Scholar]
- [28].Glasow A, Bornstein SR, Chrousos GP, Brown JW, Scherbaum WA. Detection of Ob-receptor in human adrenal neoplasms and effect of leptin on adrenal cell proliferation, Hormone and metabolic research. Hormon- und Stoffwechselforschung. 1999;31:247–251. doi: 10.1055/s-2007-978726. [DOI] [PubMed] [Google Scholar]
- [29].Hikita M, Bujo H, Hirayama S, Takahashi K, Morisaki N, Saito Y. Differential regulation of leptin receptor expression by insulin and leptin in neuroblastoma cells. Biochem Biophys Res Commun. 2000;271:703–709. doi: 10.1006/bbrc.2000.2692. [DOI] [PubMed] [Google Scholar]
- [30].Morash B, Johnstone J, Leopold C, Li A, Murphy P, Ur E, Wilkinson M. The regulation of leptin gene expression in the C6 glioblastoma cell line. Molecular and cellular endocrinology. 2000;165:97–105. doi: 10.1016/s0303-7207(00)00259-8. [DOI] [PubMed] [Google Scholar]
- [31].Karaduman M, Bilici A, Ozet A, Sengul A, Musabak U, Alomeroglu M. Tissue leptin levels in patients with breast cancer. J BUON. 2010;15:369–372. [PubMed] [Google Scholar]
- [32].Coskun U, Gunel N, Toruner FB, Sancak B, Onuk E, Bayram O, Cengiz O, Yilmaz E, Elbeg S, Ozkan S. Serum leptin, prolactin and vascular endothelial growth factor (VEGF) levels in patients with breast cancer. Neoplasma. 2003;50:41–46. [PubMed] [Google Scholar]
- [33].Han C, Zhang HT, Du L, Liu X, Jing J, Zhao X, Yang X, Tian B. Serum levels of leptin, insulin, and lipids in relation to breast cancer in china. Endocrine. 2005;26:19–24. doi: 10.1385/ENDO:26:1:019. [DOI] [PubMed] [Google Scholar]
- [34].Miyoshi Y, Funahashi T, Tanaka S, Taguchi T, Tamaki Y, Shimomura I, Noguchi S. High expression of leptin receptor mRNA in breast cancer tissue predicts poor prognosis for patients with high, but not low, serum leptin levels. Int J Cancer. 2006;118:1414–1419. doi: 10.1002/ijc.21543. [DOI] [PubMed] [Google Scholar]
- [35].Petridou E, Papadiamantis Y, Markopoulos C, Spanos E, Dessypris N, Trichopoulos D. Leptin and insulin growth factor I in relation to breast cancer (Greece) Cancer Causes Control. 2000;11:383–388. doi: 10.1023/a:1008903727238. [DOI] [PubMed] [Google Scholar]
- [36].Sauter ER, Garofalo C, Hewett J, Hewett JE, Morelli C, Surmacz E. Leptin expression in breast nipple aspirate fluid (NAF) and serum is influenced by body mass index (BMI) but not by the presence of breast cancer, Hormone and metabolic research. Hormon- und Stoffwechselforschung. 2004;36:336–340. doi: 10.1055/s-2004-814490. [DOI] [PubMed] [Google Scholar]
- [37].Tessitore L, Vizio B, Pesola D, Cecchini F, Mussa A, Argiles JM, Benedetto C. Adipocyte expression and circulating levels of leptin increase in both gynaecological and breast cancer patients. Int J Oncol. 2004;24:1529–1535. [PubMed] [Google Scholar]
- [38].Harris HR, Tworoger SS, Hankinson SE, Rosner BA, Michels KB. Plasma leptin levels and risk of breast cancer in premenopausal women. Cancer Prev Res (Phila) 2011;4:1449–1456. doi: 10.1158/1940-6207.CAPR-11-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Maccio A, Madeddu C, Gramignano G, Mulas C, Floris C, Massa D, Astara G, Chessa P, Mantovani G. Correlation of body mass index and leptin with tumor size and stage of disease in hormone-dependent postmenopausal breast cancer: preliminary results and therapeutic implications. J Mol Med (Berl) 2010;88:677–686. doi: 10.1007/s00109-010-0611-8. [DOI] [PubMed] [Google Scholar]
