Abstract
Translocase I (MraY/MurX) is an essential enzyme in growth of the vast majority of bacteria that catalyzes the transformation from UDP-MurNAc-pentapeptide (Park’s nucleotide) to prenyl-MurNAc-pentapeptide (lipid I), the first membrane-anchored peptidoglycan precursor. MurX has been received considerable attentions to the development of new TB drugs due to the fact that the MurX inhibitors kill exponentially growing Mycobacterium tuberculosis (Mtb) much faster than clinically used TB drugs. Lipid I isolated from Mtb contains the C50-prenyl unit that shows very poor water-solubility, and thus, this chemical characteristic of lipid I renders MurX enzyme assays impractical for screening and lacks reproducibility of the enzyme assays. We have established a scalable chemical synthesis of Park’s nucleotide-Nε-dansylthiourea 2 that can be used as a MurX enzymatic substrate to form lipid I analogues. In our investigation of minimum structure requirement of the prenyl phosphate in the MraY/MurX-catalyzed lipid I analogue synthesis with 2, we found that neryl phosphate (C10-phosphate) can be recognized by MraY/MurX to generate the water-soluble lipid I analogue in quantitative yield under the optimized conditions. Herein, we report a rapid and robust analytical method for quantifying MraY/MurX inhibitory activity of library molecules.
Keywords: Mur X, Mra Y, Translocase I, Mycobacterium tuberculosis, Water-soluble lipid I, Park’s nucleotide, MraY assay, HTS, MraY inhibitors
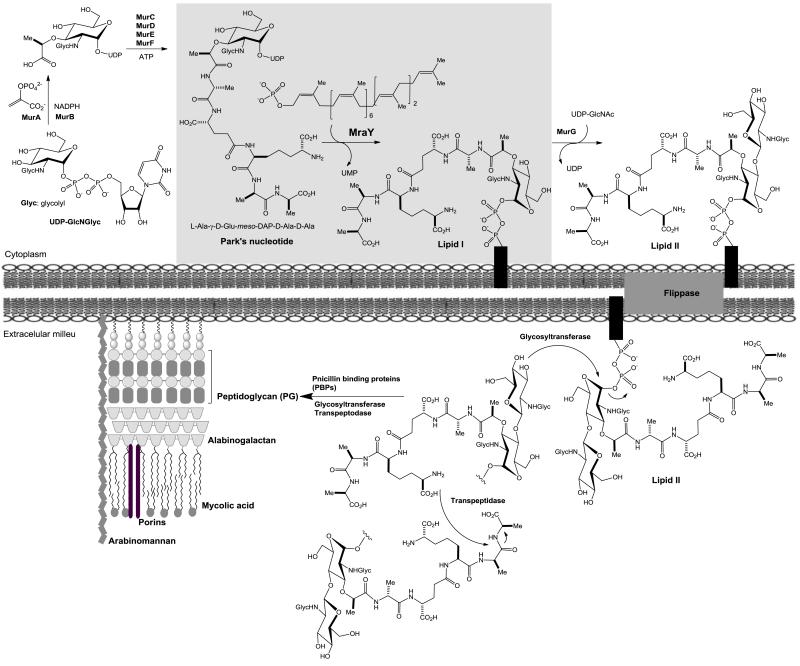
The eradication of tuberculosis remains a prominent challenge for basic, translational, and clinical research scientists [1]. Once thought to be under control, tuberculosis case reports are increasing world-wide and the disease poses a major global public health threat. In 2011, 8.7 million people were infected with Mycobacterium tuberculosis (Mtb) and 1.4 million people died from TB [2-3]. One-third of the 42 million people living with HIV/AIDS worldwide are co-infected with Mtb [4-5]. Clinical responses of multidrug-resistant (MDR)-TB patients to the 1st line drugs have been poor, and in some cases there is no response at all. The WHO estimated that 650,000 new cases of MDR-TB emerge each year, and 27 countries around the world account for 86% of the MDR-TB burden. An outbreak of extensively-drug resistant (XDR)-Mtb was reported in 2006 [3,6]. For MDR strains of Mtb, treatment length of TB chemotherapy can be at least 20-28 months. The treatment of XDR-TB takes substantially longer than MDR-TB [4,7]. Thus, it is significantly important to discover promising approaches to shorten current TB drug regimen. In in vitro time-kill assessment experiments, FDA-approved TB drugs required 11 to 14 days to kill exponentially growing Mtb at 2-4×MIC concentrations. On the other hand, several translocase I (MraY/MurX, hereafter referred to as “MurX” for Mtb translocase I) inhibitors have been known to kill >95% of Mtb in 2-5 days at MIC or 2-4×MIC concentrations [8-9]. Since peptidoglycan (PG) is an essential bacterial cell-wall polymer, the machinery for PG biosynthesis provides a unique and selective target for antibiotic action. The biosynthesis of PG of E. coli has been discussed extensively in reviews by van Heijenoort [10-12]. Most of the genes involved in peptidoglycan biosynthesis in E. coli are known and orthologs have been identified in the Gram-positive genomes. However, very few genes responsible for the unique features of mycobacterial peptidoglycan to diversify the cell wall structure have been known. Detailed analyses of the components of mycobacterial PG revealed that it contains a variety of modified molecules including 1) an N-glycolyl (NGlyc) in addition to N-acetyl (NAc) group on the muramic acid (Mur), 2) amidation of the carboxylic acids in the peptide moieties of PG, and 3) additional glycine or serine residues [13-15]. Interestingly, the N-glycolylated muramic acid predominates in mycobacteria [13] (Fig 1). To date, only a few enzymes in PG biosynthesis such as the transpeptidase of penicillin binding proteins (PBPs) have been studied extensively. Thus, the machinery for PG synthesis is still considered to be a source of unexploited drug targets. However, most of drugs associated with cell-wall biosynthesis may not reduce treatment time of a TB drug regimen because the dormant or non-replicating Mtb are not actively synthesizing cell-walls [16]. On the contrary, a fast bactericidal effect of MurX inhibitors is very attractive to develop new TB drugs that reduce the time frame for effective anti-TB chemotherapy [8]. MurX catalyzes the transformation of UDP-MurNGlyc-pentapeptide and UDP-MurNAc-pentapeptide (Park’s nucleotide) to the corresponding lipid I using decaprenyl (C50) phosphate in Mycobacterium spp. [17-18]. This process is believed to be a reversible process in which E. coli MraY catalyzes an exchange reaction between UMP and lipid I to form Park’s nucleotide in vitro [19].
Fig 1.
Biosynthesis of peptidoglycan in Mycobacterium tuberculosis.
Isolation and quantitation of Park’s nucleotide and lipid I from in vitro MraY/MurX assay reaction mixtures are time-consuming processes [17]. In addition, preparation of Mtb Park’s nucleotide via semi-purified Mur enzymes is not amenable to multigram scale-up and the acquisition cost of enough decaprenyl phosphate for medium- to high-throughput screenings is very high. To date, several screening methods for MraY/MurX inhibitors have been reported that includes; 1) monitoring the transfer of phosphoryl-MurNAc-pentapeptide using fluorescent or radiolabeled Park’s nucleotide and/or undecaprenyl phosphate [19], 2) measuring the exchange reaction between [3H]UMP to Park’s nucleotide that requires separation of [3H]uridine after the treatment of alkaline phosphatase [20,21], 3) an indirect assay using a coupled MraY-MurG that requires biotinylated Park’s nucleotide and [14C]UDP-GlcNAc [22], 4) an assay using HP20ss hydrophobic beads for isolating the generated radiolabeled lipid I [23], 5) a microplate-based assay using a radiolabeled-Park’s nucleotide [24], and 6) a scintillation proximity assay using wheat germ agglutinin-coated beads to capture the lipid I from a radiolabeled-Park’s nucleotide [25]. Although a several assay methods were reported to be amenable to a HTS assay for MraY [19,25,26], in our hands, extraction of water-insoluble lipid I derivative from assay media is essential. In our attempt at developing reliable in vitro MraY/MurX assay, we concluded that the reported assays need further optimization to be robust statistical methods that can identify MraY/MurX inhibitors routinely with IC50 values. We established an efficient synthetic method for the generation of sufficient amount of fluorescent Park’s nucleotide probes for HTS [27,28], and tested the Park’s nucleotide probes in MurX-catalyzed lipid I analogue synthesis with decaprenyl and truncated prenyl phosphates. Surprisingly, under the optimized conditions the water-soluble lipid I-neryl (C10) analogue could be biosynthesized efficiently with the Park’s nucleotide probes and neryl phosphate. In the present work, we report a convenient and reliable enzyme assay for MurX to identify antimycobacterial MurX inhibitor molecules.
