Abstract
Human apolipoprotein E (APOE) exists in three isoforms ɛ2, ɛ3, and ɛ4, of which APOE4 is the main genetic risk factor of Alzheimer's disease (AD). As cerebrovascular defects are associated with AD, we tested whether APOE genotype has an impact on the integrity and function of the blood–brain barrier (BBB) in human APOE-targeted replacement mice. Using the quantitative in situ brain perfusion technique, we first found lower (13.0% and 17.0%) brain transport coefficient (Clup) of [3H]-diazepam in APOE4 mice at 4 and 12 months, compared with APOE2 and APOE3 mice, reflecting a decrease in cerebral vascularization. Accordingly, results from immunohistofluorescence experiments revealed a structurally reduced cerebral vascularization (26% and 38%) and thinner basement membranes (30% and 35%) in 12-month-old APOE4 mice compared with APOE2 and APOE3 mice, suggesting vascular atrophy. In addition, APOE4 mice displayed a 29% reduction in [3H]-d-glucose transport through the BBB compared with APOE2 mice without significant changes in the expression of its transporter GLUT1 in brain capillaries. However, an increase of 41.3% of receptor for advanced glycation end products (RAGE) was found in brain capillaries of 12-month-old APOE4 mice. In conclusion, profound divergences were observed between APOE genotypes at the cerebrovascular interface, suggesting that APOE4-induced BBB anomalies may contribute to AD development.
Keywords: Alzheimer's disease, blood–brain barrier, cerebral blood flow, cerebrovascular disease, glucose
Introduction
Carrying the allele ɛ4 of the apolipoprotein E (APOE) gene is the major genetic risk factor of Alzheimer's disease (AD).1 ApoE is chiefly involved in the transport of cholesterol through its interaction with low-density lipoprotein receptor-related protein-1 (LRP1), lipoprotein receptor with 11 binding repeats (LR11, also known as SOLRA) and other apoE receptors.2 ApoE is a polymorphic protein, which exists in humans as three isoforms ɛ2, ɛ3, and ɛ4 with worldwide frequencies of 6.4±5.1%, 78.3±12.1%, and 14.5±8.5%, respectively.1,3 Prevalence of APOE4 allele is about 37% in AD patients, a proportion three times higher than in the general population.4 The three isoforms differ from each other only by the amino acids located at positions 112 and 158, leading to alterations in apoE structure and in its affinity toward its ligands and receptors, and thus in its role in neuropathologic conditions.5 However, despite massive research effort in recent years, the exact AD-relevant loss or gain of function resulting from apoE4 expression remains poorly defined.1
It is increasingly recognized that AD and cerebrovascular diseases (CVD) share key risk factors such as hypertension, cerebral hypoperfusion, diabetes, hypercholesterolemia, and APOE4 carriage.6,7 The blood–brain barrier (BBB) is a biologic barrier comprises endothelial cells surrounded by the basement membrane, pericytes and astrocytes, having a central role in brain homeostasis.8,9 Because the BBB is an obligatory interface between the cardiovascular system and the brain, its dysfunction is suspected to underlie AD-related cerebrovascular defects.10 For example, increased cerebrospinal fluid/serum ratios of blood-borne macromolecules have been interpreted as evidence of impaired BBB permeability in AD.11 Morphologic abnormalities of brain capillaries, as well as evidence of cerebrovascular dysfunction such as decreased cerebral blood flow or lower brain glucose metabolism, have also been documented.10,12 Supportive observations have also been gathered in animal models of Aβ or tau AD-like neuropathology.13, 14, 15 Finally, BBB-expressed transporters such as the receptor for advanced glycation end products (RAGE) and LRP1 are thought to regulate Aβ transport in and out of the brain.2,16 Incidentally, an upregulation of RAGE and a downregulation of LRP1 were shown in the AD brain where both changes could contribute to the accumulation and deposition of Aβ.17,18
However, recent data suggest that APOE4 expression exerts detrimental effects on the cerebrovascular system including BBB impairments.10 Indeed, human apoE4 expression in the mouse, compared with apoE2 and apoE3, results in altered BBB permeability and reduced cerebral blood flow.19 Furthermore, apoE has been shown to have a role in Aβ deposition in cerebral parenchyma and microvessels, in an isoform-dependent manner.1 In addition, accumulating evidence suggest that apoE, through its binding to LRP1, mediates the clearance of Aβ across the BBB, and that the APOE4 allele contributes to cerebral accumulation of Aβ.1,20 Finally, we recently found that APOE4 expression leads to reduced uptake of docosahexaenoic acid through the BBB, which may explain why APOE4 carriers do not benefit from this dietary polyunsaturated fatty acid.4
On the basis of these series of data, we hypothesized that the ɛ4 allele of APOE impairs the morphology and functional properties of the BBB. To verify this, we quantified by in situ brain perfusion the passage of diazepam and glucose through the BBB in mice carrying the different alleles of human APOE. In addition, we evaluated the thickness of the basement membrane and relative vessel density and quantified several key transporters of the BBB in the cerebral vessels of mice expressing the human APOE2, 3, or 4 allele.
Materials and methods
Animals
Male and female APOE (E2, E3, and E4) targeted replacement mice were purchased from Taconic (Hudson, NY, USA) and then reproduced in our laboratory. In these models, the murine APOE gene of C57BL6 mice was replaced by one of three human APOE alleles (E2, E3, or E4).21 Animals were killed at 2, 4, or 12 months of age, or 5 months for the mice used in the human immunoglobulins (hIgG) biodistribution study. All mice had free access to standard laboratory food and water and were kept on a 12-hour light-dark cycle at 22±3°C. All experiments were performed in accordance with the Canadian Council on Animal Care and were approved by the Institutional Committee of the Centre Hospitalier de CHU de Québec.
In Situ Brain Perfusion
The in situ brain perfusion technique measures the volume of distribution and transport coefficient (Clup) of compounds in the brain after an intracarotid perfusion. Since 100% of the perfusate reaches the BBB, distribution and transport parameters can be readily determined. The cerebrovascular volume is assessed in parallel during the same experiment using a vascular space marker such as [14C]-sucrose (412 mCi/mmol, Moravek Biochemicals, Brea, CA, USA) (0.3 μCi/mL), which does not cross the BBB. The surgery was performed as described previously.4,8,13,22,23 Briefly, mice were anesthetized by intraperitoneal injection of xylazine/ketamine (8/140 mg/kg). Then, the right common carotid artery and the right external carotid artery were ligated at the heart side and at the level of bifurcation, respectively. The right common carotid artery was then catheterized with polyethylene tubing filled with heparin (25 IU/mL). Before perfusion, the thoracic cavity was opened, the heart was cut, and the perfusion was started immediately at a flow rate of 2.5 mL/min. The different ligatures ensure that the entire perfusate containing labeled compound reaches the BBB directly after its perfusion into the internal carotid artery. The perfusion fluid consisted of bicarbonate-buffered physiologic saline (mmol/L): 128 NaCl, 24 NaHCO3, 4.2 KCl, 2.4 NaH2PO4, 1.5 CaCl2, 0.9 MgCl2, and 9 d-glucose. The solution was gassed with 95% O2 and 5% CO2 to obtain a pH of 7.4 and heated to 37°C. The passive diffusion was measured by perfusing [3H]-diazepam (83.7 Ci/mmol, Perkin-Elmer, Boston, MA, USA) (0.3 μCi/mL) for 60 seconds, where the diazepam is a highly lipophilic drug that readily crosses the BBB in a flow-limited manner.13 The passage of glucose was measured by perfusing the mice with [3H]-d-glucose (20 Ci/mmol, Perkin-Elmer) (0.3 μCi/mL) for 20 seconds. Each mouse was coperfused with [14C]-sucrose (0.3 μCi/mL), a vascular space marker, to evaluate the physical integrity of the BBB for each mouse individually. The perfusion was terminated by decapitating the mouse. The right cerebral hemispheres and aliquots of the perfusion fluid were collected and weighted. Tissue samples were digested in 1 mL of Solvable (Perkin-Elmer, Waltham, MA, USA) at 50°C overnight, and then cooled to room temperature and mixed with 9 mL of Ultima Gold scintillation cocktail (Perkin-Elmer). Total isotopes were determined in a Packard Tri-Carb model 1900TR liquid scintillation analyzer.