- [40].Jarde T, Caldefie-Chezet F, Damez M, Mishellany F, Penault-Llorca F, Guillot J, Vasson MP. Leptin and leptin receptor involvement in cancer development: a study on human primary breast carcinoma. Oncology reports. 2008;19:905–911. [PubMed] [Google Scholar]
- [41].Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewska J, Russo A, Sulkowski S, Surmacz E. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin Cancer Res. 2006;12:1447–1453. doi: 10.1158/1078-0432.CCR-05-1913. [DOI] [PubMed] [Google Scholar]
- [42].Ishikawa M, Kitayama J, Nagawa H. Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin Cancer Res. 2004;10:4325–4331. doi: 10.1158/1078-0432.CCR-03-0749. [DOI] [PubMed] [Google Scholar]
- [43].Hu X, Juneja SC, Maihle NJ, Cleary MP. Leptin--a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst. 2002;94:1704–1711. doi: 10.1093/jnci/94.22.1704. [DOI] [PubMed] [Google Scholar]
- [44].Dieudonne MN, Machinal-Quelin F, Serazin-Leroy V, Leneveu MC, Pecquery R, Giudicelli Y. Leptin mediates a proliferative response in human MCF7 breast cancer cells. Biochem Biophys Res Commun. 2002;293:622–628. doi: 10.1016/S0006-291X(02)00205-X. [DOI] [PubMed] [Google Scholar]
- [45].Laud K, Gourdou I, Pessemesse L, Peyrat JP, Djiane J. Identification of leptin receptors in human breast cancer: functional activity in the T47-D breast cancer cell line. Molecular and cellular endocrinology. 2002;188:219–226. doi: 10.1016/s0303-7207(01)00678-5. [DOI] [PubMed] [Google Scholar]
- [46].Frankenberry KA, Skinner H, Somasundar P, McFadden DW, Vona-Davis LC. Leptin receptor expression and cell signaling in breast cancer. Int J Oncol. 2006;28:985–993. [PubMed] [Google Scholar]
- [47].Ray A, Nkhata KJ, Cleary MP. Effects of leptin on human breast cancer cell lines in relationship to estrogen receptor and HER2 status. Int J Oncol. 2007;30:1499–1509. [PubMed] [Google Scholar]
- [48].Saxena NK, Taliaferro-Smith L, Knight BB, Merlin D, Anania FA, O’Regan RM, Sharma D. Bidirectional crosstalk between leptin and insulin-like growth factor-I signaling promotes invasion and migration of breast cancer cells via transactivation of epidermal growth factor receptor. Cancer Res. 2008;68:9712–9722. doi: 10.1158/0008-5472.CAN-08-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Somasundar P, Yu AK, Vona-Davis L, McFadden DW. Differential effects of leptin on cancer in vitro. J Surg Res. 2003;113:50–55. doi: 10.1016/s0022-4804(03)00166-5. [DOI] [PubMed] [Google Scholar]
- [50].Garofalo C, Sisci D, Surmacz E. Leptin interferes with the effects of the antiestrogen ICI 182,780 in MCF-7 breast cancer cells. Clin Cancer Res. 2004;10:6466–6475. doi: 10.1158/1078-0432.CCR-04-0203. [DOI] [PubMed] [Google Scholar]
- [51].Halter SA, Dempsey P, Matsui Y, Stokes MK, Graves-Deal R, Hogan BL, Coffey RJ. Distinctive patterns of hyperplasia in transgenic mice with mouse mammary tumor virus transforming growth factor-alpha. Characterization of mammary gland and skin proliferations. The American journal of pathology. 1992;140:1131–1146. [PMC free article] [PubMed] [Google Scholar]
- [52].Cleary MP, Phillips FC, Getzin SC, Jacobson TL, Jacobson MK, Christensen TA, Juneja SC, Grande JP, Maihle NJ. Genetically obese MMTV-TGF- alpha/Lep(ob)Lep(ob) female mice do not develop mammary tumors. Breast Cancer Res Treat. 2003;77:205–215. doi: 10.1023/a:1021891825399. [DOI] [PubMed] [Google Scholar]