Materials and methods
Chemical materials and methods
Difco Middlebrook 7H10 agar, Middlebrook 7H9 broth, Tryptic soy agar, Tryptic soy broth, MOPS, tris(hydroxymethyl)aminomethane, 2-mercaptoethanol, sucrose and triton-X 100 were purchased from Sigma-Aldrich. ADC enrichment was purchased from Fisher Scientific. Magnesium chloride and potassium chloride were obtained from VWR. All reagents and solvents were commercial grade and were used as received without further purification unless otherwise noted. Flash chromatography was performed with Whatman silica gel (Purasil 60 Å, 230-400 Mesh). Analytical thin-layer chromatography was performed with 0.25 mm coated commercial silica gel plates (EMD, Silica Gel 60F254) visualizing at 254 nm, or developed with ceric ammonium molybdate or anisaldehyde solutions by heating on a hot plate. 1H-NMR spectral data were obtained using 400, and 500 MHz instruments. 13C-NMR spectral data were obtained using 100 and 125 MHz instruments. For all NMR spectra, δ values are given in ppm and J values in Hz.
MurX/MraY assay substrates
Park’s nucleotide-Nε-dansylthiourea 2, neryl-lipid I-Nε-dansylthiourea 5, and neryl phosphate (6) were chemically synthesized from the corresponding starting materials.
Neryl phosphate (6)
To a solution of phosphoric acid (98 mg, 1.0 mmol), pyridine (0.40 mL, 5.0 mmol) and nerol (1.8 mL, 10 mmol) was added triethylamine (0.28 mL, 2.0 mmol). After being stirred for 30 min., acetic anhydride (0.19 mL, 2.0 mmol) was added to the reaction mixture. The reaction mixture was stirred at 80 °C for 12h, and the reaction was cooled to room temperature. The reaction was quenched with water (5 mL) and stirred for 1h at 80 °C. The reaction mixture was cooled to room temperature, and the aqueous phase was extracted with ether (5mL × 3). Lyophilization of the aqueous phase gave the crude product. Purification by DOWEX 50WX8 afforded neryl phosphate (6)-mono ammounium salt (0.17g, 74%) as a white solid [29]. This reaction could readily be scaled up to multi grams of neryl phosphate-mono ammonium salt. 1H NMR (400 MHz, D2O2) δ 5.33 (td, J = 7.3, 1.6 Hz, 1H), 5.14 – 5.04 (m, 1H), 4.27 (t, J = 7.5 Hz, 2H), 2.10 – 2.00 (m, 4H), 1.67 (d, J = 1.2 Hz, 3H), 1.59 (s, 3H), 1.53 (s, 3H); 13C NMR (101 MHz, D2O) δ 142.75, 133.95, 123.84, 120.62, 120.54, 61.88, 61.83, 31.18, 25.92, 24.81, 22.57, 16.91; 31P NMR (162 MHz, D2O) δ 3.72; LRMS (EI) calcd for C10H20O4P (M+H+): 235.11, found: 235.05.
Park’s nucleotide-Nε-dansylthiourea 2
Park’s nucleotide was synthesized according to the previously reported procedure [27]. To a stirred solution of N-acetyl Park’s nucleotide (3.0 mg, 2.6 μmol) in 0.1 M aqueous NaHCO3 solution (0.10 mL) was added 5-(dimethylamino)-N-(4-isothiocyanatophenyl)naphthalene-1-sulfonamide (3.0 mg, 7.8 μmol) in DMF (0.05 mL). After being stirred for 2.5h at room temperature, the reaction mixture was filtered. The filtrate was purified by reverse-phase HPLC [column: HYPERSIL GOLD™ (175 A, 12 μm, 250 × 10 mm), solvents: a gradient elution of 0 : 100 to 30 : 70 CH3CN : 0.05 M aqueous NH4HCO3 over 30 min, flow rate: 2.0 mL/min, UV: 350 nm] to afford 2 (2.4 mg, 60%, the retention time: 29 min). Similarly, N-acetyl Park’s nucleotide (500 mg) was converted to 2 in 75% yield. 1H NMR (400 MHz, D2O) δ 8.43 (d, J = 8.6 Hz, 1H), 8.39 (d, J = 8.5 Hz, 1H), 8.23 (d, J = 7.3 Hz, 1H), 7.89 (d, J = 8.1 Hz, 1H), 7.67 (t, J = 8.1 Hz, 1H), 7.59 (t, J = 8.0 Hz, 1H), 7.37 (d, J = 7.6 Hz, 1H), 6.98 – 6.93 (m, 4H), 5.94 (d, J = 3.7 Hz, 1H), 5.89 (d, J = 8.1 Hz, 1H), 5.45 (dd, J = 7.2, 3.1 Hz, 1H), 5.28 (dd, J = 7.2, 2.9 Hz, 1H), 4.33 (d, J = 3.3 Hz, 2H), 4.30 (d, J = 7.3 Hz, 1H), 4.27 – 4.22 (m, 2H), 4.22 – 4.16 (m, 3H), 4.16 – 4.08 (m, 3H), 4.07 (q, J = 7.2 Hz, 1H), 3.96 – 3.90 (m, 1H), 3.88 – 3.71 (m, 3H), 3.65 – 3.57 (m, 1H), 3.48 – 3.36 (m, 2H), 3.33 (s, 1H), 2.82 (s, 6H), 2.30 – 2.22 (m, 2H), 2.21 (s, 3H), 2.16 – 2.05 (m, 1H), 1.90 – 1.79 (m, 1H), 1.78 – 1.63 (m, 2H), 1.53 – 1.42 (m, 2H), 1.37 (d, J = 7.1 Hz, 3H), 1.36 (d, J = 6.7 Hz, 3H), 1.31 (d, J = 7.3 Hz, 3H), 1.28 (d, J = 7.3 Hz, 3H); LRMS (EI) calcd for C59H83N12O28P2S2 (M+H+): 1533.44, found: 1533.80.
Neryl Lipid I-Nε-dansylthiourea 5
Lipid I-neryl analogue was synthesized according to the reported procedure with a minor modification [27-28]. Thiourea formation of lipid I-neryl analogue was performed under the same conditions for the synthesis of 2. The crude product was purified by reverse-phase HPLC [column: HYPERSIL GOLD™ (175 A, 12 μm, 250 × 10 mm). solvent: a gradient elution of 20 : 80 to 50 : 50 CH3CN : 0.05 M aqueous NH4HCO3 over 30 min, flow rate: 2.0 mL/min, UV 350 nm] to afford 5 (2.0 mg, 70%, the retention time: 20 min). 1H NMR (400 MHz, D2O) δ 8.46 (d, J = 8.6 Hz, 1H), 8.35 (d, J = 8.8 Hz, 1H), 8.29 (d, J = 7.8 Hz, 1H), 7.73 (t, J = 8.1 Hz, 1H), 7.62 (t, J = 8.2 Hz, 1H), 7.42 (d, J = 7.3 Hz, 1H), 7.04 – 7.00 (m, 4H), 5.45 – 5.41 (m, 1H), 5.39 – 5.35 (m, 1H), 5.08 – 5.01 (m, 2H), 4.44 – 4.38 (m, 2H), 4.30 (q, J = 6.9 Hz, 2H), 4.23 (q, J = 7.1 Hz, 2H), 4.18 – 4.04 (m, 6H), 3.96 – 3.90 (m, 2H), 3.86 (dd, J = 12.0, 1.9 Hz, 1H), 3.81 (d, J = 4.4 Hz, 1H), 3.78 (d, J = 5.5 Hz, 1H), 3.74 (d, J = 9.3 Hz, 1H), 3.60 (t, J = 9.6 Hz, 1H), 3.49 – 3.40 (m, 2H), 2.85 (s, 6H), 2.29 – 2.23 (m, 2H), 2.05 – 1.97 (m, 2H), 1.94 (s, 3H), 1.89 – 1.80 (m, 2H), 1.74 (d, J = 28.0 Hz, 2H), 1.66 (s, 3H), 1.59 (s, 3H), 1.51 (s, 3H), 1.38 (d, J = 6.9 Hz, 3H), 1.36 (d, J = 6.7 Hz, 3H), 1.31 (d, J = 7.2 Hz, 3H), 1.28 (d, J = 7.2 Hz, 3H); LRMS (EI) calcd for C60H89N10O23P2S2 (M+H+): 1443.50, found: 1443.90.
