Abstract
Background
The course of dilated cardiomyopathy (DCM) leading to heart failure in children varies; survival with conventional treatment is 64% at 5 years. Heart transplantation (HTx) enables improved survival; however, outcomes from listing for transplant are not well described. This study reports survival of patients with DCM from listing with the availability of mechanical bridge to transplant.
Methods
Patients with a primary diagnosis of DCM (n = 1,098) were identified from a multi-institutional, prospective, registry of patients aged < 18 years listed for HTx from January 1, 1993, to December 31, 2006.
Results
Characteristics of DCM patients at listing included a mean age of 7.3 years; 51% male, 64% white ethnicity, 77% United Network for Organ Sharing status I, 66% on inotropic support, 28% mechanically ventilated, and 15% on mechanical support. Waitlist mortality was 11%, and 75% underwent HTx at 2 years after listing. Overall 10-year survival after listing was 72%, with higher risk of death associated with arrhythmias, mechanical ventilation, and extracorporeal membrane oxygenation (ECMO) support, but not ventricular assist device (VAD) support. Survival at 10 years post-HTx was 72%, with a higher risk of death associated with black race, older age, mechanical ventilation, longer ischemic time, and earlier era of transplant.
Conclusions
Transplantation for DCM in the pediatric population offers enhanced survival compared with the natural history. Overall waitlist mortality for DCM is low, with the exception of patients on ECMO, mechanically ventilated, or with arrhythmias. DCM patients fared well after transplant, making HTx a key therapeutic intervention.
Dilated cardiomyopathy (DCM) is a myocardial disorder characterized by dilation of the left ventricle and systolic dysfunction that usually results in congestive heart failure.1-3 DCM has an annual reported incidence in childhood (< 18 years old) of 5.7 per million population.4 DCM may occur due to a variety of causes, including arrhythmia, myocarditis, anthracycline toxicity, or in association with generalized muscle disease.5 In 66% of patients, however, the underlying diagnosis is unknown. 4 Overall, the 5-year survival is no more than 64%, but survival is variable given the wide variation in etiology, with potential for both recovery and deterioration.6-8
DCM secondary to arrhythmia is managed by treatment of the primary arrhythmia. For the remainder, generic anti-failure treatment with diuretics, angiotensin-converting enzyme (ACE) inhibition, and β-blockers are the mainstay of treatment. If this fails, then treatment is escalated to include intravenous inotropic therapy, ventilation, and mechanical support, including extracorporeal membrane oxygenation (ECMO), ventricular assist device (VAD), or intra-aortic balloon pump (IABP).
Heart transplantation (HTx) is offered for those DCM patients in whom recovery is considered unlikely, and has been shown to improve outcomes.9 It is now the most common indication (60%) for pediatric HTx in the International Society of Heart and Lung Transplantation (ISHLT) Registry, and the outcome with HTx is well known, with graft half-life exceeding 50% at 13 years.10 Outcome from listing is less well described, and there is varying practice with regards to indications and timing of listing for HTx. The aims of this study were to describe the clinical characteristics and course of pediatric patients with DCM after listing for HTx and to define risk factors for serious adverse events and death while awaiting transplantation.
METHODS
Patient Selection and Data Collection
The Pediatric Heart Transplant Study (PHTS) is a prospective multi-institutional research database that enrolls all patients listed for HTx aged < 18 years old. Detailed listing data, transplant data, donor information, and initial immunosuppression are collected. Follow-up data collected include the details of major outcomes, defined as death, repeat HTx, rejection, infection, coronary artery disease, and malignancies. Currently, 30 institutions participate in the database. Institutional Review Board approval was obtained at each institution. The Data Collection and Analysis Center is located at the University of Alabama at Birmingham.