- [53].Cleary MP, Juneja SC, Phillips FC, Hu X, Grande JP, Maihle NJ. Leptin receptor-deficient MMTV-TGF-alpha/Lepr(db)Lepr(db) female mice do not develop oncogene-induced mammary tumors. Exp Biol Med (Maywood) 2004;229:182–193. doi: 10.1177/153537020422900207. [DOI] [PubMed] [Google Scholar]
- [54].Lee WM, Lu S, Medline A, Archer MC. Susceptibility of lean and obese Zucker rats to tumorigenesis induced by N-methyl-N-nitrosourea. Cancer Lett. 2001;162:155–160. doi: 10.1016/s0304-3835(00)00635-2. [DOI] [PubMed] [Google Scholar]
- [55].Hakkak R, Holley AW, Macleod SL, Simpson PM, Fuchs GJ, Jo CH, Kieber-Emmons T, Korourian S. Obesity promotes 7,12-dimethylbenz(a)anthracene- induced mammary tumor development in female zucker rats. Breast Cancer Res. 2005;7:R627–633. doi: 10.1186/bcr1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Levin BE, Triscari J, Sullivan AC. Relationship between sympathetic activity and diet-induced obesity in two rat strains. The American journal of physiology. 1983;245:R364–371. doi: 10.1152/ajpregu.1983.245.3.R364. [DOI] [PubMed] [Google Scholar]
- [57].Levin BE, Triscari J, Hogan S, Sullivan AC. Resistance to diet-induced obesity: food intake, pancreatic sympathetic tone, and insulin. The American journal of physiology. 1987;252:R471–478. doi: 10.1152/ajpregu.1987.252.3.R471. [DOI] [PubMed] [Google Scholar]
- [58].Levin BE, Brown KL, Vincent G. Increased potency and binding of mazindol to putative brain anorectic receptors in obesity-prone rats. Brain Res. 1994;668:171–179. doi: 10.1016/0006-8993(94)90522-3. [DOI] [PubMed] [Google Scholar]
- [59].Cleary MP, Grande JP, Maihle NJ. Effect of high fat diet on body weight and mammary tumor latency in MMTV-TGF-alpha mice. Int J Obes Relat Metab Disord. 2004;28:956–962. doi: 10.1038/sj.ijo.0802664. [DOI] [PubMed] [Google Scholar]
- [60].Cleary MP, Grande JP, Juneja SC, Maihle NJ. Diet-induced obesity and mammary tumor development in MMTV-neu female mice. Nutr Cancer. 2004;50:174–180. doi: 10.1207/s15327914nc5002_7. [DOI] [PubMed] [Google Scholar]
- [61].Potter JD, Cerhan JR, Sellers TA, McGovern PG, Drinkard C, Kushi LR, Folsom AR. Progesterone and estrogen receptors and mammary neoplasia in the Iowa Women’s Health Study: how many kinds of breast cancer are there? Cancer Epidemiol Biomarkers Prev. 1995;4:319–326. [PubMed] [Google Scholar]
- [62].Enger SM, Ross RK, Paganini-Hill A, Carpenter CL, Bernstein L. Body size, physical activity, and breast cancer hormone receptor status: results from two case- control studies. Cancer Epidemiol Biomarkers Prev. 2000;9:681–687. [PubMed] [Google Scholar]
- [63].Huang WY, Newman B, Millikan RC, Schell MJ, Hulka BS, Moorman PG. Hormone-related factors and risk of breast cancer in relation to estrogen receptor and progesterone receptor status. Am J Epidemiol. 2000;151:703–714. doi: 10.1093/oxfordjournals.aje.a010265. [DOI] [PubMed] [Google Scholar]
- [64].Ray A, Nkhata KJ, Grande JP, Cleary MP. Diet-induced obesity and mammary tumor development in relation to estrogen receptor status. Cancer Lett. 2007;253:291–300. doi: 10.1016/j.canlet.2007.02.005. [DOI] [PubMed] [Google Scholar]