Bacterial strains and growth of bacteria
Mycobacterium tuberculosis (H37Rv) was obtained through BEI Resources, NIAID/NIH. Mycobacterium smegmatis (ATCC 607), Staphylococcus aureus (ATCC BAA-1556), and Escherichia coli K-12 (ATCC 29425) were obtained from ATCC. A single colony of bacterial strain was obtained on a Difco Middlebrook 7H10 nutrient agar enriched with 10% oleic acid, albumin, dextrose and catalase (OADC for M. tuberculosis), albumin, dextrose and catalase (ADC for M. smegmatis), and on Tryptic Soy agar (for E. coli and S. aureus). Seed cultures were obtained in Middlebrook 7H9 broth enriched with OADC (for M. tuberculosis), ADC (for M. smegmatis), and in Tryptic Soy broth (for E. coli and S. aureus), respectively. Each bacterium was grown to mid-log phase.
Preparation of membrane fraction P-60 containing MurX/MraY
M. tuberculosis cells were harvested by centrifugation (4,700 RPM) at 4 °C followed by washing with 0.9% saline solution (thrice) and ~5g of pellet (wet weight) was collected. The washed cell pellets were suspended in homogenization buffer (containing 50 mM MOPS of pH = 8, 0.25 M sucrose, 10 mM MgCl2 and 5 mM 2-mercaptoethanol) and disrupted by probe sonication on ice (10 cycles of 60s on and 90s off). The resulting suspension was centrifuged at 1,000 ×g for 10 min at 4 °C to remove unbroken cells. The supernatant was centrifuged at 25,000 ×g for 40 min at 4 °C (3 to 4 times). All pellets in each tube were pooled and a second sonication was performed (10 cycles of 60s on and 90s off). The lysate was centrifuged once at 25,000 ×g for 1h and the supernatant was subjected to ultracentrifugation at 60,000 ×g for 1h at 4 °C. The supernatant was discarded and the membrane fraction containing MurX enzyme (P-60) was suspended in the TRIS-HCl buffer (pH 7.5, containing 2-mercaptoethanol) [30,31]. Total protein concentrations are about 8~10 mg/mL [32]. Aliquots were stored in eppendorf tubes at −80°C. Similarly, the membrane fractions containing MraY enzyme (P-60) were prepared from M. smegmatis, S aureus, and E. coli, respectively.
MurX/MraY assay
Park’s nucleotide-Nε-dansylthiourea 2 (2 mM stock solution; 3.75 μL (75 μM)), MgCl2 (0.5 M; 10 μL (50 mM)), KCl (2 M, 10 μL (200 mM)), triton X100 (0.5%; 11.25 μL), tris-buffer (pH = 8; 50mM, 2.5 μL), neryl phosphate (6, 10 mM, 45 μL), and inhibitor (0-100 μM, in DMSO (2.5 μL)) were place in a 500 μL Eppendorf tube. To a stirred reaction mixture, P-60 (15 μl) was added (the total volume of the reaction mixture: 100 μL). The reaction mixture was incubated for 1h at room temperature (26 °C), and quenched with CHCl3 (200 μL). Two phases were mixed via vortex and centrifuged at 25,000 ×g for 10 min. The upper aqueous phase was assayed via reverse-phase HPLC. The water phase (10 μl) was injected into HPLC (solvent: CH3CN:0.05 M aq. NH4HCO3 = 25 : 75, UV: 350 nm, flow rate: 0.5 mL/min, Column: Kinetex 5u C8 100Å, 150 × 4.60mm), and the area of the peak for lipid I-neryl derivative 5 was quantified to obtain the IC50 value. The IC50 values were calculated from plots of the percent product inhibition versus the inhibitor concentration.
Kinetic parameter evaluation via MurX/MraY activity assay
Evaluation of kinetic parameters were performed through MurX- or M. smegmatis MraY-catalyzed lipid I synthesis. Km and Vmax were determined by Michaelis-Menten enzyme kinetics. The correlation (Michaelis-Menten plot) between the concentrations of Park’s nucleotide-dansylthiourea 2 (x axis) and rate (V) of lipid I formation (y axis) was obtained using GraphPad Prism Software [33-34].
Compounds
All antimycobacterial molecules screened against MurX were synthesized in our laboratory except for tunicamycin (sigma) and vancomycin (sigma). All molecules were diluted with DMSO to be the concentration of 1 mg/100 μL (stock solution). The MurX assays developed here were tolerated to 2.5% of DMSO concentrations in total volume of the reaction solution (100 μL). The maximum tolerated concentration for DMSO in MurX-catalyzed neryl lipid I-Nε-dansylthiourea 5 has not been determined.
Determination of MICs
M. tuberculosis was cultured to be an optical density of 0.4-0.5. Each compound (8 μL) stored in DMSO (1 mg/100 μL) was placed in a sterile 96 well plate and a serial dilution was conducted with the culturing broth (total volume of 100 μL). The bacterial suspension (100 μL) was added to each well (total volume of 200 μL). The bacterial culture in a plate treated or non-treated with compounds was incubated for 14 days at 37 °C in a shaking incubator (120 rpm). Resazurin (0.01%, 20 μL) was added to each well and incubated at 37 °C for 5h. The MIC values were determined according to NCCLS method (pink = growth, blue = no visible growth). The absorbance of each well was also measured at 570 nm and 600 nm via a microplate reader.
Results and discussion
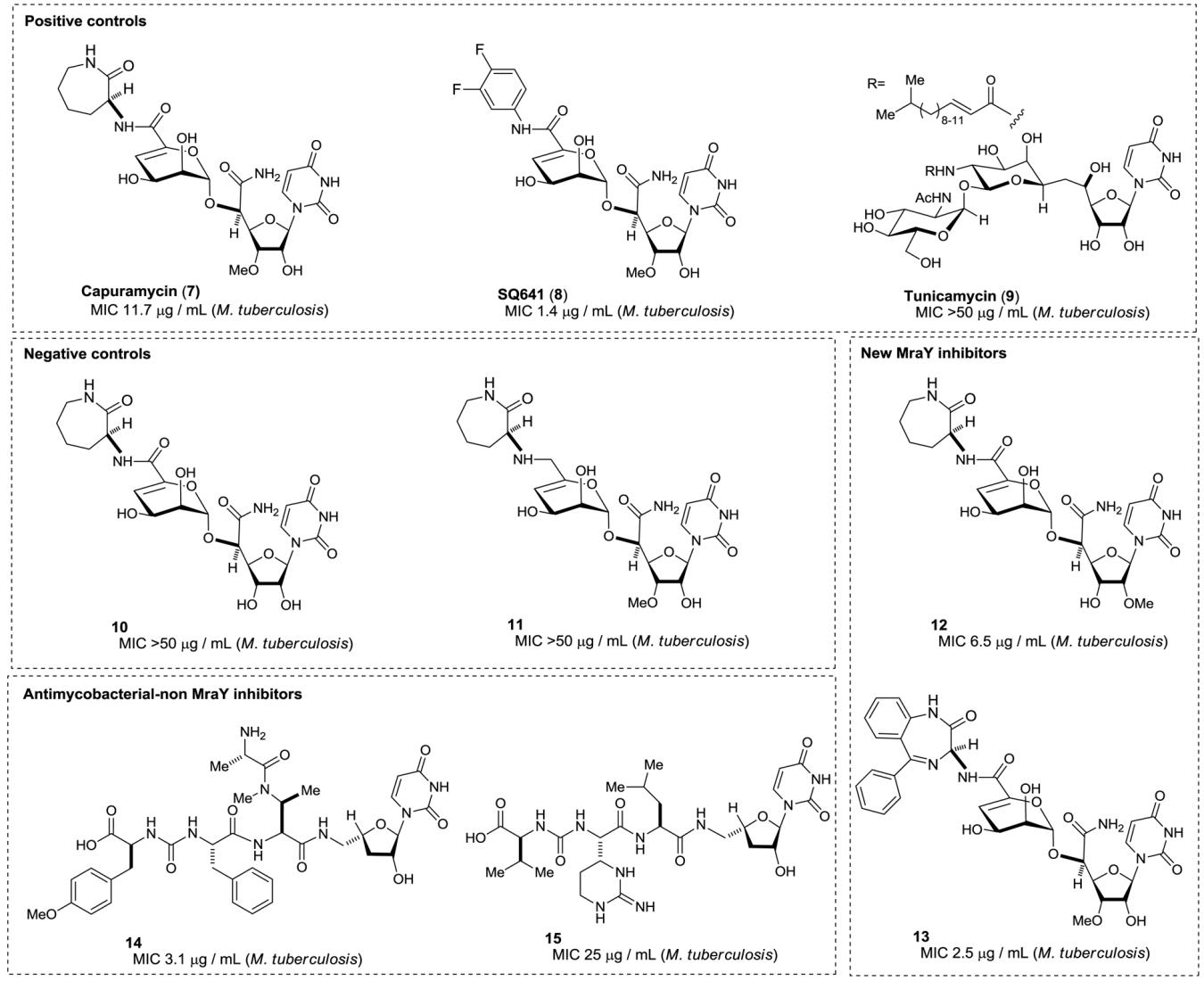
A number of selective MraY inhibitors from natural sources have been reported [35-41]. The major source of future developments resides in the nucleoside based inhibitors, which are subdivided into 5 classes. Tunicamycin is a relatively weak MurX inhibitor [26], and did not show strong antimycobacterial activity. Caprazamycin, muraymycins, liposidomycin, capuramycin, mureidomycins, pacidamycins and their congeners were reported to exhibit significant MraY enzyme inhibitory activity in vitro [35]. Capuramycin showed activity specific to Mycobacterium spp. Antimicrobial spectrum focused against Mtb (selective antimycobacterial agent) is preferable for TB chemotherapy due to the fact that TB chemotherapy requires a long regimen, so that broad-spectrum anti-TB agents may cause resistant to other bacteria during TB chemotherapy. Most of the reported MraY inhibitors were competitive to Park’s nucleotide but not to prenyl-phosphate. Because capuramycin is a well-characterized MraY/MurX inhibitor that showed activities focused against Mycobacterium spp. [40], we selected positive and negative controls from capuramycin derivatives to develop a convenient MurX assay method, and the new MurX assay developed here was planned to demonstrate by screen antimycobacterial uridyl-peptide library molecules.