Data Analysis
Survival after listing was compared between 1,098 patients with DCM and 1,827 patients without CM. The primary diagnosis among the non-CM patients was congenital heart disease. Standard Kaplan-Meier and parametric analyses were used for survival analysis. Competing outcomes methods were used to analyze outcome after listing.11
Multivariate analysis in the hazard-function domain was used to identify risk factors for death while waiting, death after transplant, and overall survival after listing, including death while waiting and death after transplant.12 Variables entered into the multivariable risk factor analysis for death after listing included demographics at listing, blood type, United Network of Organ Sharing (UNOS) listing status, etiology of CM, surgical history, other clinical diagnoses and comorbidities, and hemodynamics at listing. Variables entered into the multivariable risk factor analysis for death after HTx included demographics and UNOS status at transplantation, blood type, etiology of CM, surgical history at listing, other clinical diagnoses and comorbidities, hemodynamics at listing, transplant era (1995 vs 2005), ischemic time, donor variables (demographics, blood type, medical history), and donor-recipient mismatch variables (race, gender, age, blood group, body surface area ratio).
RESULTS
Patient Characteristics at Listing
Among 1,320 children listed for CM in the PHTS Registry, 1,098 met diagnostic criteria for DCM (increased left ventricular end diastolic dimension with low fractional shortening) and comprised 83% of those with a CM diagnosis. Demographic details and UNOS status at listing are given in Table 1. The underlying etiology was reported as idiopathic in 81%.
Table 1.
Characteristics of 1,098 Patients With Dilated Cardiomyopathy
| Listed for transplant | Received transplant | |||
|---|---|---|---|---|
| Characteristics | Totala | No. undefined(%) or mean ± SE |
Totala | No. undefined(%) or mean ± SE |
| Demographics | ||||
| Male | 1095 | 555 (51) | 815 | 416 (51) |
| White | 1089 | 698 (64) | 811 | 517 (64) |
| Age, years | 1098 | 7.3 ± 0.189 | 817 | 8.1 ± 0.217 |
| Status | ||||
| Status 1 | 1064 | 814 (77) | 786 | 669 (85) |
| Inotropes | 1098 | 720 (66) | 817 | 552 (68) |
| Ventilator | 1098 | 305 (28) | 817 | 164 (20) |
| ECMO | 1098 | 92 (8) | 817 | 64 (8) |
| VAD | 1098 | 74 (7) | 817 | 107 (13) |
| Condition at listing | ||||
| Arrhythmia | 1098 | 250 (23) | ||
| Failure to thrive | 1098 | 131 (12) | ||
| Renal insufficiency | 1098 | 38 (3) | ||
| Asthma | 1098 | 33 (3) | ||
| CVA | 1098 | 22 (2) | ||
| Diabetes | 1098 | 5 (<1) | ||
| Etiology of DCM | ||||
| Idiopathic | 888 (80.9) | |||
| Myocarditisb | 133 (12.1) | |||
| Adriamycin toxicity |
34 (3.1) | |||
| Postpartum | 3 (3.6) | |||
| Other | 40 (3.6) | |||
CVA, cerebrovascular accident; DCM, dilated cardiomyopathy; ECMO, extracorporeal membrane oxygenation; SE, standard error; VAD, ventricular assist device.
Number of patients with data available.
Acute myocarditis vs DCM at any time after an episode of myocarditis were unable to be separated within the database and both patient types contribute to this group of patients.
Overall Outcomes
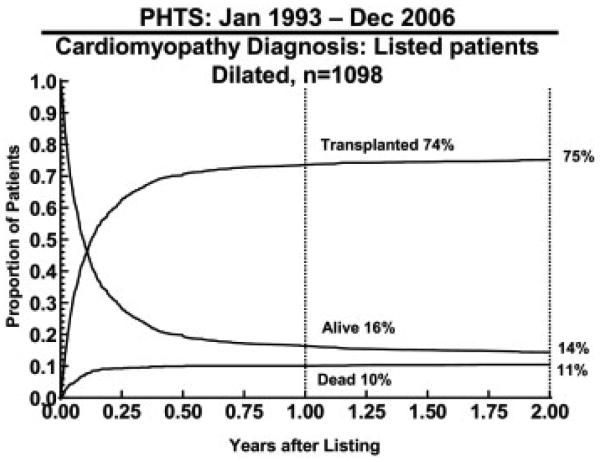
The mean follow-up time among survivors was 4.9 years, with a maximum of 13.9 years. Overall 10-year survival from listing for patients with DCM was 72% (Figure 1). At the end of 2 years, 75% had received an allograft, 11% died waiting, and 14% remained alive awaiting HTx. Competing risk analysis demonstrated that most patients had achieved this outcome by 1 year after listing (Figure 2).