- [65].Amjadi F, Javanmard SH, Zarkesh-Esfahani H, Khazaei M, Narimani M. Leptin promotes melanoma tumor growth in mice related to increasing circulating endothelial progenitor cells numbers and plasma NO production. J Exp Clin Cancer Res. 2011;30:21. doi: 10.1186/1756-9966-30-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Endo H, Hosono K, Uchiyama T, Sakai E, Sugiyama M, Takahashi H, Nakajima N, Wada K, Takeda K, Nakagama H, Nakajima A. Leptin acts as a growth factor for colorectal tumours at stages subsequent to tumour initiation in murine colon carcinogenesis. Gut. 2011;60:1363–1371. doi: 10.1136/gut.2010.235754. [DOI] [PubMed] [Google Scholar]
- [67].Otvos L, Jr., Kovalszky I, Riolfi M, Ferla R, Olah J, Sztodola A, Nama K, Molino A, Piubello Q, Wade JD, Surmacz E. Efficacy of a leptin receptor antagonist peptide in a mouse model of triple-negative breast cancer. Eur J Cancer. 2011;47:1578–1584. doi: 10.1016/j.ejca.2011.01.018. [DOI] [PubMed] [Google Scholar]
- [68].Rene Gonzalez R, Watters A, Xu Y, Singh UP, Mann DR, Rueda BR, Penichet ML. Leptin-signaling inhibition results in efficient anti-tumor activity in estrogen receptor positive or negative breast cancer. Breast Cancer Res. 2009;11:R36. doi: 10.1186/bcr2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. The Journal of biological chemistry. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- [70].Miyoshi Y, Funahashi T, Kihara S, Taguchi T, Tamaki Y, Matsuzawa Y, Noguchi S. Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res. 2003;9:5699–5704. [PubMed] [Google Scholar]
- [71].Bondanelli M, Margutti A, Ambrosio MR, Plaino L, Cobellis L, Petraglia F, degli Uberti EC. Blood growth hormone-binding protein levels in premenopausal and postmenopausal women: roles of body weight and estrogen levels. J Clin Endocrinol Metab. 2001;86:1973–1980. doi: 10.1210/jcem.86.5.7485. [DOI] [PubMed] [Google Scholar]
- [72].Mantzoros C, Petridou E, Dessypris N, Chavelas C, Dalamaga M, Alexe DM, Papadiamantis Y, Markopoulos C, Spanos E, Chrousos G, Trichopoulos D. Adiponectin and breast cancer risk. J Clin Endocrinol Metab. 2004;89:1102–1107. doi: 10.1210/jc.2003-031804. [DOI] [PubMed] [Google Scholar]
- [73].Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- [74].Korner A, Pazaitou-Panayiotou K, Kelesidis T, Kelesidis I, Williams CJ, Kaprara A, Bullen J, Neuwirth A, Tseleni S, Mitsiades N, Kiess W, Mantzoros CS. Total and high-molecular-weight adiponectin in breast cancer: in vitro and in vivo studies. J Clin Endocrinol Metab. 2007;92:1041–1048. doi: 10.1210/jc.2006-1858. [DOI] [PubMed] [Google Scholar]
- [75].Chen DC, Chung YF, Yeh YT, Chaung HC, Kuo FC, Fu OY, Chen HY, Hou MF, Yuan SS. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett. 2006;237:109–114. doi: 10.1016/j.canlet.2005.05.047. [DOI] [PubMed] [Google Scholar]
- [76].Ryan AS, Berman DM, Nicklas BJ, Sinha M, Gingerich RL, Meneilly GS, Egan JM, Elahi D. Plasma adiponectin and leptin levels, body composition, and glucose utilization in adult women with wide ranges of age and obesity. Diabetes Care. 2003;26:2383–2388. doi: 10.2337/diacare.26.8.2383. [DOI] [PubMed] [Google Scholar]
- [77].Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- [78].Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- [79].Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement- related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Tsao TS, Murrey HE, Hug C, Lee DH, Lodish HF. Oligomerization state- dependent activation of NF-kappa B signaling pathway by adipocyte complement-related protein of 30 kDa (Acrp30) The Journal of biological chemistry. 2002;277:29359–29362. doi: 10.1074/jbc.C200312200. [DOI] [PubMed] [Google Scholar]