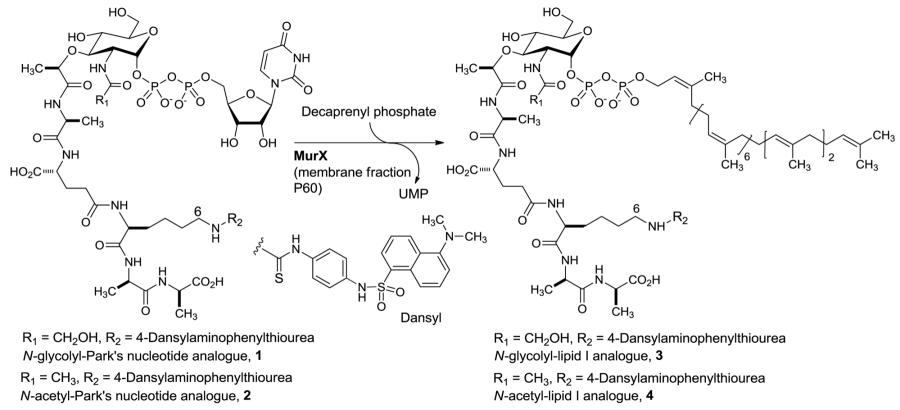
N-Glycolylated muramic (MurNGlyc) acid predominates in M. tuberculosis and MurNGlyc is the major muramyl building blocks in mycobacterial cell walls (Fig 1) [13,41]. Thus, it is of importance to characterize specificity of MurX against N-glycolyl Park’s nucleotide and N-acetyl Park’s nucleotide for discovery of novel antimycobacterial MurX inhibitors. In order to understand the tolerance of MurX in the structure of Park’s nucleotide, we have synthesized a series of Park’s nucleotide analogues according to our established chemo-enzymatic or total chemical syntheses [17,27,28]. We and other groups have demonstrated that the dansyl- or FITC-conjugated N-acetyl Park’s nucleotides can be applied to MraY enzyme assays with the MraY obtained from Gram-negative and some Gram-positive organisms [41]. In our preliminary studies, the membrane fraction containing MraY or MurX enzyme (P-60) obtained from E. coli, S. aureus, M. smegmatis, or Mtb could transfer the fluorescent-Park’s nucleotide conjugates to the corresponding undecaprenyl lipid I analogues with undecaprenyl phosphate in 5-25% yields after 1h incubation [31]. These results were in accordance with the data previously reported by several other groups [19,20]. It was experimentally proved that MurX was also tolerated in the structure of the C6-position (Nε) of lysine moiety of Park’s nucleotides.
In order to further study tolerance of MurX against the structure of the N-acyl moiety of muramic acid in Park’s nucleotide, we examined time-course experiments of lipid I syntheses with N-glycolyl Park’s nucleotide-Nε-dansylthiourea 1 and N-acyl Park’s nucleotide-Nε-dansylthiourea 2. MurX-catalyzed syntheses of lipid I analogues with 1 and 2 are summarized in Table 1. All reactions were performed in duplicate under the same reaction conditions except for the concentrations of decaprenyl phosphate and reaction time. The lipid I analogues 3 and 4 were chemically synthesized as reference molecules for HPLC studies [27-28]. Direct comparison of the product yields for 3 and 4 revealed that Park’s nucleotide-Nε-dansylthiourea 1 and 2 were converted to the corresponding lipid I analogues 3 and 4, respectively, by MurX without noticeable difference in reaction rate and product yield (Entry 1 vs. 4 and 2 vs. 5 in Table 1). Increasing concentration of decaprenyl phosphate (from 2 to 10 equivalents against 1 or 2) did not dramatically increase the product yield (Entry 3 vs. 6 in Table 1). Kinetic studies revealed that N-glycolyl Park’s nucleotide-Nε-dansylthiourea 1 and N-acyl Park’s nucleotide-Nε-dansylthiourea 2 have a similar binding affinity towards MurX with the Km values of 18.05 and 17.95 μM, respectively [19]. Although Mtb predominantly uses N-glycolyl Park’s nucleotide for the biosynthesis of peptidoglycan through N-glycolyl lipid I [13], MurX recognizes N-acetyl Park’s nucleotide equally. These structural tolerances of the Park’s nucleotide binding domain of MurX implies the possibility of further simplification of Park’s nucleotide for development of a convenient MurX/MraY assay.
Table 1.
MurX-catalyzed syntheses of lipid I analogues from N-glycolyl- and N-acetyl-Park’s nucleotides
| |||||
|---|---|---|---|---|---|
|
| |||||
| Entry1 | Park’s nucleotide | Decaprenyl phosphate (equivalents against 1 or 2) |
Lipid I | Reaction time (h) |
Yield (%)2 |
| 1 | 1 | 2 | 3 | 1 | 15-20 |
| 2 | 1 | 2 | 3 | 3 | 15-20 |
| 3 | 1 | 10 | 3 | 1 | 20-25 |
| 4 | 2 | 2 | 4 | 1 | 15-20 |
| 5 | 2 | 2 | 4 | 3 | 15-25 |
| 6 | 2 | 10 | 4 | 1 | 20-25 |
Reaction conditions: Park’s nucleotide (2 mM; 3.75 μL), MgCl2 (0.5 M; 10 μL); KCl (2 M, 10 μL), Triton X100 (0.1%; 11.25 μl), Tris-buffer (pH = 8; 50mM, 5 μL), decaprenyl phosphate (10 mM, 2 or 10 equivalents against 1 or 2), P-60 (15 μL), 26 °C, 1 or 3h.;
n-butanol extract was analyzed by HPLC (column: Kinetex 5u C8 100A, 150×4.60 mm, solvent: CH3CN : 0.05 M aq. NH4HCO4, flow rate: 0.5 mL / min.