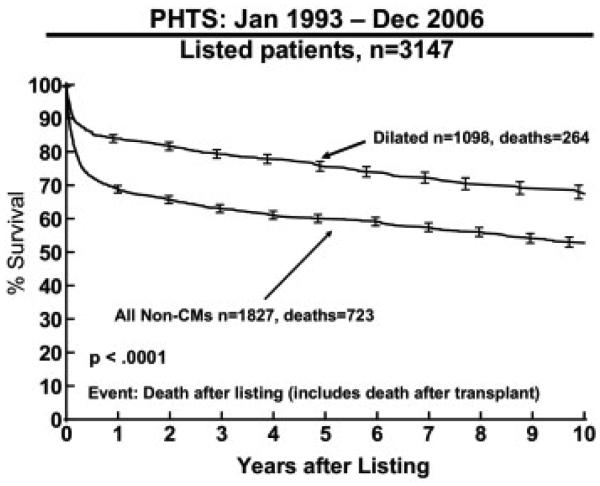
Figure 1.
Kaplan-Meier survival curve for patients with dilated cardiomyopathy (CM) listed for transplantation from the Pediatric Heart Transplant Study (PHTS) Registry data.
Figure 2.
Competing outcomes for patients with dilated cardiomyopathy listed for transplantation from the Pediatric Heart Transplant Study (PHTS) Registry data.
Waitlist Mortality and Causes of Death
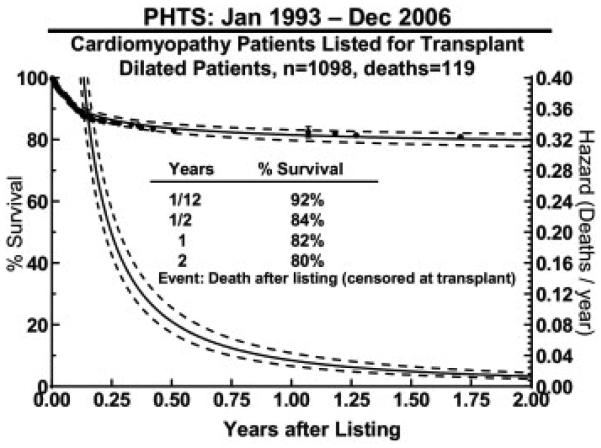
Overall waitlist mortality was low at 11% at 2 years after listing. Figure 3 illustrates the hazard function for death after listing (censored at transplant), showing that most deaths occurred early after listing. In multivariable analysis, significant factors for waitlist mortality included history of ventilation (relative risk [RR], 3.02; p < 0.001), ECMO (RR, 2.17; p = 0.001) or arrhythmias (RR, 1.53; p = 0.03). Etiology, race, gender, age, size, blood group, UNOS status, inotropic or VAD support, surgical history, cardiac index, and pulmonary vascular resistance at listing were not independently associated with waitlist mortality. Death while waiting was most commonly due to heart failure (45%), with other reasons detailed in Table 2.
Figure 3.
Survival and hazard curves (dotted line, 70% confidence intervals) for death after listing for patients with dilated cardiomyopathy (censored at transplant) calculated from the Pediatric Heart Transplant Study (PHTS) Registry data.
Table 2.