- [81].Tworoger SS, Eliassen AH, Kelesidis T, Colditz GA, Willett WC, Mantzoros CS, Hankinson SE. Plasma adiponectin concentrations and risk of incident breast cancer. J Clin Endocrinol Metab. 2007;92:1510–1516. doi: 10.1210/jc.2006-1975. [DOI] [PubMed] [Google Scholar]
- [82].Petridou E, Mantzoros C, Dessypris N, Koukoulomatis P, Addy C, Voulgaris Z, Chrousos G, Trichopoulos D. Plasma adiponectin concentrations in relation to endometrial cancer: a case-control study in Greece. J Clin Endocrinol Metab. 2003;88:993–997. doi: 10.1210/jc.2002-021209. [DOI] [PubMed] [Google Scholar]
- [83].Dal Maso L, Augustin LS, Karalis A, Talamini R, Franceschi S, Trichopoulos D, Mantzoros CS, La Vecchia C. Circulating adiponectin and endometrial cancer risk. J Clin Endocrinol Metab. 2004;89:1160–1163. doi: 10.1210/jc.2003-031716. [DOI] [PubMed] [Google Scholar]
- [84].Michalakis K, Williams CJ, Mitsiades N, Blakeman J, Balafouta-Tselenis S, Giannopoulos A, Mantzoros CS. Serum adiponectin concentrations and tissue expression of adiponectin receptors are reduced in patients with prostate cancer: a case control study. Cancer Epidemiol Biomarkers Prev. 2007;16:308–313. doi: 10.1158/1055-9965.EPI-06-0621. [DOI] [PubMed] [Google Scholar]
- [85].Li H, Stampfer MJ, Mucci L, Rifai N, Qiu W, Kurth T, Ma J. A 25-year prospective study of plasma adiponectin and leptin concentrations and prostate cancer risk and survival. Clinical chemistry. 2010;56:34–43. doi: 10.1373/clinchem.2009.133272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Wei EK, Giovannucci E, Fuchs CS, Willett WC, Mantzoros CS. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. J Natl Cancer Inst. 2005;97:1688–1694. doi: 10.1093/jnci/dji376. [DOI] [PubMed] [Google Scholar]
- [87].Ishikawa M, Kitayama J, Kazama S, Hiramatsu T, Hatano K, Nagawa H. Plasma adiponectin and gastric cancer. Clin Cancer Res. 2005;11:466–472. [PubMed] [Google Scholar]
- [88].Pfeiler G, Hudelist G, Wulfing P, Mattsson B, Konigsberg R, Kubista E, Singer CF. Impact of AdipoR1 expression on breast cancer development. Gynecologic oncology. 2010;117:134–138. doi: 10.1016/j.ygyno.2009.12.018. [DOI] [PubMed] [Google Scholar]
- [89].Kaklamani VG, Sadim M, Hsi A, Offit K, Oddoux C, Ostrer H, Ahsan H, Pasche B, Mantzoros C. Variants of the adiponectin and adiponectin receptor 1 genes and breast cancer risk. Cancer Res. 2008;68:3178–3184. doi: 10.1158/0008-5472.CAN-08-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Gialamas SP, Petridou ET, Tseleni-Balafouta S, Spyridopoulos TN, Matsoukis IL, Kondi-Pafiti A, Zografos G, Mantzoros CS. Serum adiponectin levels and tissue expression of adiponectin receptors are associated with risk, stage, and grade of colorectal cancer. Metabolism: clinical and experimental. 2011;60:1530–1538. doi: 10.1016/j.metabol.2011.03.020. [DOI] [PubMed] [Google Scholar]
- [91].Byeon JS, Jeong JY, Kim MJ, Lee SM, Nam WH, Myung SJ, Kim JG, Yang SK, Kim JH, Suh DJ. Adiponectin and adiponectin receptor in relation to colorectal cancer progression. Int J Cancer. 2010;127:2758–2767. doi: 10.1002/ijc.25301. [DOI] [PubMed] [Google Scholar]
- [92].Chou SH, Tseleni-Balafouta S, Moon HS, Chamberland JP, Liu X, Kavantzas N, Mantzoros CS. Adiponectin receptor expression in human malignant tissues. Horm Cancer. 2010;1:136–145. doi: 10.1007/s12672-010-0017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Bub JD, Miyazaki T, Iwamoto Y. Adiponectin as a growth inhibitor in prostate cancer cells. Biochem Biophys Res Commun. 2006;340:1158–1166. doi: 10.1016/j.bbrc.2005.12.103. [DOI] [PubMed] [Google Scholar]