The vascular volume (Vvasc, μL/g) was evaluated by measuring the distribution volume of [14C]-sucrose using the following equation: Vvasc=(Xvascular marker/Cvascular marker), in which X (dpm/g) is the total quantity of radioactivity found in the right cerebral hemisphere, and C (dpm/μL) is the marker concentration in the perfusate. If the internal diameter of cerebral blood vessels is not changed, then an increase in the Vvasc indicates a disruption of the BBB, leading to enhanced paracellular permeability.
The brain transport coefficient (Clup, μL/g s) of [3H]-diazepam and [3H]-d-glucose was calculated as described previously22 using the following equation:
 |
where Xtissue (dpm/g) is the measured amount of [3H] test compound in the right hemisphere, Cperf (dpm/μL) is the test compound concentration in the perfusion fluid, and T (seconds) is the perfusion time.
Tissue total radioactivity was corrected for ‘vascular' contamination with:Xtissue=Xtot−(Vvasc × Cperf), where Xtot (dpm/g) represents the total amount of labeled compound within the tissue parenchyma and the ‘vascular' compartment.
Immunohistofluorescence
Twelve-month-old APOE2, E3, and E4 mice were anesthetized deeply and then perfused transcardially with 50 mL of ice-cold 0.1 mol/L phosphate-buffered saline (PBS) buffer followed by 50 mL of ice-cold fixative (4% paraformaldehyde in PBS 0.1mol/L, pH 7.4). After the two perfusions, brains were collected and postfixed overnight at 4°C in the same fixative buffer, then transferred into a 20% sucrose/0.05% sodium azide solution for 48 hours until equilibration and kept frozen at −80°C until sectioning. Brains were embedded in optimal cutting temperature (Sakura Finetek, Torrance, CA, USA) and brain slices (12 μm) were cut with a cryostat, thaw-mounted onto superfrost plus slides (Fisher Scientific Company, Ottawa, ON, Canada), desiccated overnight at 4°C, and stored at −80°C until assayed. Experiments were performed on five to six animals per group and on at least twelve sections per animal.
The immunohistofluorescence technique was adapted from previous studies.13,24 Briefly, slides were washed in 0.1 mol/L PBS two times for 5 minutes to clean the remaining optimal cutting temperature and blocked with 0.1 mol/L PBS containing 10% normal horse serum and 0.2% Triton X-100 for 1 hour at room temperature. Sections were then incubated overnight at 4°C in a humid chamber with primary antibody in the blocking solution: goat anti collagen IV (1:400; Millipore Bioscience Research Reagents, Temecula, CA, USA). After incubation with primary antibody, slides were washed three times in 0.1 mol/L PBS and then incubated for 2 hours in 0.1 mol/L PBS solution containing 0.05% Triton X-100, 1% normal horse serum and Alexa Fluor 488-conjugated donkey anti-goat antibody (1:500; Invitrogen, Burlington, ON, Canada). Finally, slides were washed three additional times and coverslips were mounted with Mowiol medium. The collagen IV-stained sections were examined with the Simple PCI version 5.0 software (Hamamatsu, Sewickley, PA, USA) linked to a Nikon eclipse 90i microscope (Nikon Instruments, Toronto, ON, Canada) using appropriate filters for Alexa Fluor 488 (excitation, 480/30 nm; emission, 535/40 nm). The apparent thickness of the collagen-positive basement membrane of microvessels and the relative density of cerebral vascularization were measured directly on digital pictures under the same conditions of illumination and magnification using the ImageJ software (National Institutes of Health, Bethesda, MD, USA). Quantifications were performed in hippocampal CA2 and CA3 areas (located between bregma −1.46 to bregma −1.70) in all sections, as the hippocampus is one of the earliest brain regions affected in AD. To measure the apparent thickness of the basement membrane of cerebral capillaries, a total of 15 pictures per animal were taken at × 100 magnification and they were distributed between 12 different brain slices (from bregma −1.46 to bregma −1.70). The quantification of the relative density of blood vessels labeled with anti-collagen IV antibody was performed by a blind investigator using 4 to 5 images per animal ( × 20 magnification, bregma −1.46 to bregma −1.70). The ImageJ software (NIH, Bethesda, MD, USA) was used with settings allowing the measurement of all blood microvessels in a single defined area. Relative cerebral vascularization was determined as a percentage (%) of collagen IV-positive blood vessels surface area per field (600 × 600 μm) and the results are expressed as mean± standard error of the mean (s.e.m.).
Assessment of Blood–Brain Barrier Permeability with Human Immunoglobulins
APOE2 (n=6, 5.1±1.1 months of age) and APOE4 (n=6, 4.9±0.9 months of age) mice were injected intraperitoneally with three doses of 30 mg hIgG (Gamunex, Grifols Canada, Mississauga, ON, Canada) per mouse, 96, 24, and 1 hour(s) before killing. Mice were killed by intracardiac perfusion with 50 mL of ice-cold 0.1 mol/L PBS containing protease and phosphatase inhibitors, under deep anesthesia. Then, plasma, spleen, liver, and brain were collected. The hippocampus and the cortex were dissected, snap frozen on dry ice, and stored at −80°C until used. Organs were homogenized in 5 (cortex and hippocampus) and 10 volumes (spleen and liver) of lysis buffer and processed as described below in the protein extraction section. The concentration of hIgG in each tissue was determined by specific enzyme-linked immunosorbent assay using hIgG Fc-specific antibodies for capture and the corresponding horseradish peroxidase-conjugated antibodies for detection (Jackson ImmunoResearch Laboratories, West Grove, PA, USA).24
Capillary Depletion
Capillary depletion technique was used to isolate brain microvessels by density gradient centrifugation.25 Twelve-month-old APOE2, E3, and E4 mice were deeply anesthetized and then perfused transcardially with 50 mL of ice-cold 0.1 mol/L PBS. Then, the brain was collected and directly transferred into ice-cold 0.1 mol/L PBS where cerebellum, meninges, brain stem, and large superficial blood vessels were removed. The brain was gently homogenized in ice-cold Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) using a Potter homogenizer. The homogenate was centrifuged at 500 g for 10 minutes at 4°C, the supernatant was excluded and the pellet was homogenized in 5 mL of ice-cold DMEM containing 25% bovine serum albumin (BSA) and centrifuged at 1,500 g for 20 minutes at 4°C. The resulting pellet was gently homogenized in 1 mL of ice-cold DMEM containing 10% FBS and the homogenate was filtered through a 60-μm filter. The filtrate was centrifuged at 12,000 g for 45 minutes at 4°C. The pellet containing the microvessels was washed in ice-cold 0.1 mol/L PBS and centrifuged again at 12,000 g for 20 minutes at 4°C. The supernatant was discarded and the pellets were stored at −80°C until processed for western blotting analysis.