Primary Causes of Death While Waiting for Heart Transplantation (n = 119)
| Cause | No. (%) |
|---|---|
| Heart failure/shock | 54 (45.4) |
| CVA/neurologic | 16 (13.5) |
| Multisystem organ failure | 12 (10.1) |
| Sudden cardiac death | 11 (9.2) |
| Infection | 6 (5.0) |
| Respiratory failure | 3 (2.5) |
| Hemorrhage | 3 (2.5) |
| Thrombus/embolism | 2 (1.7) |
| PHT/right ventricular failure | 2 (1.7) |
| Hepatic or renal failure | 2 (1.6) |
| Arrhythmia | 2 (1.7) |
| Unknown | 6 (5.0) |
CVA, cerebrovascular accident; PHT, pulmonary hypertension.
Transplant Outcomes
Among 1,098 patients listed for HTx, 817 (74%) underwent HTx. The demographic details and support at transplant are detailed in Table 1. The mean waiting time was 9.6 months. The clinical status of the patients changed between that at listing and that at transplant: Patients in UNOS status 1 category increased from 77% to 85% and VAD usage increased from 7% to 13%. There was little change in inotropic requirement or ECMO support, however, and the need for mechanical ventilation reduced by 8%.
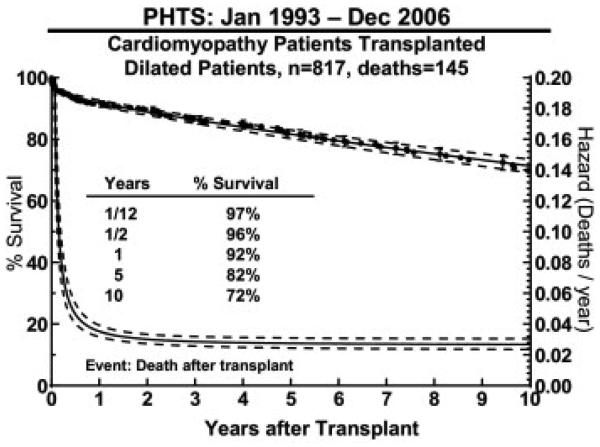
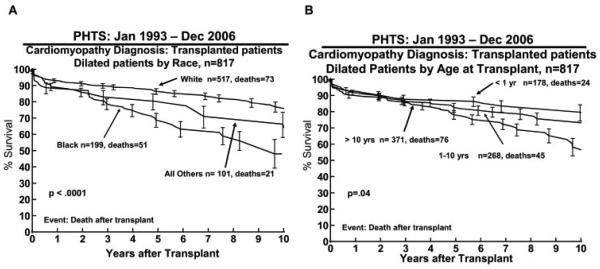
A total of 145 (18%) died after HTx, giving a 10-year post-transplant survival of 72% (Figure 4). The causes of death after HTx are detailed in Table 3. Significant risk factors for death after transplant in multivariable analysis included black race (10-year survival 48% for black race vs 75% white race; RR, 3.00; p < .0001; Figure 5A), older age (10-year-old 10-year survival of 58% vs 1-year-old 10-year survival of 80%; RR, 1.4; p < .04 Figure 5B), mechanical ventilation at transplant (RR, 3.09; p < 0.001), longer ischemic time (RR 1.23; p = 0.03 for an additional hour of ischemic time), and earlier era of transplant (RR, 1.76; p = 0.004 for transplant in 1995 vs in 2005, Table 4).
Figure 4.
Survival and hazard curves (dotted line, 70% confidence intervals) for death after transplant in patients with dilated cardiomyopathy calculated from the Pediatric Heart Transplant Study (PHTS) Registry data.
Table 3.
Primary Causes of Death for 145 Transplant Recipients
| Cause | No. (%) |
|---|---|
| Rejection | 41 (28.3) |
| Allograft vasculopathy | 22 (15.2) |
| Early/non-specific graft failure | 3 17 (11.7) |
| Sudden cardiac death | 17 (11.7) |
| Infection | 15 (10.3) |
| CVA/neurologic | 7 (4.8) |
| Hemorrhage | 5 (3.5) |
| Respiratory failure | 5 (3.5) |
| Arrhythmia | 3 (2.1) |
| Multisystem organ failure | 3 (2.1) |
| Other | 3 (2.1) |
| PTLD | 2 (1.4) |
| Unknown | 5 (3.5) |
CVA, cerebrovascular accident; PTLD, post-transplant lymphoproliferative disorder
Figure 5.