- [94].Zakikhani M, Dowling RJ, Sonenberg N, Pollak MN. The effects of adiponectin and metformin on prostate and colon neoplasia involve activation of AMP- activated protein kinase. Cancer Prev Res (Phila) 2008;1:369–375. doi: 10.1158/1940-6207.CAPR-08-0081. [DOI] [PubMed] [Google Scholar]
- [95].Cong L, Gasser J, Zhao J, Yang B, Li F, Zhao AZ. Human adiponectin inhibits cell growth and induces apoptosis in human endometrial carcinoma cells, HEC-1- A and RL95 2. Endocrine-related cancer. 2007;14:713–720. doi: 10.1677/ERC-07-0065. [DOI] [PubMed] [Google Scholar]
- [96].Ishikawa M, Kitayama J, Yamauchi T, Kadowaki T, Maki T, Miyato H, Yamashita H, Nagawa H. Adiponectin inhibits the growth and peritoneal metastasis of gastric cancer through its specific membrane receptors AdipoR1 and AdipoR2. Cancer Sci. 2007;98:1120–1127. doi: 10.1111/j.1349-7006.2007.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Kang JH, Lee YY, Yu BY, Yang BS, Cho KH, Yoon DK, Roh YK. Adiponectin induces growth arrest and apoptosis of MDA-MB-231 breast cancer cell. Archives of pharmacal research. 2005;28:1263–1269. doi: 10.1007/BF02978210. [DOI] [PubMed] [Google Scholar]
- [98].Wang Y, Lam JB, Lam KS, Liu J, Lam MC, Hoo RL, Wu D, Cooper GJ, Xu A. Adiponectin modulates the glycogen synthase kinase-3beta/beta-catenin signaling pathway and attenuates mammary tumorigenesis of MDA-MB-231 cells in nude mice. Cancer Res. 2006;66:11462–11470. doi: 10.1158/0008-5472.CAN-06-1969. [DOI] [PubMed] [Google Scholar]
- [99].Dos Santos E, Benaitreau D, Dieudonne MN, Leneveu MC, Serazin V, Giudicelli Y, Pecquery R. Adiponectin mediates an antiproliferative response in human MDA-MB 231 breast cancer cells. Oncology reports. 2008;20:971–977. [PubMed] [Google Scholar]
- [100].Dieudonne MN, Bussiere M, Dos Santos E, Leneveu MC, Giudicelli Y, Pecquery R. Adiponectin mediates antiproliferative and apoptotic responses in human MCF7 breast cancer cells. Biochem Biophys Res Commun. 2006;345:271–279. doi: 10.1016/j.bbrc.2006.04.076. [DOI] [PubMed] [Google Scholar]
- [101].Arditi JD, Venihaki M, Karalis KP, Chrousos GP. Antiproliferative effect of adiponectin on MCF7 breast cancer cells: a potential hormonal link between obesity and cancer, Hormone and metabolic research. Hormon- und Stoffwechselforschung. 2007;39:9–13. doi: 10.1055/s-2007-956518. [DOI] [PubMed] [Google Scholar]
- [102].Li G, Cong L, Gasser J, Zhao J, Chen K, Li F. Mechanisms underlying the anti-proliferative actions of adiponectin in human breast cancer cells, MCF7-dependency on the cAMP/protein kinase-A pathway. Nutr Cancer. 2011;63:80–88. doi: 10.1080/01635581.2010.516472. [DOI] [PubMed] [Google Scholar]
- [103].Grossmann ME, Nkhata KJ, Mizuno NK, Ray A, Cleary MP. Effects of adiponectin on breast cancer cell growth and signaling. Br J Cancer. 2008;98:370–379. doi: 10.1038/sj.bjc.6604166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Grossmann ME, Ray A, Dogan S, Mizuno NK, Cleary MP. Balance of adiponectin and leptin modulates breast cancer cell growth. Cell research. 2008;18:1154–1156. doi: 10.1038/cr.2008.293. [DOI] [PubMed] [Google Scholar]