Protein Extraction
The protein extraction was adapted from previous studies.4,8,24 Briefly, the pellet containing the microvessels was weighed and total proteins were extracted by homogenization in eight volumes of lysis buffer (150 mmol/L NaCl, 10 mmol/L NaH2PO4, 1% Triton X-100, 0.5% SDS, and 0.5% deoxycholate, pH 7.4) containing Complete protease inhibitors (Roche, Indianapolis, IN, USA), 10 mg/mL pepstatin A, and phosphatase inhibitors (1 mmol/L sodium pyrophosphate, 50 mmol/L sodium fluoride). The obtained suspension was sonicated briefly (3 × 10 seconds) and centrifuged at 100,000 g for 20 minutes at 4°C. Protein concentration was determined with the BCA assay and the supernatant was stored at −80°C until western blotting analysis.
Western Blotting
Equal amounts of proteins per sample were added to Laemmli's loading buffer, heated to 70°C for 10 minutes before loading (15 μg protein per lane), and subjected to sodium dodecyl sulfate-polyacrylamide (SDS) gel electrophoresis. Proteins were electroblotted onto PVDF membranes (Immobilon, Millipore, MA, USA) before blocking in 5% nonfat dry milk, 0.5% BSA, and 0.1% Tween-20 in 0.1 mol/L PBS for 1 hour at room temperature. The membranes were washed three times for 10 minutes in 0.1 mol/L PBS containing 0.1% Tween-20. Then, membranes were incubated overnight at 4°C with primary antibodies diluted in 0.1 mol/L PBS containing 0.1% Tween-20, 5% nonfat dry milk, and 0.5% BSA. The dilutions of primary antibodies were: 1:10,000 for mouse anti-GAPDH (ABM, Richmond, BC, Canada), 1:500 for rabbit anti-occludin (Invitrogen), 1:500 for rabbit anti-claudin-5 (Santa Cruz Biotechnology, Dallas, TX, USA), 1:4,000 for mouse anti-Glut1 (Abcam, Toronto, ON, Canada), 1:1,000 for mouse anti-LR11 (BD Bioscience, Mississauga, ON, Canada), 1:1,000 for rat anti-RAGE (R&D Systems, Minneapolis, MN, USA), 1:20,000 for rabbit anti-LRP1 (Abcam) and 1: 2,000 for rabbit anti-human apoE (Novus Biologicals, Oakville, ON, Canada). The next day, membranes were washed three times for 10 minutes in 0.1 mol/L PBS containing 0.1% Tween-20 and then incubated for 1 hour at room temperature with appropriate horseradish peroxidase-labeled secondary antibodies diluted in 0.1 mol/L PBS containing 0.1% Tween-20 and 1% BSA. The dilutions of secondary antibodies were 1:100,000 for goat anti-mouse (Jackson, West Grove, PA, USA), 1:60,000 for goat anti-rat (Jackson), and 1:60,000 for goat anti-rabbit (Jackson). The membranes were again washed three times for 10 minutes in 0.1 mol/L PBS containing 0.1% Tween-20 and probed with chemiluminescence reagents (Lumiglo Reserve, KPL, Gaithersburg, MD, USA). Immunoblots were analyzed with a KODAK Imaging Station 4000 MM Digital Imaging System (Molecular Imaging Software version 4.0.5f7, Carestream Health, Rochester, NY, USA).
Statistical Analysis
Data are shown as means±s.e.m. Groups were compared using one-way analysis of variance (ANOVA) followed by Dunnett's multiple comparison test. Statistical significance was set as follows: *P<0.05; **P<0.01; ***P<0.001. All statistical analyses were performed with the Prism5 software (GraphPad Software, San Diego, CA, USA).