Kaplan-Meier survival curves calculated for transplant recipients (A) stratified by race and (B) stratified by age using data from the Pediatric Heart Transplant Study (PHTS) Registry. Range bars show the confidence interval.
Table 4.
Risk Factors for 145 Deaths After Transplant in 817 Patients With Dilated Cardiomyopathy Diagnosisa
| Early phase | Constant phase | |||
|---|---|---|---|---|
| Factor | RR | p-value | RR | p-value |
|
|
||||
| Death after transplant | ||||
| Older age | — | — | 1.40b | 0.04 |
| Black race | — | — | 3.00 | <0.001 |
| Ventilator at transplant | 3.09 | <0.001 | — | — |
| Longer ischemic time | 1.23c | 0.03 | — | — |
| Earlier year of transplant |
1.76d | 0.004 | — | — |
RR, relative risk.
Pediatric Heart Transplant Study data from January 1993 to December 2006
Compares increased risk from age 1 to 10 years.
Compares increased risk with additional hour of ischemic time.
Compares increased risk of a transplant in 1995 vs 2005.
Era Effect
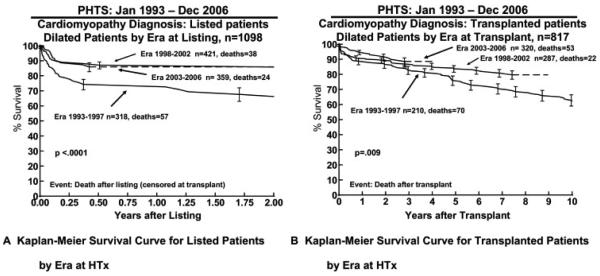
There was a significant era effect on outcomes after listing and after HTx. In the early era, listed DCM patients had an increased risk of dying whilst waiting (Figure 6A), fewer patients achieved HTx (Table 5), and transplant survival was worse (Figure 6B).
Figure 6.
Era effect on survival for (A) listed patients and (B) transplant recipients calculated from the Pediatric Heart Transplant Study (PHTS) Registry data. Range bars show the confidence interval.
Table 5.
Outcome 1 Year After Listing by Era
| Era | No. | Received HTx, % | Alive waiting, % | Died waiting, % |
|---|---|---|---|---|
| 1993–1997 | 318 | 68 | 16 | 16 |
| 1998–2002 | 421 | 75 | 17 | 8 |
| 2003–2006 | 359 | 77 | 16 | 7 |
HTx, heart transplant.
Patients Awaiting Transplant
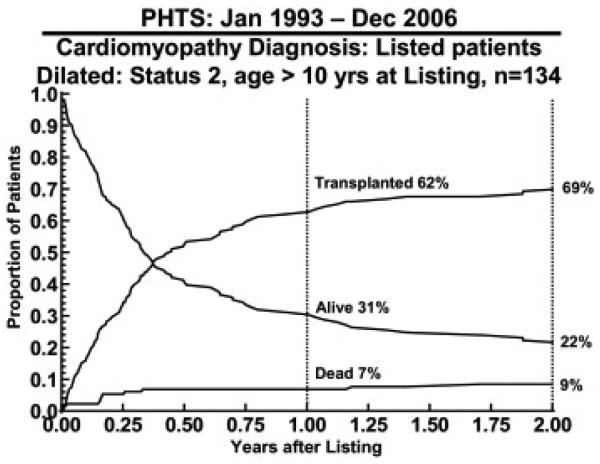
At 1 and 2 years after listing, the largest group of patients that remained on the list comprised 134 patients (12%) who were aged 10 years or older and were at UNOS status 2. Of these, 31% were alive and awaiting transplant 1 year after listing and 22% at 2 years after listing (Figure 7).