- [105].Kamada Y, Matsumoto H, Tamura S, Fukushima J, Kiso S, Fukui K, Igura T, Maeda N, Kihara S, Funahashi T, Matsuzawa Y, Shimomura I, Hayashi N. Hypoadiponectinemia accelerates hepatic tumor formation in a nonalcoholic steatohepatitis mouse model. J Hepatol. 2007;47:556–564. doi: 10.1016/j.jhep.2007.03.020. [DOI] [PubMed] [Google Scholar]
- [106].Fujisawa T, Endo H, Tomimoto A, Sugiyama M, Takahashi H, Saito S, Inamori M, Nakajima N, Watanabe M, Kubota N, Yamauchi T, Kadowaki T, Wada K, Nakagama H, Nakajima A. Adiponectin suppresses colorectal carcinogenesis under the high-fat diet condition. Gut. 2008;57:1531–1538. doi: 10.1136/gut.2008.159293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Lam JB, Chow KH, Xu A, Lam KS, Liu J, Wong NS, Moon RT, Shepherd PR, Cooper GJ, Wang Y. Adiponectin haploinsufficiency promotes mammary tumor development in MMTV-PyVT mice by modulation of phosphatase and tensin homolog activities. PLoS One. 2009;4:e4968. doi: 10.1371/journal.pone.0004968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Denzel MS, Hebbard LW, Shostak G, Shapiro L, Cardiff RD, Ranscht B. Adiponectin deficiency limits tumor vascularization in the MMTV-PyV-mT mouse model of mammary cancer. Clin Cancer Res. 2009;15:3256–3264. doi: 10.1158/1078-0432.CCR-08-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Landskroner-Eiger S, Qian B, Muise ES, Nawrocki AR, Berger JP, Fine EJ, Koba W, Deng Y, Pollard JW, Scherer PE. Proangiogenic contribution of adiponectin toward mammary tumor growth in vivo. Clin Cancer Res. 2009;15:3265–3276. doi: 10.1158/1078-0432.CCR-08-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Otani K, Kitayama J, Yasuda K, Nio Y, Iwabu M, Okudaira S, Aoki J, Yamauchi T, Kadowaki T, Nagawa H. Adiponectin suppresses tumorigenesis in Apc(Min)(/+) mice. Cancer Lett. 2010;288:177–182. doi: 10.1016/j.canlet.2009.06.037. [DOI] [PubMed] [Google Scholar]
- [111].Man K, Ng KT, Xu A, Cheng Q, Lo CM, Xiao JW, Sun BS, Lim ZX, Cheung JS, Wu EX, Sun CK, Poon RT, Fan ST. Suppression of liver tumor growth and metastasis by adiponectin in nude mice through inhibition of tumor angiogenesis and downregulation of Rho kinase/IFN-inducible protein 10/matrix metalloproteinase 9 signaling. Clin Cancer Res. 2010;16:967–977. doi: 10.1158/1078-0432.CCR-09-1487. [DOI] [PubMed] [Google Scholar]
- [112].Ashizawa N, Yahata T, Quan J, Adachi S, Yoshihara K, Tanaka K. Serum leptin-adiponectin ratio and endometrial cancer risk in postmenopausal female subjects. Gynecologic oncology. 2010;119:65–69. doi: 10.1016/j.ygyno.2010.07.007. [DOI] [PubMed] [Google Scholar]
- [113].Yamaji T, Iwasaki M, Sasazuki S, Tsugane S. Interaction between adiponectin and leptin influences the risk of colorectal adenoma. Cancer Res. 2010;70:5430–5437. doi: 10.1158/0008-5472.CAN-10-0178. [DOI] [PubMed] [Google Scholar]
- [114].Nkhata KJ, Ray A, Schuster TF, Grossmann ME, Cleary MP. Effects of adiponectin and leptin co-treatment on human breast cancer cell growth. Oncology reports. 2009;21:1611–1619. doi: 10.3892/or_00000395. [DOI] [PubMed] [Google Scholar]
- [115].Grossmann ME, Mizuno NK, Bonorden MJ, Ray A, Sokolchik I, Narasimhan ML, Cleary MP. Role of the adiponectin leptin ratio in prostate cancer. Oncol Res. 2009;18:269–277. doi: 10.3727/096504009x12596189659367. [DOI] [PubMed] [Google Scholar]
- [116].Mistry T, Digby JE, Desai KM, Randeva HS. Leptin and adiponectin interact in the regulation of prostate cancer cell growth via modulation of p53 and bcl-2 expression. BJU Int. 2008;101:1317–1322. doi: 10.1111/j.1464-410X.2008.07512.x. [DOI] [PubMed] [Google Scholar]