Results
The Brain Uptake of [3H]-Diazepam Was Reduced in APOE4 Mice at 4 and 12 Months
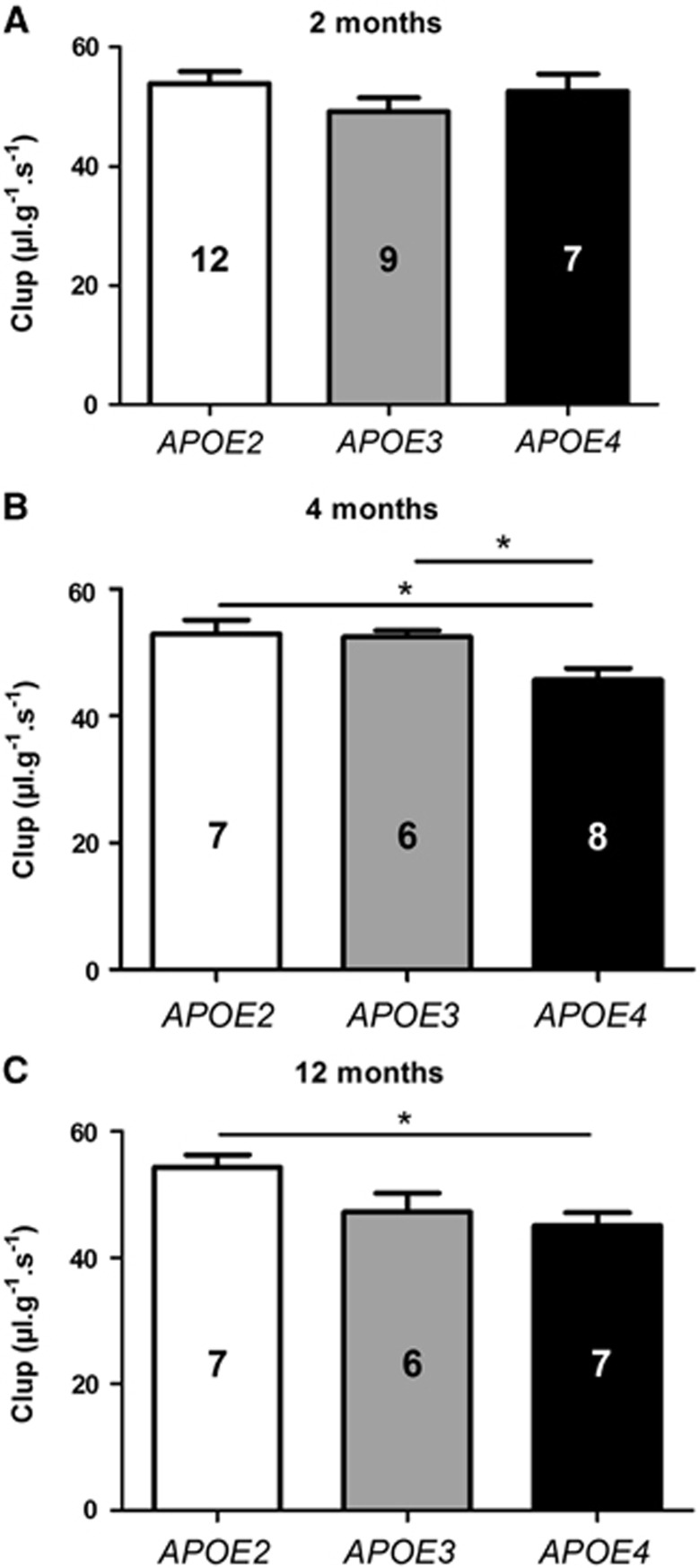
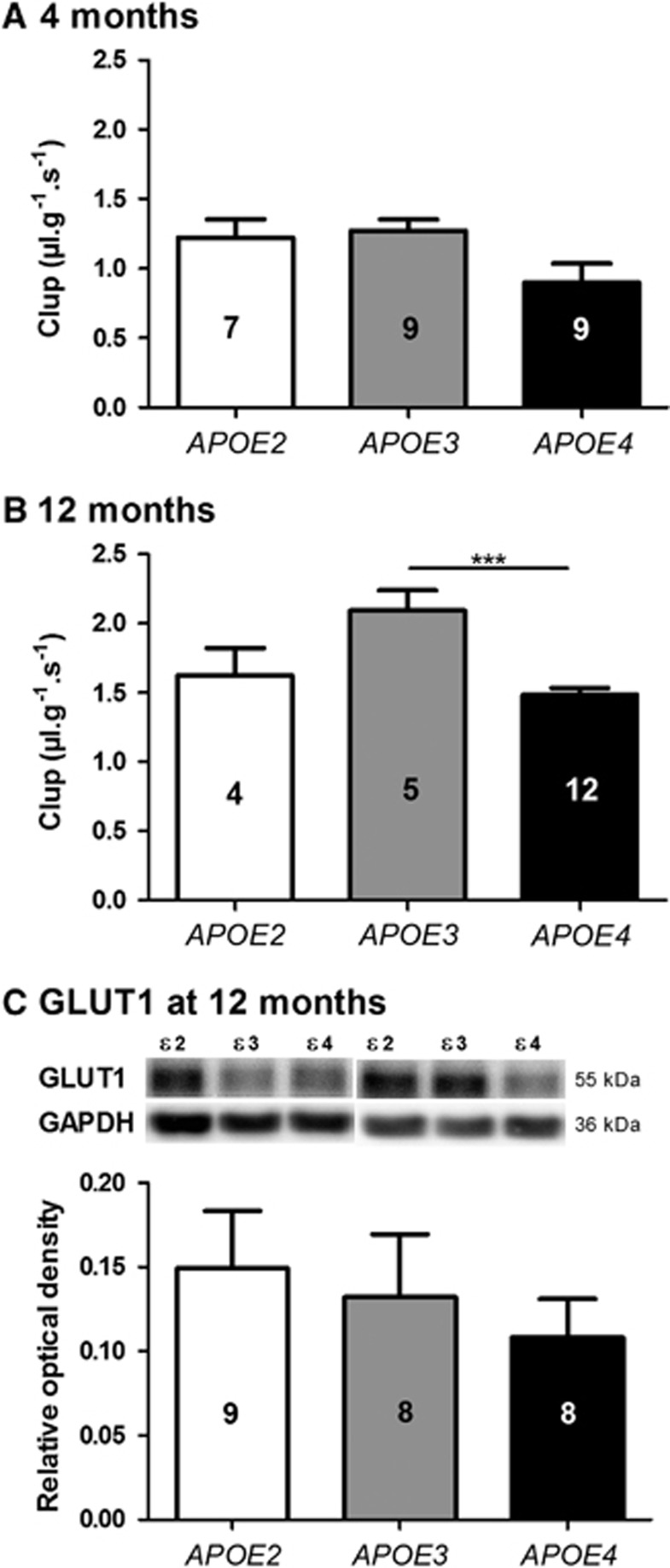
To detect global alterations in the cerebral vascularization according to the APOE genotype, the Clup of [3H]-diazepam was assessed using in situ brain perfusion technique. Diazepam is highly diffusible in biologic membranes. As both the perfused concentration of diazepam and the pump-controlled fluid velocity remain constant, its passage via the BBB depends on the surface of cerebral vascularization.13,22,23 Reductions of 13.7% (P<0.05) and 13.0% (P<0.05) of the Clup of [3H]-diazepam were found in APOE4 mice at 4 months compared with APOE2 and APOE3, respectively. The extent of [3H]-diazepam Clup reduction reached 17.0% (P<0.05) at 12 months compared with APOE2 mice (Figure 1). These results are consistent with a smaller BBB surface area and a less extensive cerebral vascularization in the APOE4 mice.
Figure 1.
The brain transport coefficient (Clup, μL/g s) of [3H]-diazepam was decreased in APOE4 mice. Clup was determined in APOE2, APOE3, and APOE4 mice at (A) 2, (B) 4, and (C) 12 months of age using the in situ brain perfusion technique. Data are shown as mean± standard error of the mean (s.e.m.). Statistical analyses: one-way analysis of variance (ANOVA) followed by Dunnett's multiple comparison test *P<0.05 (n=6 to 12).
Reduction of Relative Vessel Density and Thinner Basement Membrane in 12-Month-Old APOE4 Mice
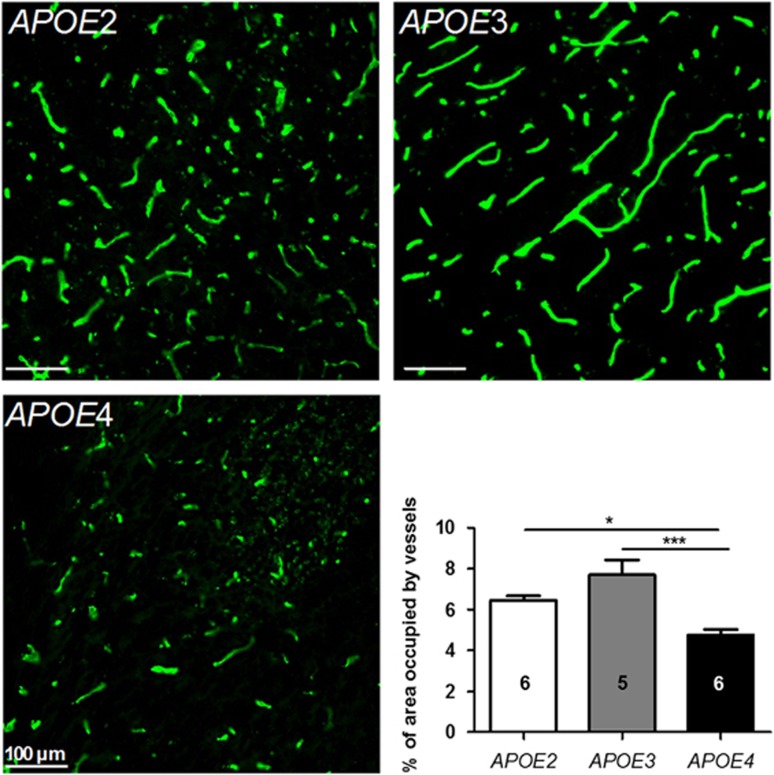
To determine whether the changes in surface area were associated with detectable morphologic alterations, the relative density of hippocampal blood vessels was evaluated in 12-month-old APOE mice of each isoform group, using a semi-quantitative immunohistofluorescence method. As shown in Figure 2, collagen IV-positive vessels were shorter and more fragmented in APOE4 than in APOE2 and APOE3 mice. The quantification of area occupied by these vessels in the CA2-CA3 region located between bregma −1.46 and bregma −1.70 revealed that the relative density of collagen IV-labeled blood vessels was lower by 26.1% (P<0.05) and 38.0% (P<0.001) in APOE4 mice compared with APOE2 and APOE3 mice, respectively (Figure 2). Accordingly, these results are consistent with the in situ brain perfusion of diazepam and support a reduction of cerebral vascularization in APOE4 mice compared with APOE2 and APOE3.
Figure 2.
Relative vessel density in the hippocampus was reduced in APOE4 mice. Area occupied by vessels was determined by immunohistofluorescence using anti-collagen IV antibody in 12-month-old APOE2, APOE3, and APOE4 mice. Image analyses were performed in the CA2-CA3 region of hippocampal slices located between bregma −1.46 and bregma −1.70. Data are shown as mean±standard error of the mean (s.e.m.). Statistical analyses: one-way analysis of variance (ANOVA) followed by Dunnett's multiple comparison test. *P<0.05; ***P<0.001 (n=5 to 6).