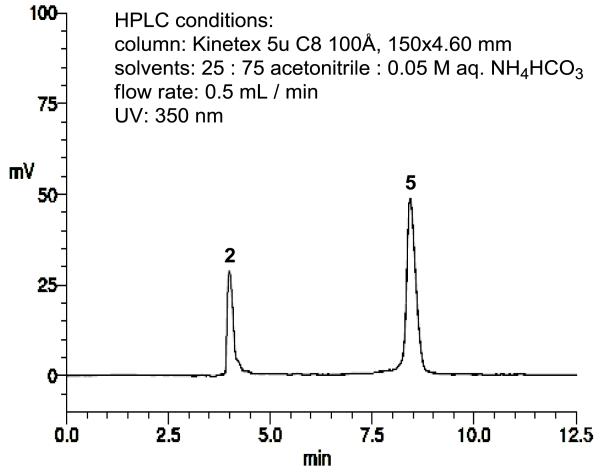
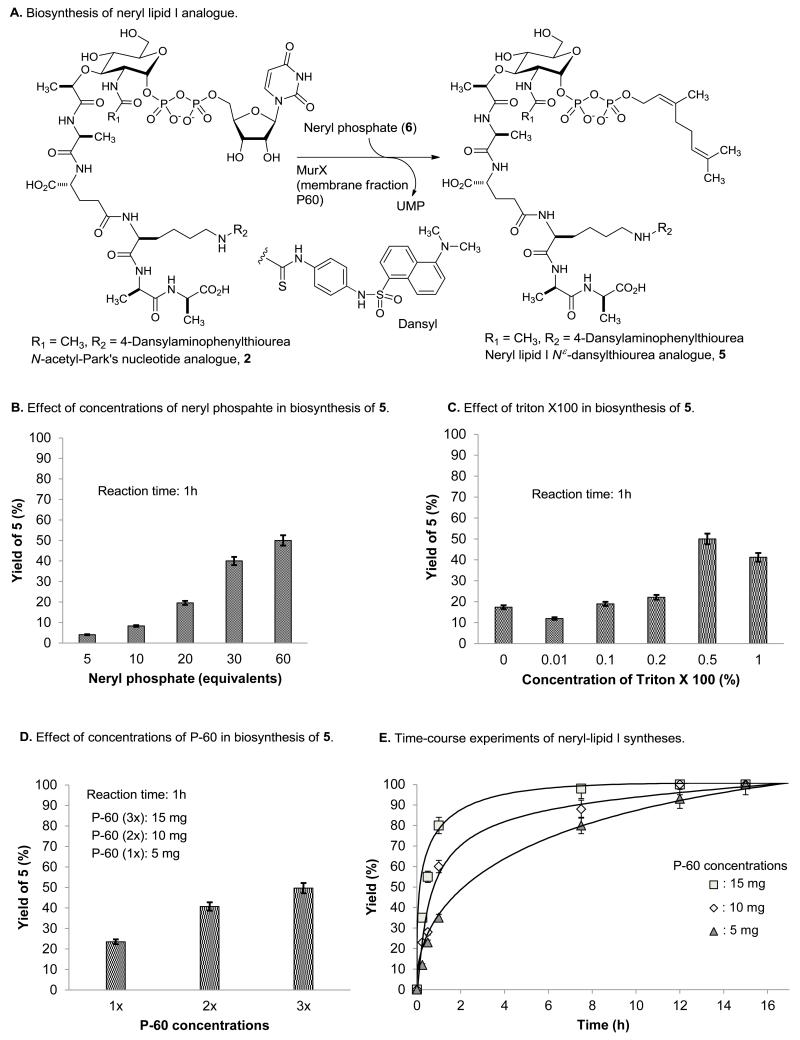
Mycobacterium spp. uses decaprenyl phosphate (C50-P) for the biosynthesis of lipid I [15]. Due to the fact that C50-lipid I does not dissolve in water media, thus, isolation and quantitation of the generated lipid I in the assay reaction mixtures are time-consuming processes (vide supra). In our hands, it is extremely difficult to develop medium- and high-throughput screening (HTS) for MurX/MraY assay using decaprenyl phosphate. We accomplished a practical chemical synthesis of neryl-lipid I and its fluorescent probes, and characterized their physicochemical properties. Neryl-lipid I-Nμ-dansylthiourea 5 showed excellent water-solubility (>50 μg/mL) and 5 can readily be analyzed via reverse-phase HPLC without gradient elution method (retention time: <9 min.) (Fig 3). MurX-catalyzed lipid I analog synthesis with Park’s nucleotide-Nε-dansylthiourea 2 and neryl phosphate (5 equivalent against 2) in the presence of 0.5% of triton × furnished neryl-lipid I-Nε-dansylthiourea 5 in 3-5% yield after 1h [40]. Under the same reaction conditions, the yield of 5 was proportionated to the concentrations of neryl phosphate (6); the same reaction with 60 equivalents of neryl phosphate furnished 5 in greater than 50% yield in 1h (Fig 2B). As summarized in Fig 2C, effect of a phase transfer catalyst, triton X100 was observed in the transformation of 2 to 5 [42]; 0.5% of triton X100 was determined to be the ultimate phase transfer catalyst of concentration for biosynthesis of lipid I-Nε-dansylthiourea analogue 5 with 2 and neryl phosphate (6). In short reaction times, application of higher concentrations of MurX enzyme (P-60) dramatically increased the reaction rate in biosynthesis of the lipid I analogue (Fig 2D). However, Park’s nucleotide-Nε-dansylthiourea 2 could be transformed to the neryl lipid I analogue 5 in greater than 90% yield even at lower concentrations of P-60 after 12h (Fig 2E). Among the other reaction parameters examined for MurX-catalyzed neryl-lipid I synthesis, the reaction temperatures did not noticeably affect the reaction rate within a temperature range between 22 and 37 °C. Thus, MurX assays can be performed conveniently at the ambient air temperatures. It is worth mentioning that all data obtained with P-60 membrane fraction from Mtb could be reproduced with that from M. smegmatis.
Fig 3.
HPLC chromatogram of Park’s nucleotide-Nε-dansyl analogue 2 and neryl lipid I-Nε-dansyl analogue 5.
Fig 2.
MurX-catalyzed biosynthesis of neryl lipid I analog 5.
A: biosynthesis of neryl lipid I analogue 5 from Park’s nucleotide analogue 2 and neryl phosphate 6, B: effect of concentrations of neryl phosphate in biosynthesis of 5, C: effect of a phase-transfer catalyst, triton x100 in biosynthesis of 5, D: effect of concentrations of MurX-containing membrane fraction (P-60), E: time-course experiments of neryl-lipid I synthesis.
MurX/MraY assays
The MraY-catalyzed transformation of lipid I from Park’s nucleotide is believed to be a reversible process [19,43]. On the contrary, under the optimized conditions MurX-catalyzed neryl-lipid I synthesis from Park’s nucleotide-Nε-dansylthiourea 2 is not an equilibrium reaction; 2 could be completely consumed to form 5 within 15h (Fig 2E). The neryl-lipid I analogue 5 can readily be dissolved in water or the assay media. Without extraction of the MurX/MraY enzymatic product from the reaction mixtures, the assay media can be assayed directly via reverse-phase HPLC for quantitation. Separation of Park’s nucleotide analogue 2 and neryl-lipid I analogue 5 could be performed via a C8- or C18-reverse phase column with a fixed solvent system (CH3CN : 0.05 M aq. NH4HCO3 = 25 : 75) at flow rate of 0.5 mL/min. Under these assay conditions, the retention times of Park’s nucleotide 2 and neryl-lipid I 5 were 4.0 min. and 7.9 min., respectively (Fig 3). On the other hand, separation of Park’s nucleotide and decaprenyl-lipid I required a gradient method for HPLC analyses [19]; the retention time of decaprenyl-lipid I, 4 (Table 2) was 60 min under our optimized HPLC conditions (C18-reverse phase column, solvent systems: (MeOH : 0.05 M aq. NH4HCO3 = 85 : 15 to 100% MeOH, flow rate: 2.0 mL/min). The Km value for Park’s nucleotide-dansylthiourea 2 was 18.29 μM at the concentrations of 450 μM of neryl phosphate; this was very similar to the Km values obtained with decaprenyl or undeacprenyl phosphate (Km: 18.05 μM) [19]. The Vmax for neryl-lipid I synthesis by MurX was determined to be 2.69 × 10−3 μM/sec through the Michaelis-Menten plot. As stated above, significant difference in MurX/MraY-catalyzed neryl-lipid I synthesis is that the transformation from Park’s nucleotide 2 to neryl-lipid I 5 is not a reverse process and neryl-lipid I 5 is biosynthesized in greater than 80% yield in 8h (Fig 2E). Although the synthesis of neryl-lipid I 5 could be achieved with Park’s nucleotide-Nε-dansylthiourea 2 in over 50% yield within 1h via the purified MraY enzymes, unlike P-60 membrane fraction, the reactions did not attain over 65% yield even after 15h due probably to instability of the purified MraY under the assay reaction conditions. Thus, MurX/MraY assays have been conveniently performed using P-60 membrane fractions obtained from Mtb or M. smegmatis.
Table 2.