- [117].Carroll PA, Healy L, Lysaght J, Boyle T, Reynolds JV, Kennedy MJ, Pidgeon G, Connolly EM. Influence of the metabolic syndrome on leptin and leptin receptor in breast cancer. Mol Carcinog. 2011;50:643–651. doi: 10.1002/mc.20764. [DOI] [PubMed] [Google Scholar]
- [118].Yan L, DeMars LC. Effects of dietary fat on spontaneous metastasis of Lewis lung carcinoma in mice. Clin Exp Metastasis. 2010;27:581–590. doi: 10.1007/s10585-010-9347-7. [DOI] [PubMed] [Google Scholar]
- [119].White PB, True EM, Ziegler KM, Wang SS, Swartz-Basile DA, Pitt HA, Zyromski NJ. Insulin, leptin, and tumoral adipocytes promote murine pancreatic cancer growth. J Gastrointest Surg. 2010;14:1888–1893. doi: 10.1007/s11605-010-1349-x. discussion 1893-1884. [DOI] [PubMed] [Google Scholar]
- [120].Nkhata KJ, Ray A, Dogan S, Grande JP, Cleary MP. Mammary tumor development from T47-D human breast cancer cells in obese ovariectomized mice with and without estradiol supplements. Breast Cancer Res Treat. 2009;114:71–83. doi: 10.1007/s10549-008-9991-7. [DOI] [PubMed] [Google Scholar]
- [121].Cleary MP, Jacobson MK, Phillips FC, Getzin SC, Grande JP, Maihle NJ. Weight-cycling decreases incidence and increases latency of mammary tumors to a greater extent than does chronic caloric restriction in mouse mammary tumor virus- transforming growth factor-alpha female mice. Cancer Epidemiol Biomarkers Prev. 2002;11:836–843. [PubMed] [Google Scholar]
- [122].Cleary MP, Hu X, Grossmann ME, Juneja SC, Dogan S, Grande JP, Maihle NJ. Prevention of mammary tumorigenesis by intermittent caloric restriction: does caloric intake during refeeding modulate the response? Exp Biol Med (Maywood) 2007;232:70–80. [PubMed] [Google Scholar]
- [123].Rogozina OP, Bonorden MJ, Grande JP, Cleary MP. Serum insulin-like growth factor-I and mammary tumor development in ad libitum-fed, chronic calorie- restricted, and intermittent calorie-restricted MMTV-TGF-alpha mice. Cancer Prev Res (Phila) 2009;2:712–719. doi: 10.1158/1940-6207.CAPR-09-0028. [DOI] [PubMed] [Google Scholar]
- [124].Dogan S, Rogozina OP, Lokshin A, Grande JP, Cleary MP. Effects of chronic vs. intermittent calorie restriction on mammary tumor incidence and serum adiponectin and leptin levels in MMTV-TGF-α mice at different ages. Oncology Letters. 2010;1:167–176. doi: 10.3892/ol_00000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Rogozina OP, Bonorden MJ, Seppanen CN, Grande JP, Cleary MP. Effect of chronic and intermittent calorie restriction on serum adiponectin and leptin and mammary tumorigenesis. Cancer Prev Res (Phila) 2011;4:568–581. doi: 10.1158/1940-6207.CAPR-10-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Jiang H, Yu J, Guo H, Song H, Chen S. Upregulation of survivin by leptin/STAT3 signaling in MCF-7 cells. Biochem Biophys Res Commun. 2008;368:1–5. doi: 10.1016/j.bbrc.2007.04.004. [DOI] [PubMed] [Google Scholar]
- [127].Yin N, Wang D, Zhang H, Yi X, Sun X, Shi B, Wu H, Wu G, Wang X, Shang Y. Molecular mechanisms involved in the growth stimulation of breast cancer cells by leptin. Cancer Res. 2004;64:5870–5875. doi: 10.1158/0008-5472.CAN-04-0655. [DOI] [PubMed] [Google Scholar]
- [128].Soma D, Kitayama J, Yamashita H, Miyato H, Ishikawa M, Nagawa H. Leptin augments proliferation of breast cancer cells via transactivation of HER2. J Surg Res. 2008;149:9–14. doi: 10.1016/j.jss.2007.10.012. [DOI] [PubMed] [Google Scholar]