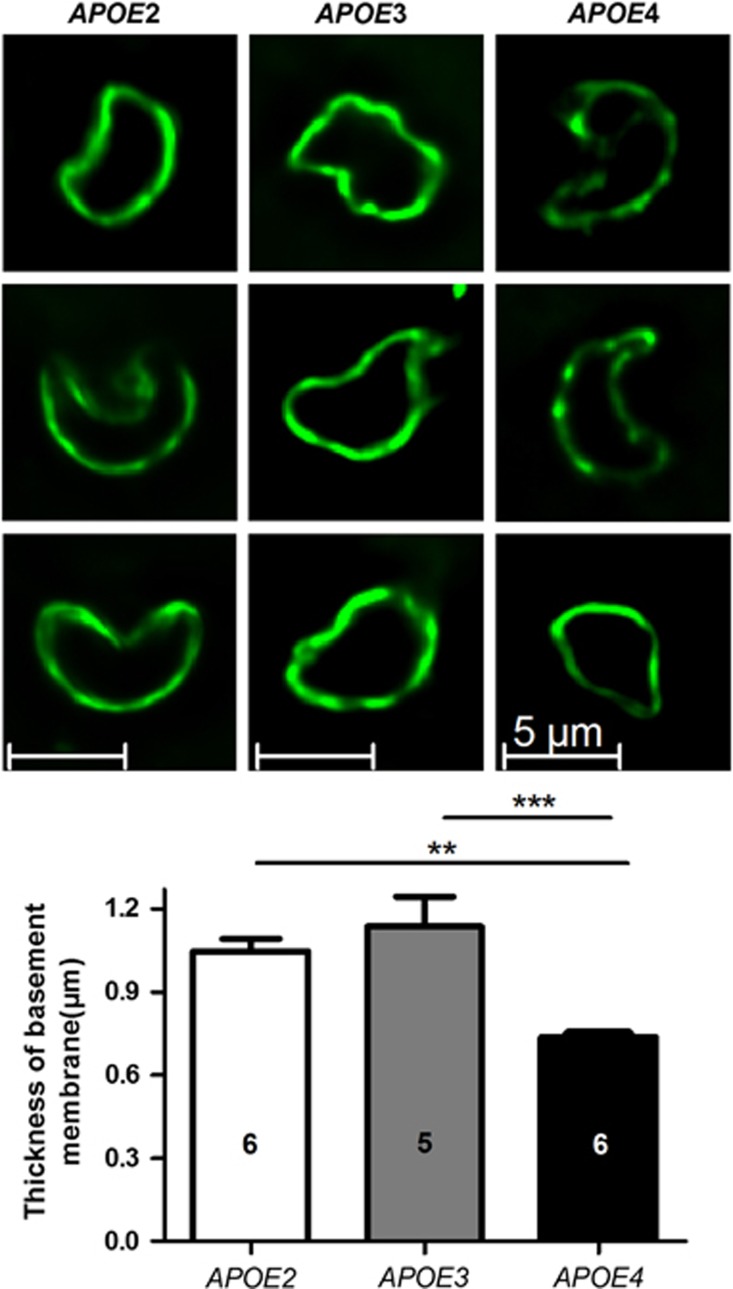
To further investigate morphologic changes of blood microvessels in APOE mice, we have measured the apparent thickness of the basement membrane using collagen IV immunohistofluorescence. We observed thinner basement membranes in 12-month-old APOE4 mice compared with APOE2 (−29.6%, P<0.01) and APOE3 (−35.1%, P<0.001), respectively, which may lead to larger luminal diameters in many cases of 12-month-old APOE4 mice (Figure 3).
Figure 3.
The apparent thickness of the basement membrane of brain capillaries (μm) was reduced in APOE4 mice, as assessed using immunohistofluorescence with an anti-collagen IV antibody in 12-month-old APOE2, APOE3, and APOE4 mice. Data are shown as mean thickness±standard error of the mean (s.e.m.). Statistical analyses: one-way analysis of variance (ANOVA) followed by Dunnett's multiple comparison test. **P<0.01; ***P<0.001 (n=5 to 6).
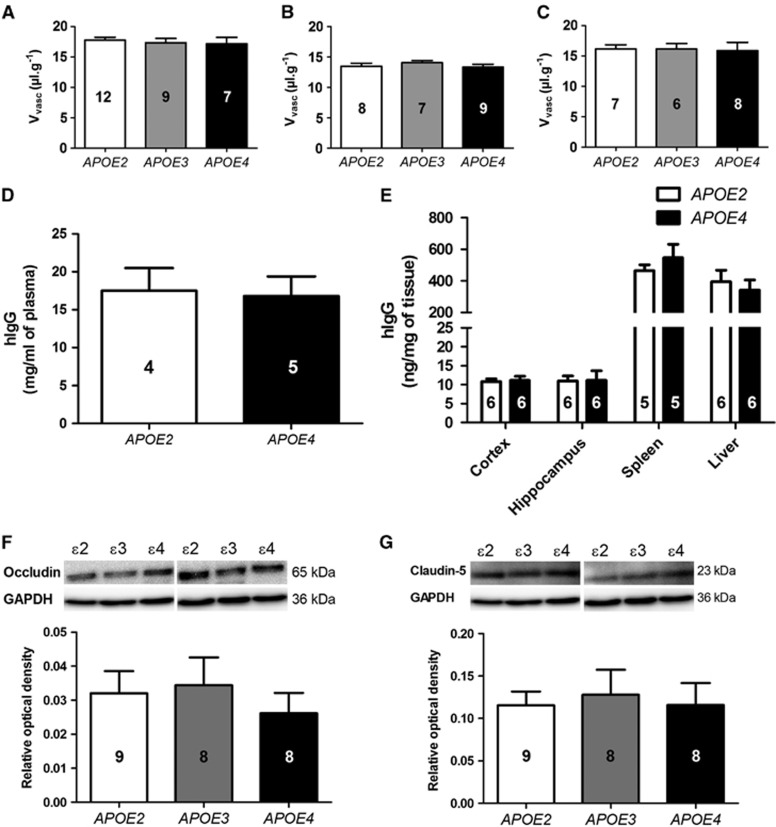
The Cerebrovascular Volume Did Not Differ Between APOE2, E3, and E4 Mice
Shrunken cerebral vascularization and lower relative vessel density would be expected to be associated with a reduced Vvasc. However, thinner basement membranes observed in 12-month-old APOE4 mice should be indicative of larger luminal volumes. To verify how these different factors combine to alter the brain vascular volume, animals were perfused with [14C]-sucrose, a molecule that is used as a vascular space marker as it does not cross the BBB.13,22 Importantly, Vvasc values remain comparable between the three groups of APOE-targeted replacement mice at 2, 4, and 12 months (Figures 4A–4C). This suggests that reduction in relative vessel density may be compensated by an increase in the diameter of blood capillaries or increased paracellular diffusion of sucrose through the BBB, resulting in unchanged apparent Vvasc in 12-month-old APOE4 mice. To verify the second hypothesis, we further investigated the permeability of the BBB in APOE4 versus APOE2 mice, using a subchronic treatment with hIgG. Since hIgG are large molecules, they do not cross the BBB, or only in very small amount (<0.01% of injected dose).24 As shown in Figures 4D and 4E, no difference in hIgG concentrations in the hippocampus and the cortex was found between APOE2 and APOE4 mice after three intraperitoneal injections of 30 mg hIgG 96, 24, and 1 hour(s) before killing. These data corroborate [14C]-sucrose data and confirm that no major breakdown of BBB integrity occurred in APOE4 mice. To further assess BBB integrity in APOE4 mice, two tight junctions proteins (occludin and claudin-5) were quantified in the capillary fractions of 12-month-old APOE2, E3, and E4 mice using western blot analyses. Again, no difference was observed across the three groups, consistent with a relatively intact BBB in APOE4 mice (Figures 4F and 4G).
Figure 4.