Assay of positive- and negative-controls, and a library of uridyl peptides against MurX
| ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| entry | Compound | MurX inhibition (%)a | IC50 (μM)b | |||||
|
|
||||||||
| 0.01 μM | 0.1 μM | 1 μM | 10 μM | 100 μM | ||||
| 1 | Capuramycin (7) | - | 36.6 | 88.4 | 100 | 100 | 0.152 ± 0.0125 | (0.150-0.0180)c |
| 2 | SQ641 (8) | - | 40 | 95 | 100 | - | 0.109 ± 0.00845 | (0.0150-0.100)c |
| 3 | Tunicamycin (9) | - | 9 | 42 | 72 | 72 | 2.73 ± 0.138 | (2.40-2.95)c |
| 4 | 10 | - | 0 | 0 | 0 | 0 | ND | |
| 5 | 11 | - | 0 | 0 | 0 | 0 | ND | |
| 6 | Vancomycin | - | 0 | 0 | 0 | 0 | ND | |
| 7 | 12 | 3.8 | 81.2 | 100 | 100 | - | 0.105 ± 0.00330 | |
| 8 | 13 | - | 40 | 95 | 100 | - | 0.0950 ± 0.00395 | |
| 9 | 14 | - | 0 | 0 | 0 | 0 | ND | |
| 10 | 15 | - | 0 | 0 | 0 | 0 | ND | |
| 11 | DMSO | 0 | 0 | 0 | 0 | 0 | ND | |
1) Reaction conditions: Park’s nucleotide-Nε-dansylthiourea 2 (75 μM; 3.75 μL), MgCl2 (0.5 M; 10 μL); KCl (2 M, 10 μL), Triton X100 (0.1%; 11.25 μl), Tris-buffer (pH = 8; 50 mM, 2.5 μL), neryl phosphate 6 (10 mM, 45 μL), inhibitor molecule (0-100 μM in DMSO (2.5 μL)), P-60 (15 μL), 26 °C, 3h.; The Km 18.29 μM (Park’s nucleotide 2).
The IC50 values were obtained three times and the standard error of the mean was calculated.
The IC50 values in parentheses were reported in the literatures (see, references 18, 19, 24, 25, 45) and/or were obtained via the assay conditions summazried in Table 1.; Reaction conditions: Park’s nucleotide-Nε-dansylthiourea 2 (75 μM; 3.75 μL), MgCl2 (0.5 M; 10 μL); KCl (2 M, 10 μL), Triton X100 (0.1%; 11.25 μl), Tris-buffer (pH = 8; 50 mM, 5 μL), decaprenyl phosphate (10 mM, 2 equivalents against 2), inhibitor molecule (0-100 μM in DMSO (2.5 μL)), P-60 (15 μL), 26 °C, 3h.; The Km 18.05 μM (Park’s nucleotide 2).
The fluorescence characteristics of the Nε-dansylthiourea moiety in the enzymatic substrate and product can be applied to fluorescence-based analytical technics in order to quantitate the inhibition of lipid I biosynthesis. However, signal-to-noise ratio of HPLC analyses using UV (350 nm) is excellent enough for quantitation of 10 μL of the assay mixtures. The range of linearity was established by injections (via an auto sampler) of six concentrations of Park’s nucleotide-Nε-dansylthiourea 2 and neryl-lipid I-Nε-dansylthiourea 5 (r ≥ 0.9), and limit of detection was determined to be much lower than 1 μM concentrations.
Validation of MurX assays with antimycobacterial uridyl peptides
In order to determine usefulness of the MurX assay developed here, we examined several known MraY inhibitor molecules and negative controls, and demonstrated effectiveness of this MurX assay by screening our uridyl peptide library molecules. Known MraY inhibitors, capuramycin (7) and SQ641 (8) exhibited strong enzyme inhibitory activity against MurX (entries 1 and 2 in Table 2); the IC50 values of 6 and 7 were 0.152 and 0.109 μM, respectively [45]. Interestingly, these antimycobacterial MraY inhibitors 7 and 8 showed a 10-fold decrease in enzyme inhibitory activity against E. coli MraY. MurX enzyme inhibitory activity of tunicamycin (9) was determined to be the IC50 value of 2.73 μM in which the observed activity of 9 was closely related to the data reported in the literatures (2.40-2.95 μM) [19]. In our screening of series of MraY inhibitors in enzyme and bacterial growth inhibitory assays, the MurX inhibitors that showed the IC50 value of above 10 μM did not exhibit significant bactericidal activity against Mtb [46-48]. Thus, we set up an IC50 threshold of 10 μM to distinguish exploitable antimycobacterial MurX inhibitors from other antimycobacterial molecules. The analogues of capuramycin 10 and 11 have been used as negative controls in our program. These molecules did not show MurX inhibitory activity even at 100 μM concentrations (entries 4 and 5 in Table 2). Although vancomycin showed the good activity in MraY-MurG coupled assays, it could be confirmed that vancomycin did not inhibit MurX even at 100 μM concentrations. We evaluated over 50 synthetic uridine-glycosyl peptides and uridyl peptides that showed the MIC values of <25 μg/mL against Mtb in the MurX assay screening at three to five different concentrations. Among the identified new MurX inhibitors two molecules 12 and 13 are worthwhile highlighting. The 2′-methyl isomer 12 and amino-benzodiazepinone analogue 13 showed increased MurX inhibitory activity compared to capuramycin 10 (entry 7 and 8 in Table 2). On the other hand, the pacidamycin analogue 14 and muraymycin analogue 15 did not exhibit MurX inhibitory activity even at 100 μM concentrations [49-50]. Thus, we concluded that dihydrouridyl analogues 14 and 15 exhibited antimycobacterial activity by targeting the other essential enzyme(s) for growth of Mtb.
Thus, the HPLC-based assay of MurX/MraY investigated here can be performed with standard analytical devises and will be adapted to medium- to high-throughput formats with close to ideal Z′-factors (0.5-1.0) [44]. The Z′-factor was estimated from the data summarized in Table 2; an estimated Z′-factor was 0.84, and thus, the new MurX/MraY assay method described here is considered to be an excellent assay. We are currently generating a relatively large number of library molecules containing known MurX/MraY inhibitors to examine robustness of the described assay method.
Conclusion
We have demonstrated MurX/MraY-catalyzed synthesis of neryl-lipid I-Nε-dansylthiourea 5 from Park’s nucleotide-Nε-dansylthiourea 2 with neryl phosphate (6). Biosynthesis of neryl-lipid I analogue 5 was achieved, for the first time, in excellent yield with the MurX-containing membrane fraction (P-60) [51-52]. Similarly, neryl-lipid I-Nε-dansylthiourea 5 could be biosynthesized via the different sources of MraY enzymes such as M. smegmatis, E. coli, and S. aureus. However, the purified MraY enzymes seem to be denaturing under the assay conditions developed for P-60 membrane fractions. We are currently investigating the assay conditions that stabilize the purified MraY enzymes for the high-yield transformation from Park’s nucleotide-Nε-dansylthiourea 2 to neryl-lipid I-Nε-dansylthiourea 5. A water-soluble lipid I generated in MurX assay media could be quantitated conveniently via reverse-phase HPLC without sophisticated extraction procedures. Signal-to-noise ratio of HPLC analyses of 2 and 5 is significantly high without using fluorescence detector. Furthermore, difference in the retention times between Park’s nucleotide-Nε-dansylthiourea 2 and neryl-lipid I-Nε-dansylthiourea 5 was more than 3.5 min and each assay analysis could be completed within 10 min. We developed convenient methods for preparation of MurX/MraY enzymatic substrates, Park’s nucleotide-Nε-dansylthiourea 2 and neryl phosphate (6), and thus, the substrates for the assays are available to screen a relatively large number of molecules in our laboratory. In order to determine usefulness of the MurX/MraY assay protocols developed here, we screened a 50-membered library including new uridyl peptides, and known positive- and negative-controls. MurX enzyme inhibitory activity of all positive-controls showed approximately equal to the IC50 values obtained with the previously reported methods. The negative-control molecules did not exhibit MurX inhibitory activity even at high concentrations. Because the assay method described here quantitates the MurX/MraY substrate and product simultaneously in each assay vial without extraction or separation, the errors in qualitative analyses of assay caused by quantitation of single-molecule (remaining molecule or converted molecule) and/or by complicated work-up procedures are diminished. In the screening of a small library of molecules using the described method, to date, a false positive or false negative result has not been identified. Some dihydropacidamycins were reported to exhibit mycobacterial growth inhibitory activity in vitro [50]. However, their MurX enzyme inhibitory activities have not been thoroughly investigated. We observed an interesting trend in a series of (2R,5R)-(aminomethyl)-3-hydroxytetrahydrofuranyl)uridine derivatives; the dihydropacidamycin analogues (represented by 14 and 15) possessing antimycobacterial activity did not exhibit Mtb, M. smegmatis, and E. coli MraY enzyme inhibitory activities even at high concentrations. We have been studying the molecular target for antimycobacterial non-MurX/MraY inhibitors identified in this program. High reliability for the MurX/MraY assay protocols described here will be a variable asset to identify selective MurX inhibitor molecules for development of new antibacterial agents. Assay protocol and enzymatic substrates developed under this program will be provided to the scientific community.