Figure 7.
Competing outcomes for listed status 2 patients with dilated cardiomyopathy who were aged older than 10 years at listing calculated from the Pediatric Heart Transplant Study (PHTS) Registry data.
DISCUSSION
Idiopathic DCM accounted for 33% of all the patients listed in the PHTS database. This is in accord with Maron et al,3 who also comment that it is the third most common cause of heart failure and the most frequent cause of HTx. Idiopathic DCM (83%) was also by far the most common of the cardiomyopathies listed. This was, however, a higher proportion than the 56% found in Australia.13
The 11% waitlist mortality of these patients was similar to the 12% reported by Stanford University Medical Center.14 Unlike the Stanford experience, where there was no difference in survival from listing between patients with CM and congenital heart disease, patient survival with DCM was better than all other non-CM patients listed in PHTS, as reported in the overall experience from PHTS (Figure 1).15 This may be due to an era effect, because the Stanford data reflected their experience between 1977 and 1996. The difference in survival between DCM and non-CM (predominantly congenital heart disease) occurs early, with the Kaplan-Meier survival curves running parallel after the first 3 months. The outcome for DCM was also better than that observed for restrictive and hypertrophic CMs.16,17
The etiology of DCM was only identified 20% of patients (Table 1) and was not an independent risk factor for waitlist mortality. Myocarditis has been shown to have a better outcome than idiopathic DCM 4; however, this information is from the Pediatric Cardiomyopathy Registry, with entry at diagnosis rather than at listing for transplant.
Predicting the outcome of DCM patients listed for HTx would be very helpful in counseling families and referring physicians. Unfortunately, patient demographics and clinical features at listing for patients with DCM were mostly unhelpful in predicting outcome in our cohort. Not surprisingly, those sick enough to require ventilation or ECMO support were less likely to survive; however, UNOS status alone was not a predictor. Arrhythmias were also a poor prognostic factor, and this was noted in the Rio de Janeiro experience.18 The ISHLT Registry10 also reported the increased relative risk of 1.26 for ventilation in non-congenital patients.
VAD support did not adversely influence survival in our study, which may be because VAD allows a more prolonged period of support and extends the window of opportunity for HTx to occur. The ISHLT Registry data10 reported a small increased relative risk of 1.44 of VAD, although this did not achieve statistical significance. The details of outcomes of VAD patients in the PHTS database have been reported elsewhere.19 Clinical details such as echocardiographic, electrocardiographic, or radiologic investigations were not part of the Registry data collection and so could not be used to predict outcomes.
Transplantation has improved the survival of patients with DCM. This study shows 72% survival at 10 years compared with 64% survival at 5 years in those without recourse to transplantation.6-8 Those patients who survived to HTx waited more than 9 months, and it is not surprising that they required increased support whilst waiting (Table 1). The adverse influence of older age 10 and black ethnicity20 on HTx survival has been reported before. However, late outcomes for patients undergoing HTx for DCM are no different than outcomes for those who receive an allograft for congenital heart disease.21
There was an era effect, with the percentage receiving a HTx improving between the earlier and later eras from 68% to 77% and the percentage dying whilst waiting reducing from 16% to 7% (Table 5). This may be partly due to the 1999 organ allocation revision (which created status 1A and 1B so that hearts would be offered first to the patients at highest risk of death) and the increasing use of ABO-blood group incompatible organs for infants.22,23 Mechanical support, particularly with VAD, is able to significantly extend the wait time to transplant but VAD was not available in the early era. Survival after HTx is also better in later eras and has also been shown in the ISHLT Registry data.10 In general, this is thought to be mainly due to better peri-operative management, because the late slope of the survival curves is similar.