The calculated vascular volume (Vvasc, μL/g) was not different between the groups. Vvasc was determined in APOE2, APOE3, and APOE4 mice at (A) 2, (B) 4, and (C) 12 months by in situ brain perfusion of the vascular marker [14C]-sucrose. The biodistribution of human immunoglobulins (hIgG) was comparable in (D) the plasma and (E) tissue homogenates of 5-month-old APOE2 and APOE4 mice after three injections of 30 mg hIgG, 96, 24, and 1 hour(s) before kiling. Western blot quantification of (F) occludin and (G) claudin-5 in brain capillary fractions did not evidence any difference between the three APOE genotypes at 12 months of age. All data represent mean±standard error of the mean (s.e.m.). The number of mice in each group is indicated in the bars of the histograms. Statistical analyses: one-way analysis of variance (ANOVA) followed by Dunnett's multiple comparison test (A, B, C, F, and G) or Student's unpaired t test (D and E).
Reduced Transport of [3H]-d-glucose in APOE4 Mice at 12 Months
One of the key roles of the BBB is to regulate the uptake of glucose, the main source of energy of cerebral tissues.12 To assess the BBB functional integrity in APOE mice, we measured the uptake of [3H]-d-glucose using in situ brain perfusion. The total concentration of perfused glucose was 9 mmol/L (9 mmol/L of d-glucose+15 nmol/L of [3H]-d-glucose), close to physiologic glycemia in the mouse but less than the estimated kM of 17 mmol/L determined with the same technique.22 In these conditions, a reduction of 29.0% (P<0.001) of [3H]-d-glucose Clup was detected in APOE4 mice compared with APOE3 mice at 12 months of age, but not at 4 months (Figures 5A and 5B). To determine whether the main BBB glucose transporter GLUT1 was involved, we measured by western blot GLUT1 levels in brain capillary extracts from each group. However, no significant reduction was observed in APOE4 mice (Figure 5C).
Figure 5.
The brain transport coefficient (Clup, μL/g s) of [3H]d-glucose was decreased in 12-month-old APOE4 mice. Clup was determined in APOE2, APOE3, and APOE4 mice at (A) 4 and (B) 12 months of age by in situ brain perfusion technique. Data are shown as mean±standard error of the mean (s.e.m.). Statistical analyses: one-way analysis of variance followed by Dunnett's multiple comparison test. ***P<0.001 (n=4 to 12). (C) GLUT1 expression was not significantly different between the groups. GLUT1 expression was measured by western blot in 12-month-old mice. Data are shown as mean±s.e.m. (n=8 to 9 mice).
Overexpression of Receptor for Advanced Glycation End Products in the Blood–Brain Barrier of APOE4 Mice Without Major Changes in Expression of Lipoprotein Receptor-Related Protein-1
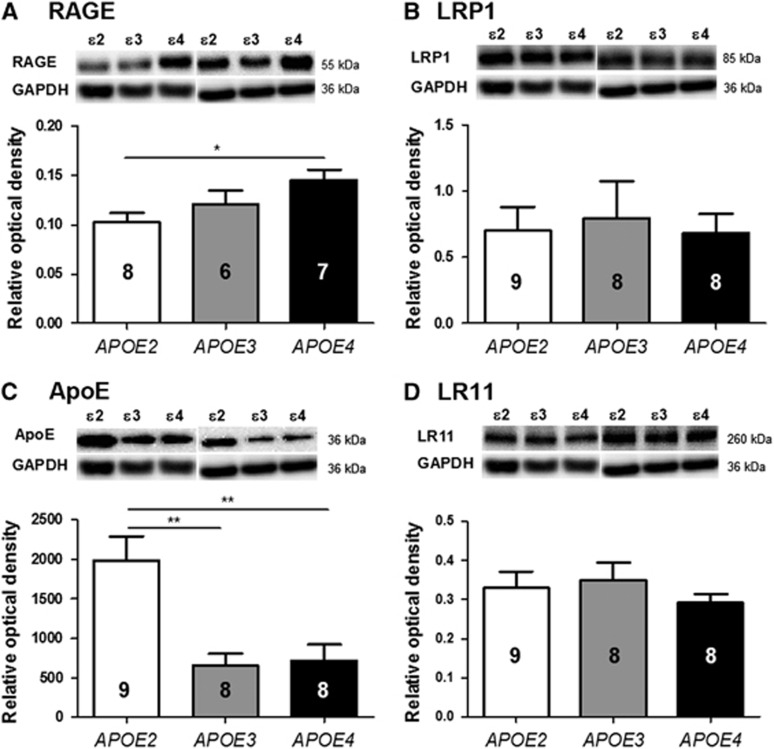
The imbalance between the influx and the efflux (clearance) of Aβ through the BBB is considered as an important factor in AD pathogenesis.26 Thus, the expression of the two key transporters implicated in these processes, RAGE and LRP1, respectively, was measured in the capillary fractions of 12-month-old mice from each group. Whereas the expression of RAGE was increased by 41.3% (P<0.05) in APOE4 mice compared with APOE2 mice, no change in LRP1 was detected (Figures 6A and 6B). Moreover, the quantity of apoE was reduced by 59.4% (P<0.01) in the capillaries of APOE4 mice compared with APOE2 (Figure 6C), consistent with earlier reports in total brain homogenates of mice and human.4,27 Finally, the expression of apoE receptor LR11 was not changed across the different groups (Figure 6D).
Figure 6.
Brain capillary concentration of receptor for advanced glycation end products (RAGE) was increased in APOE4 mice. (A) RAGE, (B) lipoprotein receptor-related protein-1 (LRP1), (C) ApoE, and (D) LR11 were measured by western blot in the brain capillaries of 12-month-old mice. Data are shown as mean± standard error of the mean (s.e.m.). Statistical analyses: one-way ANOVA followed by Dunnett's multiple comparison test. *P<0.05; **P<0.01; (n=6 to 9 mice).
Discussion
The aim of the present study was to investigate the effect of APOE alleles on the functional and morphologic properties of the BBB, to elucidate the role of APOE in AD pathogenesis. Our results suggest that BBB-related parameters and cerebrovascular morphology are critically affected by the genotype of human APOE in mice, in an age-dependent manner. Altogether, in situ brain perfusion of [3H]-diazepam and [3H]-d-glucose as well as immunohistofluorescence analysis indicate that apoE4 expression in the mouse leads to a reduction in cerebral vascularization accompanied by thinner vascular walls and decreased glucose uptake, compared with APOE2 or APOE3 mice. Since brain function relies on an optimal cerebrovascular system, our results suggest that APOE4-induced acceleration of AD pathogenesis might result from its detrimental effects on the BBB.
A first key observation reported here is the significant decrease in the passage of [3H]-diazepam across the BBB, which was detected in APOE4 mice, without concomitant changes in vascular volume. This is consistent with our previous work showing decreased uptake of another highly diffusible compound docosahexaenoic acid in APOE4 mice.4 Since diazepam enters the brain by passive diffusion requiring neither a transporter nor energy, the measured brain uptake of [3H]-diazepam depends on two factors: (1) infusion rate and (2) surface permeability.13,14,25 Indeed, lipophilic solutes cross the BBB through a transcellular pathway, a process referred to as flow-limited transport.9,14,28 However, a unique advantage of the in situ brain perfusion technique is to allow complete control on not only the perfusate content and perfusion time, but also the flow rate, by adjusting the pump.8 Since 100% of the perfused [3H]-diazepam has direct access to the BBB, in accordance with Fick's first law of diffusion, a decrease in [3H]-diazepam uptake can only be explained by a reduced surface permeability of the BBB.13,22 Finally, considerable changes in BBB integrity have been ruled out by experiments conducted with perfused [14C]-sucrose, systemically injected hIgG and two tight junctions proteins (occludin and claudin-5). Therefore, a decrease in the total BBB surface area accessible to the perfused [3H]-diazepam in the brain remains the most likely explanation for the observed uptake decrease in APOE4 mice.