Acknowledgments
The National Institutes of Health is greatly acknowledged for financial support of this work (AI084411). We also thank University of Tennessee for generous financial support. NMR data were obtained on instruments supported by the NIH Shared Instrumentation Grant. The following reagent was obtained through BEI Resources, NIAID, NIH: Mycobacterium tuberculosis, Strain H37Rv and Gamma-Irradiated Mycobacterium tuberculosis, NR-14819. The authors gratefully acknowledge Drs. William Clemons (California Institute Technology) and Crick (Colorado State University) for useful discussions.
References
- [1].Stover CK, Warrener P, VanDevater DR, Sherman DR, Arain TM, Langhorne MH, Anderson SW, Towell JA, Yuan Y, McMurray DN, Kreiswirth BN, Barry CE, Baker WR. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature. 2000;405:962–966. doi: 10.1038/35016103. [DOI] [PubMed] [Google Scholar]
- [2].Lamichhanea G, Freundlichb JS, Ekinsc S, Wickramaratnea N, Nolana ST, Bishaia WR. Essential metabolites of Mycobacterium tuberculosis and their mimics. mBio. 2011;2:1–10. doi: 10.1128/mBio.00301-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chien JY, Lai CC, Tan CK, Huang YT, Chou CH, Hung CC, Yang PC, Hsueh PR. Decline in rates of acquired multidrug-resistant tuberculosis after implementation of the directly observed therapy, short course (DOTS) and DOTS-Plus programmes in Taiwan. J. Antimicrob. Chemother. 2013;68:1910–1916. doi: 10.1093/jac/dkt103. [DOI] [PubMed] [Google Scholar]
- [4].Connolly LE, Edelstein PH, Ramakrishnan L. Why is long-term therapy required to cure tuberculosis? PLoS Med. 2007;4:435–442. doi: 10.1371/journal.pmed.0040120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dworkin J, Shah IM. Exit from dormancy in microbial organisms. Nat. Rev. Microbiol. 2010;8:890–896. doi: 10.1038/nrmicro2453. [DOI] [PubMed] [Google Scholar]
- [6].Portero JL, Rubio M. New anti-tuberculosis therapies. Expert Opin. Ther. Pat. 2007;17:617–637. [Google Scholar]
- [7].Miranda MS, Breiman A, Allain S, Deknuydt F, Altare F. The tuberculous granuloma: An unsuccessful host defense mechanism providing a safety shelter for the bacteria? Clin. Dev. Immunol. 2012:1–14. doi: 10.1155/2012/139127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Reddy VM, Einck L, Nacy CA. In Vitro antimycobacterial activities of capuramycin analogues. Antimicrob. Agents Chemother. 2008;52:719–721. doi: 10.1128/AAC.01469-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nikonenko BV, Reddy VM, Protopopova M, Bogatcheva E, Einck L, Nacy CA. Activity of SQ641, a capuramycin analog, in a murine model of tuberculosis. Antimicrob. Agents Chemother. 2009;53:3138–3139. doi: 10.1128/AAC.00366-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Auger G, van Heijenoort J, Mengin-Lecreulx D, Blanot D. A MurG assay which utilizes a synthetic analogue of lipid I. FEMS Microbiol. Lett. 2003;219:115–119. doi: 10.1016/S0378-1097(02)01203-X. [DOI] [PubMed] [Google Scholar]
- [11].van Heijenoort J. Lipid intermediates in the biosynthesis of bacterial peptidoglycan. Microbiol. Mol. Biol. Rev. 2007;71:620–635. doi: 10.1128/MMBR.00016-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bupp K, van Heijenoort J. The final step of peptidoglycan subunit assembly in Escherichia coli occurs in the cytoplasm. J. Bacteriol. 1993;175:1841–1843. doi: 10.1128/jb.175.6.1841-1843.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Raymond JB, Mahapatra S, Crick DC, Pavelka MS. Identification of the namH gene, encoding the hydroxylase responsible for the N-glycosylation of the Mycobacterial peptidoglycan. J. Biol. Chem. 2005;280:326–333. doi: 10.1074/jbc.M411006200. [DOI] [PubMed] [Google Scholar]
- [14].Mahapatra S, Scherman H, Brennan PJ, Crick DC. N-Glycolylation of the nucleotide precursors of peptidoglycan biosynthesis of Mycobacterium spp. is altered by drug treatment. J. Bacteriol. 2005;187:2341–2347. doi: 10.1128/JB.187.7.2341-2347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mahapatra S, Crick DC, Brennan PJ. Comparison of the UDP-N-acetylmuramate:L-alanine ligase enzymes from Mycobacterium tuberculosis and Mycobacterium leprae. J. Bacteriol. 2000;182:6827–6830. doi: 10.1128/jb.182.23.6827-6830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Barry CE, Blanchard JS. The chemical biology of new drugs in development for tuberculosis. Curr. Opin. Chem. Biol. 2010;14:456–466. doi: 10.1016/j.cbpa.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kurosu M, Mahapatra S, Narayanasamy P, Crick DC. Chemoenzymatic synthesis of park’s nucleotide: toward the development of high-throughput screening for MraY inhibitors. Tetrahedron Lett. 2007;48:799–803. [Google Scholar]
- [18].Timothy DH, Lloyd AJ, Roper DI. Phospho-MurNAc-pentapeptide translocase (MraY) as a target for antibacterial agents and antibacterial proteins. Infect. Disord.: Drug Targets. 2006;6:85–106. doi: 10.2174/187152606784112128. [DOI] [PubMed] [Google Scholar]
- [19].Stachyra T, Dini C, Ferrari P, Bouhss A, van Heijenoort J, Mengin-Lecreulx D, Blanot D, Biton J, Le Beller D. Fluorescence detection-based functional assay for high-throughput screening for MraY. Antimicrob. Agents Chemother. 2004;48:897–902. doi: 10.1128/AAC.48.3.897-902.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Weppner WA, Neuhaus FC. Fluorescent substrate for nascent peptidoglycan synthesis. Uridine diphosphate-N-acetylmuramyl-(Nepsilon-5-dimethylaminonaphthalene-1-sulfonyl)pentapeptide. J. Biol. Chem. 1977;252:2296–2303. [PubMed] [Google Scholar]
- [21].Geis A, Plapp R. Phospho-N-acetylmuramoyl-pentapeptide-transferase of Escherichia coli K12. Properties of the membrane-bound and the extracted and partially purified enzyme. Biochim. Biophys. Acta. 1978;527:414–424. doi: 10.1016/0005-2744(78)90355-8. [DOI] [PubMed] [Google Scholar]
- [22].Branstorma AA, Midha S, Longley CB, Han K, Baizman ER. Assay for identification of inhibitors for bacterial MraY translocase and MurG transferase. Anal. Biochem. 2000;280:315–319. doi: 10.1006/abio.2000.4530. [DOI] [PubMed] [Google Scholar]
- [23].Hyland SA, Anderson MS. A high-throughput solid-phase extraction assay capable of measuring diverse polyprenyl phosphate: sugar-1-phosphate transferases as exemplified by WecA, MraY and MurG proteins. Anal. Biochem. 2003;317:156–164. doi: 10.1016/s0003-2697(03)00088-5. [DOI] [PubMed] [Google Scholar]
- [24].Solapure SM, Raphael P, Gayathri CN, Barde SP, Chandrakala B, Das KS, deSousa SM. Development of a microplate-based scintillation proximity assay for MraY using a modified substrate. J. Biomol. Screening. 2005;10:149–156. doi: 10.1177/1087057104272007. [DOI] [PubMed] [Google Scholar]