An interesting sub-group comprises patients who remain listed 2 years after listing. These children may not have undergone HTx because other children were more ill and assumed a higher priority. They may have been listed early to accrue time on the waitlist but with heart offers turned down pending clinical deterioration, or they may remain listed because of clinical well-being, despite poor echocardiographic function, awaiting the “perfect offer.” An earlier study used the PHTS database to address the question of whether relatively well patients (UNOS status 2) should undergo HTx,24 with the conclusion that receiving an allograft conferred a survival benefit. Others, however, have pointed out that the event-free survival of patients with DCM once they are more than 24 months from diagnosis is very good.25 Further study of this group of patients is warranted.
This study has some limitations. Although this large group contains almost 1,100 patients with DCM listed for HTx, when seeking factors that influence survival, the numbers in sub-groups are small, making robust statistical statements challenging. Individual institutions contributing data to the PHTS Registry may have different criteria for listing and bridging patients to HTx. The data forms are not all-inclusive, diagnostic etiologies and mechanisms of death were not recorded, which limited the ability to identify potential key risk factors that might guide listing decisions. Despite these limitations, the information is valuable in predicting outcomes and counseling families.
In conclusion, transplantation for DCM offers enhanced survival compared with the natural history of this heterogenous diagnosis in the pediatric population. Overall waitlist mortality is lower than most other cardiomyopathy groups, with the exception of patients on ECMO, those who are mechanically ventilated, or who have significant arrhythmias. Conversely, no other variables studied, including status, diagnosis, age, and VAD, support predicted waitlist mortality. These patients fared better after transplant as well, making transplantation a key therapeutic intervention.
Disclosure Statement
None of the authors has a financial relationship with a commercial entity that has an interest in the subject of the presented manuscript.
Acknowledgements
This research was partly funded by a grant from the Children's Cardiomyopathy Foundation and the National Institutes of Health under award number: T32HL007572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors are indebted to Susan L. Myers and Margaret Tresler Foushee for their dedicated statistical assistance and input to the manuscript.
References
- 1.Andrews R, Fenton M, Ridout D, Burch M. New-onset heart failure due to heart muscle disease in childhood: a prospective study in the United Kingdom and Ireland. Circulation. 2008;117:79–84. doi: 10.1161/CIRCULATIONAHA.106.671735. [DOI] [PubMed] [Google Scholar]
- 2.Richardson P, McKenna W, Bristow M. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the definition and classification of cardiomyopathies. Circulation. 1996;93:841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 3.Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee. Circulation. 2006;113:1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 4.Towbin J, Lowe A, Colan S, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296:1867–1876. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 5.Towbin J. Pediatric myocardial disease. Pediatr Clin North Am. 1999;45:289–312. doi: 10.1016/s0031-3955(05)70119-x. View In Article | Cross Ref. [DOI] [PubMed] [Google Scholar]
- 6.Akagi T, Benson LN, Lightfoot NE, et al. Natural history of dilated cardiomyopathy in children. Am Heart J. 1991;121:1502–1506. doi: 10.1016/0002-8703(91)90158-e. [DOI] [PubMed] [Google Scholar]
- 7.Burch M, Siddiqi SA, Celermajer DS, Scott C, Bull C, Deanfield JE. Dilated cardiomyopathy in children: determinants of outcome. Br Heart J. 1994;72:246–250. doi: 10.1136/hrt.72.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arola A, Tuominen J, Ruuskanen O, Jokinenet E. Idiopathic dilated cardiomyopathy in children: prognostic indicators and outcome. Pediatrics. 1998;101:369–376. doi: 10.1542/peds.101.3.369. [DOI] [PubMed] [Google Scholar]