If true, such a change in BBB surface area found in APOE4 mice should be detectable with a microscopic approach. Indeed, the labeling of the basement membrane using a semi-quantitative immunohistofluorescence analysis confirmed a diminution in relative cerebral vascularization and basement membrane thickness surrounding brain capillary endothelial cells in 12-month-old APOE4 mice. In addition, the reduced cerebral vascularization observed here is consistent with previous reports of reduced microvascular length and decreased brain capillary density in several animal models of AD.29,30 In line with these observations, implanted glioma tumors in the brains of two transgenic mouse models of AD (Tg APPsw and Tg PS1/APPsw mice) showed a 50% decrease in blood vessel density compared with implanted tumors in the brains of wild-type mice.31 Consistently, a reduction in the basement membrane surface area was observed in postmortem data of individuals carrying APOE4 genotype comparatively to APOE3.32
As pointed out above, the volume of distribution of [14C]-sucrose after intracarotid perfusion remained close to 15 μL/g across all groups, suggesting that the reduction in cerebral vascularization was not associated with an alteration in the vascular volume. On the one hand, the absence of changes in [14C]-sucrose is surprising in the light of the massive BBB disruption previously reported in APOE4 mice, as suggested by qualitative evaluation of fluorescent leakage of IV-injected dextran through the BBB.19 Since most of in situ perfused sucrose remained entrapped in the cerebral vasculature, it would have been logical to expect that a reduced density of cerebral blood vessel would equate to a diminished total vascular content in the brain, and thus lower brain [14C]-sucrose content. A possible explanation for these apparently contradictory results is that the effect of reduced vessel density on vascular volume was compensated by either (1) an increase in luminal space due to the thinning of the vascular wall also observed here or (ii) a small increase in BBB permeability. Indeed, increased brain vessel diameters or small amount of [14C]-sucrose flowing through endothelial cells could have compensated the decreased cerebral vascularization, resulting in a total vascular volume remaining unchanged. Nevertheless, our quantitative analyses with perfused [14C]-sucrose, systemically injected hIgG and two tight junctions proteins are sufficient to rule out a massive disruption of BBB integrity in the APOE4 mice.
The unchanged cerebral concentrations of hIgG after systemic administration in APOE4 mice are also an important observation in the light of recent clinical studies designed and powered to test the clinical efficacy and safety of intravenous immunoglobulin (IVIg) in AD.33,34 Indeed, secondary analysis showed that APOE4 carriers responded better to IVIg than noncarriers,33 suggesting that APOE4 could increase the brain uptake of IVIg. Our present data in human knock-in mice suggest that the additional clinical benefits of IVIg in APOE4 carriers, if confirmed, are unlikely to be explained by higher brain bioavailability.
The series of BBB anomalies evidenced here are likely to exert significant long-term consequences on brain homeostasis. If the observed BBB impairments are associated with defective cerebral blood flow, as previously evidenced in APOE4 mice,19 then one will expect metabolic changes, reduced nutrient intake, enhanced oxidative stress, and formation of string vessels, which all could contribute to cognitive impairment.35,36 Consistent with this, the transport of [3H]-d-glucose to the brain was impaired in APOE4 mice at 12 but not at 4 months of age. Although a reduction in GLUT1 is observed in the brain of AD patients,37 no significant difference in GLUT1 expression was observed in the capillaries of APOE4 mice. In spite of the brain weight is equal to 2% of the body weight, but it consumes 25% of body glucose in awake state,38 and it is generally accepted that perturbations of glucose intake and metabolism can greatly impact brain function. Moreover, brain hypometabolism is now considered as a canonical sign of AD, mostly assessed by in vivo imaging using positron emission tomography with 2-[(18)F]fluoro-2-deoxy-d-glucose. It is detected early in the course of the disease and its extent and topography correlate with symptom severity.39 Incidentally, APOE4 carriage is also associated with cerebral glucose hypometabolism in AD-relevant cerebral regions, long before cognitive symptoms.40 Since transportation of glucose within endothelial cells is a key first step in its use as a source of energy for the brain,38 the observed reduction of glucose transport in APOE4 mice is likely to have serious consequences.
Finally, overexpression of RAGE and reduction of apoE concentration were observed in the cerebral capillaries from APOE4 mice compared with other genotypes without significant changes of LRP1 or LR11 expression. Such an upregulation of RAGE in APOE4 mice is reminiscent of what is observed in AD patients.18 Since apoE has an important role in the clearance of Aβ 2 and RAGE is involved in the reuptake of Aβ in the brain, whereas RAGE null mice did not show any cerebral accumulation of peripheral Aβ,16 the consequence of such combined alterations in apoE and RAGE at the BBB could result in hindered clearance of Aβ through the BBB.
Conclusion
Our findings suggest that human APOE isoforms have a direct impact on cerebrovascular health. The expression of human APOE4 in the mouse was found to lead to a collection of morphologic, functional and molecular changes in cerebral blood vessels, consistent with impaired blood perfusion, BBB dysfunction, and reduced Aβ clearance, which have all been confirmed to occur during AD progression. Therefore, these results suggest that APOE may modify the risk of developing AD through profound effects on BBB-related parameters and cerebrovascular function.
Acknowledgments
The authors thank Dr Vincent Emond and Dr Eric Béliveau for their editing of this article and their help to prepare the photos of brain capillaries.
The authors declare no conflict of interest.
Footnotes
Financial support was provided from the Natural Sciences and Engineering Research Council of Canada (NSERC, RGPIN 435555-2013), the ‘Institut sur la nutrition et des aliments fonctionnels (INAF)', and the Canada Foundation for Innovation.