- [25].Ravishankar S, Prasanna Kumar V, Chandrakala B, Jha RK, Solapure SM, deSousa SM. Scintillation proximity assay for inhibitors of Escherichia coli MurG and, optionally, MraY. Antimicrob. Agents Chemother. 2005;49:1410–1418. doi: 10.1128/AAC.49.4.1410-1418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shapiro AB, Jahic H, Gao N, Hajec L, Rivin O. A high-throughput, homogeneous, fluorescence resonance energy transfer-based assay for phospho-N-acetylmuramoyl-pentapeptide translocase (MraY) J. Biomol. Screening. 2012;17:662–672. doi: 10.1177/1087057112436885. [DOI] [PubMed] [Google Scholar]
- [27].Li K, Kurosu M. Synthetic studies on Mycobacterium tuberculosis specific fluorescent park’s nucleotide probe. Heterocycles. 2008;76:455–469. [Google Scholar]
- [28].Mitachi K, Mohan P, Siricilla S, Kurosu M. One-pot protection-glycosylation reactions for synthesis of lipid II analogues. Chem. - Eur. J. 2014;20:1–6. doi: 10.1002/chem.201400307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dueymes C, Pirat C, Pascal R. Facile synthesis of simple mono-alkyl phosphates from phosphoric acid and alcohols. Tetrahedron Lett. 2008;49:5300–5301. [Google Scholar]
- [30].Rezwan M, Laneelle MA, Sander P, Daffe M. Breaking down the wall: Fractionation of mycobacteria. J. Microbiol. Methods. 2007;68:2–39. doi: 10.1016/j.mimet.2006.05.016. [DOI] [PubMed] [Google Scholar]
- [31].Wolfe LM, Mahaffey SB, Kruh NA, Dobos KM. Proteomic definition of the cell wall of Mycobacterium tuberculosis. J. Proteome Res. 2010;9:5816–5826. doi: 10.1021/pr1005873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bouhss A, Crouvoisier M, Blanot D, Mengin-Lecreulx D. Purification and characterization of the bacterial MraY translocase catalyzing the first membrane step of peptidoglycan biosynthesis. J. Biol. Chem. 2004;279:29974–29980. doi: 10.1074/jbc.M314165200. [DOI] [PubMed] [Google Scholar]
- [33].Ha S, Chang E, Lo MC, Men H, Park P, Ge M, Walker S. The kinetic characterization of Escherichia coli MurG using synthetic substrate analogues. J. Am. Chem. Soc. 1999;37:8415–8426. [Google Scholar]
- [34].Ma Y, Münch D, Schneider T, Sahl HG, Bouhss A, Ghoshdastider U, Wang J, Dötsch V, Wang X, Bernhard F. Preparative scale cell-free production and quality optimization of MraY homologues in different expression modes. J. Biol. Chem. 2011;286:38844–38853. doi: 10.1074/jbc.M111.301085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Timothy DH, Lloyd AJ, Roper DI. Phospho-MurNAc-pentapeptide translocase (MraY) as a target for antibacterial agents and antibacterial proteins. Infect. Disord. : Drug Targets. 2006;6:85–106. doi: 10.2174/187152606784112128. [DOI] [PubMed] [Google Scholar]
- [36].Winn M, Goss RJM, Kimura K, Bugg TDH. Antimicrobial nucleoside antibiotics targeting cell wall assembly: Recent advances in structure–function studies and nucleoside biosynthesis. Nat. Prod. Rep. 2009;27:279–304. doi: 10.1039/b816215h. [DOI] [PubMed] [Google Scholar]
- [37].Yamashita A, Norton E, Petersen PJ, Rasmussen BA, Singh G, Yang Y, Mansour TS, Ho DM. Muraymycins, novel peptidoglycan biosynthesis inhibitors: synthesis and SAR of their analogues. Bioorg. Med. Chem. Lett. 2003;13:3345–3350. doi: 10.1016/s0960-894x(03)00671-1. [DOI] [PubMed] [Google Scholar]
- [38].Boojamra CG, Lemoine RC, Lee JC, Leger R, Stein KA, Vernier NG, Magon A, Lemovskaya O, Martin PK, Chamberland S, Lee MD, Hecker SJ, Lee VJ. Stereochemical elucidation and total synthesis of dihydropacidamycin D, a semisynthetic pacidamycin. J. Am. Chem. Soc. 2001;123:870–874. doi: 10.1021/ja003292c. [DOI] [PubMed] [Google Scholar]
- [39].Dini C. MraY inhibitors as novel antibacterial agents. Curr. Top. Med. Chem. 2005;5:1221–1236. doi: 10.2174/156802605774463042. [DOI] [PubMed] [Google Scholar]
- [40].Kurosu M, Li K. Synthetic studies towards the identification of novel capuramycin analogs with antimycobacterial activity. Heterocycles. 2009;77:217–225. [Google Scholar]
- [41].Chen KT, Kuan YC, Fu WC, Liang PH, Cheng TJR, Wong CH, Cheng WG. Rapid preparation of mycobacterium N-Glycolyl lipid I and lipid II derivatives: A biocatalytic approach. Chem. - Eur. J. 2013;19:834–838. doi: 10.1002/chem.201203251. [DOI] [PubMed] [Google Scholar]
- [42].Brandish PE, Burnham MK, Lonsdale JT, Southgate R, Inukai M, Bugg TDH. Slow binding inhibition of phospho-N-acetylmuramyl-pentapeptide-translocase (Escherichia coli) by Mureidomycin A. J. Biol. Chem. 1996;271:7609–7614. doi: 10.1074/jbc.271.13.7609. [DOI] [PubMed] [Google Scholar]
- [43].Nuhaus FC. Initial translocation reaction in biosynthesis of peptidoglycan by bacterial membranes. Acc. Chem. Res. 1971;4:297–303. [Google Scholar]
- [44].Zhang JH, Chung TDY, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screening. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- [45].Koga T, Fukuoka T, Harasaki T, Inoue H, Hotoda H, Kakuta M, Muramatsu Y, Yamamura N, Hoshi M, Hirota T. Activity of capuramycin analogs against Mycobacterium tuberculosis, Mycobacterium avium and Mycobacterium intracellular in vitro and in vivo. J. Antimicrob. Chemother. 2004;54:755–760. doi: 10.1093/jac/dkh417. [DOI] [PubMed] [Google Scholar]
- [46].Wang Y, Siricilla S, Aleiwi BA, Kurosu M. Improved synthesis of capuramycin and its analogues. Chem. - Eur. J. 2013;19:13847–13858. doi: 10.1002/chem.201302389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kurosu M, Li K, Crick DC. A concise synthesis of capuramycin. Org. Lett. 2009;11:2393–2396. doi: 10.1021/ol900458w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Aleiwi BA, Schneider CM, Kurosu M. Synthesis of ureido-muraymycidine derivatives for structure activity relationship studies of muraymycins. J. Org. Chem. 2012;77:3859–3867. doi: 10.1021/jo300205b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Boojamra CG, Lemoine RC, Blais J, Vernier NG, Stein KA, Magon A, Chamberland S, Hecker SJ, Lee VJ. Synthetic dihydropacidamycin antibiotics: A modified spectrum of activity for the pacidamycin class. Bioorg. Med. Chem. Lett. 2003;13:3305–3309. doi: 10.1016/s0960-894x(03)00682-6. [DOI] [PubMed] [Google Scholar]
- [50].Lin YI, Li Z, Francisco GD, McDonald LA, Davis RA, Singh G, Yang Y, Mansour TS. Muraymycins, novel peptidoglycan biosynthesis inhibitors: semisynthesis and SAR of their derivatives. Bioorg. Med. Chem. Lett. 2002;12:2341–1344. doi: 10.1016/s0960-894x(02)00469-9. [DOI] [PubMed] [Google Scholar]
- [51].Boonjamra CG, Lemoine RC, Blais J, Venier NG, Stein KA, Magon A, Chamberland S, Hecker SJ, Lee VJ. Synthetic dihydropacidamycin antibiotics: A modified spectrum of activity for the pacidamycin class. Bioorg. Med. Chem. Lett. 2003;13:3305–3309. doi: 10.1016/s0960-894x(03)00682-6. [DOI] [PubMed] [Google Scholar]
- [52].Breukink E, van Heusden HE, Vollmerhaus PJ, Swiezewska E, Brunner L, Walker S, Heck AJR, de Kruijff B. Lipid II as an intrinsic component of the pore induced by nisin in bacterial membrans. J. Biol. Chem. 2003;278:19898–19903. doi: 10.1074/jbc.M301463200. [DOI] [PubMed] [Google Scholar]
- [53].Men H, Park P, Ge M, Walker S. Substrate synthesis and activity assay for MurG. J. Am. Chem. Soc. 1998;120:2484–2485. [Google Scholar]