- 9.Tsirka E, Trinkaus K, Chen S, et al. Improved outcomes of pediatric dilated cardiomyopathy with utilization of heart transplantation. J Am Coll Cardiol. 2004;44:391–397. doi: 10.1016/j.jacc.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 10.Kirk R, Edwards L, Aurora P, et al. Registry of the International Society for Heart and Lung: eleventh official pediatric heart transplantation report 2008. J Heart Lung Transplant. 2008;27:970–977. doi: 10.1016/j.healun.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 11.Blackstone EH, Naftel DC, Turner ME. The decompensation of time-varying hazard into phases, each incorporating a separate stream of concomitant information. J Am Statistical Assoc. 1986;81:615–624. [Google Scholar]
- 12.McGriffin DC, Naftel DC, Kirklin JK. Depicting time-related events after cardiac surgery: Kaplan-Meier or competing risk? Asia Pacific Heart J. 1998;7:98–102. [Google Scholar]
- 13.Nugent AW, Daubeney PEF, Chondros P, et al. The epidemiology of childhood cardiomyopathy in Australia. N Engl J Med. 2003;348:1639–1646. doi: 10.1056/NEJMoa021737. [DOI] [PubMed] [Google Scholar]
- 14.Dubin AM, Rosenthal DN, Chin C, Falco DA, Gamberg P, Bernstein DL. Outcome between listing and transplantation in pediatric heart transplant candidates: comparison of congenital heart disease and dilated cardiomyopathy. Pediatr Res. 1999;45:22A. [Google Scholar]
- 15.Dipchand AI, Naftel DC, Feingold B, et al. Outcomes of children with cardiomyopathy listed for transplant: a multi-institutional study. J Heart Lung Transplant. 2009;28:1312–1321. doi: 10.1016/j.healun.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Zangwill SD, Naftel DC, L'Ecuyer T, et al. Outcomes of children with restrictive cardiomyopathy listed for heart transplant: a multi-institutional study. J Heart Lung Transplant. 2009;28:1335–1340. doi: 10.1016/j.healun.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 17.Gajarski R, Naftel DC, Pahl E, et al. Outcomes of pediatric patients with hypertrophic cardiomyopathy listed for transplant. J Heart Lung Transplant. 2009;28:1329–1334. doi: 10.1016/j.healun.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 18.Azevedo VMP, Santos MA, Albanesi Filho FM, Castier MB, Tura RB, Amino JGC. Outcome factors of idiopathic dilated cardiomyopathy in children – a long-term follow-up review. Cardiol Young. 2007;17:175–184. doi: 10.1017/S1047951107000170. [DOI] [PubMed] [Google Scholar]
- 19.Blume E, Naftel D, Bastardi H, Duncan B, Kirklin J, Webber S. Outcomes of children bridged to heart transplantation with ventricular assist devices. Circulation. 2006;113:2313–2319. doi: 10.1161/CIRCULATIONAHA.105.577601. [DOI] [PubMed] [Google Scholar]
- 20.Mahle W, Kanter K, Vincent R. Disparities in outcome for black patients after pediatric heart transplantation. J Pediatr. 2005;147:739–743. doi: 10.1016/j.jpeds.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Dionigi B, Razzouk AJ, Hasaniya NW, Chinnock RE, Bailey LL. Late outcomes of pediatric heart transplantation are independent of pre-transplant diagnosis and prior cardiac surgical intervention. J Heart Lung Transplant. 2008;27:1090–1095. doi: 10.1016/j.healun.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 22.West L, Pollock-BarZiv S, Dipchand A, et al. ABO-incompatible heart transplantation in infants. N Engl J Med. 2001;344:793–800. doi: 10.1056/NEJM200103153441102. [DOI] [PubMed] [Google Scholar]
- 23.Pollock-BarZiv S, McCrindle B, West L, Manlhiot C, VanderVliet M, Dipchand A. Competing outcomes after neonatal and infant wait-listing. J Heart Lung Transplant. 2007;26:980–985. doi: 10.1016/j.healun.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 24.Kirklin J, Naftel D, Caldwell R, et al. Should status ii patients be removed from the pediatric heart transplant waiting list? J Heart Lung Transplant. 2006;25:271–275. doi: 10.1016/j.healun.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Gagliardi M, Bevilacqua M, Bassano C, et al. Long term follow up of children with myocarditis treated by immunosuppression and of children with dilated cardiomyopathy. Heart. 2004;90:1167–1171. doi: 10.1136/hrt.2003.026641. [DOI] [PMC free article] [PubMed] [Google Scholar]