References
- Holtzman DM, Herz J, Bu G. Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006312. doi: 10.1101/cshperspect.a006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer's disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg DT, Kuzawa CW, Hayes MG. Worldwide allele frequencies of the human apolipoprotein E gene: climate, local adaptations, and evolutionary history. Am J Phys Anthropol. 2010;143:100–111. doi: 10.1002/ajpa.21298. [DOI] [PubMed] [Google Scholar]
- Vandal M, Alata W, Tremblay C, Rioux-Perreault C, Salem N, Jr, Calon F, et al. Reduction in DHA transport to the brain of mice expressing human APOE4 compared to APOE2. J Neurochem. 2014;129:516–526. doi: 10.1111/jnc.12640. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E: structure determines function, from atherosclerosis to Alzheimer's disease to AIDS. J Lipid Res. 2009;50:S183–S188. doi: 10.1194/jlr.R800069-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos P, Rosa-Neto P, Rochford J, Hamel E. Pioglitazone improves reversal learning and exerts mixed cerebrovascular effects in a mouse model of Alzheimer's disease with combined amyloid-beta and cerebrovascular pathology. PLoS ONE. 2013;8:e68612. doi: 10.1371/journal.pone.0068612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo K, Black SE, Verhoeff NP. Alzheimer's disease, cerebrovascular disease, and the beta-amyloid cascade. Can J Neurol Sci. 2012;39:712–728. doi: 10.1017/s0317167100015547. [DOI] [PubMed] [Google Scholar]
- Alata W, Paris-Robidas S, Emond V, Bourasset F, Calon F. Brain uptake of a fluorescent vector targeting the transferrin receptor: a novel application of in situ brain perfusion. Mol Pharm. 2014;11:243–253. doi: 10.1021/mp400421a. [DOI] [PubMed] [Google Scholar]
- Passeleu-Le Bourdonnec C, Carrupt PA, Scherrmann JM, Martel S. Methodologies to assess drug permeation through the blood-brain barrier for pharmaceutical research. Pharm Res. 2013;30:2729–2756. doi: 10.1007/s11095-013-1119-z. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Cerebrovascular effects of apolipoprotein E: implications for Alzheimer disease. JAMA Neurol. 2013;70:440–444. doi: 10.1001/jamaneurol.2013.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog I, Wallin A, Fredman P, Hesse C, Aevarsson O, Karlsson I, et al. A population study on blood-brain barrier function in 85-year-olds: relation to Alzheimer's disease and vascular dementia. Neurology. 1998;50:966–971. doi: 10.1212/wnl.50.4.966. [DOI] [PubMed] [Google Scholar]
- Cunnane S, Nugent S, Roy M, Courchesne-Loyer A, Croteau E, Tremblay S, et al. Brain fuel metabolism, aging, and Alzheimer's disease. Nutrition. 2011;27:3–20. doi: 10.1016/j.nut.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourasset F, Ouellet M, Tremblay C, Julien C, Do TM, Oddo S, et al. Reduction of the cerebrovascular volume in a transgenic mouse model of Alzheimer's disease. Neuropharmacology. 2009;56:808–813. doi: 10.1016/j.neuropharm.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Mehta DC, Short JL, Nicolazzo JA. Altered brain uptake of therapeutics in a triple transgenic mouse model of Alzheimer's disease. Pharm Res. 2013;30:2868–2879. doi: 10.1007/s11095-013-1116-2. [DOI] [PubMed] [Google Scholar]
- Paul J, Strickland S, Melchor JP. Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer's disease. J Exp Med. 2007;204:1999–2008. doi: 10.1084/jem.20070304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- Donahue JE, Flaherty SL, Johanson CE, Duncan JA, 3rd, Silverberg GD, Miller MC, et al. RAGE, LRP-1, and amyloid-beta protein in Alzheimer's disease. Acta Neuropathol. 2006;112:405–415. doi: 10.1007/s00401-006-0115-3. [DOI] [PubMed] [Google Scholar]
- Miller MC, Tavares R, Johanson CE, Hovanesian V, Donahue JE, Gonzalez L, et al. Hippocampal RAGE immunoreactivity in early and advanced Alzheimer's disease. Brain Res. 2008;1230:273–280. doi: 10.1016/j.brainres.2008.06.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeynes B, Provias J. The case for blood-brain barrier dysfunction in the pathogenesis of Alzheimer's disease. J Neurosci Res. 2011;89:22–28. doi: 10.1002/jnr.22527. [DOI] [PubMed] [Google Scholar]
- Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, et al. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem. 1997;272:17972–17980. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- Dagenais C, Rousselle C, Pollack GM, Scherrmann JM. Development of an in situ mouse brain perfusion model and its application to mdr1a P-glycoprotein-deficient mice. J Cereb Blood Flow Metab. 2000;20:381–386. doi: 10.1097/00004647-200002000-00020. [DOI] [PubMed] [Google Scholar]
- Ouellet M, Emond V, Chen CT, Julien C, Bourasset F, Oddo S, et al. Diffusion of docosahexaenoic and eicosapentaenoic acids through the blood-brain barrier: An in situ cerebral perfusion study. Neurochem Int. 2009;55:476–482. doi: 10.1016/j.neuint.2009.04.018. [DOI] [PubMed] [Google Scholar]
- St-Amour I, Pare I, Alata W, Coulombe K, Ringuette-Goulet C, Drouin-Ouellet J, et al. Brain bioavailability of human intravenous immunoglobulin and its transport through the murine blood-brain barrier. J Cereb Blood Flow Metab. 2013;33:1983–1992. doi: 10.1038/jcbfm.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do TM, Alata W, Dodacki A, Traversy MT, Chacun H, Pradier L, et al. Altered cerebral vascular volumes and solute transport at the blood-brain barriers of two transgenic mouse models of Alzheimer's disease. Neuropharmacology. 2014;81:311–317. doi: 10.1016/j.neuropharm.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Bell RD. The imbalance of vascular molecules in Alzheimer's disease. J Alzheimers Dis. 2012;32:699–709. doi: 10.3233/JAD-2012-121060. [DOI] [PubMed] [Google Scholar]
- Beffert U, Cohn JS, Petit-Turcotte C, Tremblay M, Aumont N, Ramassamy C, et al. Apolipoprotein E and beta-amyloid levels in the hippocampus and frontal cortex of Alzheimer's disease subjects are disease-related and apolipoprotein E genotype dependent. Brain Res. 1999;843:87–94. doi: 10.1016/s0006-8993(99)01894-6. [DOI] [PubMed] [Google Scholar]
- Bassingthwaighte JB. Blood flow and diffusion through mammalian organs. Science. 1970;167:1347–1353. doi: 10.1126/science.167.3923.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris D, Patel N, DelleDonne A, Quadros A, Smeed R, Mullan M. Impaired angiogenesis in a transgenic mouse model of cerebral amyloidosis. Neurosci Lett. 2004;366:80–85. doi: 10.1016/j.neulet.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Lee GD, Aruna JH, Barrett PM, Lei DL, Ingram DK, Mouton PR. Stereological analysis of microvascular parameters in a double transgenic model of Alzheimer's disease. Brain Res Bull. 2005;65:317–322. doi: 10.1016/j.brainresbull.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Paris D, Ganey N, Banasiak M, Laporte V, Patel N, Mullan M, et al. Impaired orthotopic glioma growth and vascularization in transgenic mouse models of Alzheimer's disease. J Neurosci. 2010;30:11251–11258. doi: 10.1523/JNEUROSCI.2586-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloway S, Gur T, Berzin T, Tavares R, Zipser B, Correia S, et al. Effect of APOE genotype on microvascular basement membrane in Alzheimer's disease. J Neurol Sci. 2002;203-204:183–187. doi: 10.1016/s0022-510x(02)00288-5. [DOI] [PubMed] [Google Scholar]
- Relkin N. Clinical trials of intravenous immunoglobulin for Alzheimer's disease. J Clin Immunol. 2014;34:S74–S79. doi: 10.1007/s10875-014-0041-4. [DOI] [PubMed] [Google Scholar]
- Relkin NR, Szabo P, Adamiak B, Burgut T, Monthe C, Lent RW, et al. 18-Month study of intravenous immunoglobulin for treatment of mild Alzheimer disease. Neurobiol Aging. 2009;30:1728–1736. doi: 10.1016/j.neurobiolaging.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Hunter JM, Kwan J, Malek-Ahmadi M, Maarouf CL, Kokjohn TA, Belden C, et al. Morphological and pathological evolution of the brain microcirculation in aging and Alzheimer's disease. PLoS ONE. 2012;7:e36893. doi: 10.1371/journal.pone.0036893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Simpson IA, Chundu KR, Davies-Hill T, Honer WG, Davies P. Decreased concentrations of GLUT1 and GLUT3 glucose transporters in the brains of patients with Alzheimer's disease. Ann Neurol. 1994;35:546–551. doi: 10.1002/ana.410350507. [DOI] [PubMed] [Google Scholar]
- Chen Z, Zhong C. Decoding Alzheimer's disease from perturbed cerebral glucose metabolism: implications for diagnostic and therapeutic strategies. Prog Neurobiol. 2013;108:21–43. doi: 10.1016/j.pneurobio.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer's disease. FDG-PET studies in MCI and AD. Eur J Nucl Med Mol Imaging. 2005;32:486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- Jagust WJ, Landau SM. Apolipoprotein E, not fibrillar beta-amyloid, reduces cerebral glucose metabolism in normal aging. J Neurosci. 2012;32:18227–18233. doi: 10.1523/JNEUROSCI.3266-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]